Abstract
The objective of this study was to investigate the modulatory effect of Chlorella vulgaris on cultured fibroblast cells derived from young and old aged individuals focusing on DNA damage, telomere length and telomerase activity. Dose-response test of the algal extract on cells in both age groups revealed that optimum viability was observed at a concentration of 50 µg/ml. Results obtained showed that Chlorella vulgaris exhibited protective effects against H2O2-induced oxidative stress as shown by the reduction in damaged DNA caused by H2O2 treatment (p<0.05) in Chlorella vulgaris pre- and post-treated groups (p<0.05). Pre-treatment of Chlorella vulgaris resulted in a significant decrease in DNA damage suggesting a bioprotective effect against free radical attacks. A decline in DNA damage was observed in post-treated cells which proves Chlorella vulgaris to present bioremediative properties. In cells induced with oxidative stress, telomere length decreased significantly coupled with a concomitant decline of telomerase activity (p<0.05). However, these reductions were prevented with prior and post treatment of Chlorella vulgaris. Therefore, we concluded that Chlorella vulgaris exhibited bioprotective effects especially in cells obtained from young donor but were more bioremediative for cells obtained from old donor as indicated by DNA damage, telomere shortening and reduction in telomerase activity.
Keywords: Chlorella vulgaris, DNA damage, telomere, telomerase, ageing
Introduction
Cellular ageing or senescence is a response towards stress stimuli resulting in the permanent withdrawal from normal cell cycle often with consequential morphological and functional transformation associated with the ensuing cellular homeostatic impairment. Senescence was first described in 1961 when Hayflick and Moorehead refuted the believe that cells are immortal in culture and showed the finite replicative ability of normal cultured fibroblast cells thus implicating a putative counting mechanism which has been dubbed by Hayflick as a ‘replicometer’ (Hayflick, 1998).
It is now generally accepted that the senescence phenomenon occurs in cells when the telomere ends of the chromosomes are shortened with each round of replication. It was shown that fibroblast cells cultured towards the end of their replicative life span have gradually eroded the telomeric ends of its chromosomes. It was also found that the mean telomere length decreased by two to three kilo base pairs (kbp) in its entire in vitro lifespan of several strains of cultured human diploid fibroblast cells (Harley et al., 1990). This, together with the discovery of the ribonucleoprotein enzyme terminal transferase called telomerase that has the ability to synthesize telomeres de novo (Greider and Blackburn, 1985) has provided the explanation for the exhaustible replicative capacity of normal somatic cells.
However, telomere erosion is not the only explanation for cellular ageing in vitro. It has been suggested that damage caused by accumulation of free radicals maybe a major contributing factor impacting various cell components thus contributing to the vitality and life span of cells. The free radical theory of ageing first suggested by Harman (1956) involving the biological action of reactive oxygen species (ROS) with the highest potential to inflict oxidative damage on a wide range of biological macromolecules (Gracy et al., 1999; Wickens, 2001). Despite its fundamental role in various intracellular reactions such as cellular metabolism and energy production, breakdown of molecular oxygen generates highly reactive agents including superoxide anions (O2-), hydroxyl radicals (·OH), hydrogen peroxide (H2O2), ozone (O3) and peroxyl radicals (ROO·) (Wickens, 2001), all of which may ultimately accelerate the process of senescence (Euralimsky and Kurz, 2005).
Although the human body has its own endogenous defence system consisting of antioxidant enzymes and small molecule antioxidants to stave off the negative effects of these reactive agents, free radicals accumulated over the human lifetime renders the endogenous system less effective. It has been suggested that this can be overcome by supplementation of an exogenous source of antioxidants mainly through diet. Many in the search for the ever elusive ‘fountain of youth’ have delved into the field of nutraceutical research, which focuses on investigating the effect of dietary antioxidants as a means to potentially reverse or forestall the deleterious effect of the ageing process (Yamagishi et al., 2005; Yuan and Walsh, 2006).
Chlorella vulgaris is a microalgae that produces proteins, vitamins and other phytonutrients with high efficiency utilizing light and carbon dioxide (Tamiya, 1957). Numerous studies in relation to the health benefits of food have proven that the increased intake of green and yellow vegetables is associated significantly with a decrease in chronic and age related diseases. Nutritional studies of chlorella have revealed that these algae produced many intracellular phytochemicals namely the carotenoids, chlorophyll, tocopherols, ubiquinone, protein and others typical of green plants (Ichioka and Endo, 1973; Endo et al., 1974; Fukuchi and Endo, 1975). These phytochemicals are attributed to the antioxidant properties of C. vulgaris.
Beneficial effects of C. vulgaris have been reported but ongoing studies in relation to ageing is of interest to determine possible protective effects that may possibly reverse the effect of ageing that occurs in vivo and in vitro. Previous studies have also reported that different aged cells have different requirement for antioxidants. In our study, we have focused on ascertaining the possible protective or remedial effect of C. vulgaris hot water extract on normal human fibroblast cells derived from different aged individuals against cellular senescence. The aim of this study was to determine DNA damage, telomere length and telomerase activity of normal skin fibroblast cell lines derived from young and old individual donors grown with and without C. vulgaris treatment pre or post H2O2-induced oxidative stress.
Materials and Method
Chlorella vulgaris hot water extracts preparation
A sub-strain of green unicellular microalgae Chlorella vulgaris Beijerinck grown in Bold's Basal Media was subjected to hot water extraction as previously described by Hasegawa et al. (1999). Briefly, dried C. vulgaris cells were suspended in distilled water at a concentration of 10% w/v, boiled at 100°C for 20 mins and then centrifuged at 10 000 rpm for 20 mins. The supernatant was subsequently lyophilized to obtain C. vulgaris (CVE) which was then added to cell culture growth medium as supplementation.
Cell culture and treatment protocols
Human diploid fibroblast (HDF) cells obtained from two donor age categories were used viz. the young (22–31 years: CRL-2361) and old (61–74 years: CRL-2054) (American Type Cell Culture, ATCC). HDFs derived from young donor (YF) were grown in Iscove's Modification of Dulbeccos Medium (IMDM) (Thermo Electron, Australia) supplemented with 10% heat-inactivated fetal calf serum, 20 mM sodium carbonate (1M), 100 IU/ml penicillin (1%), 100 IU/ml streptomycin (1%) and 0.25 µg/ml amphostate (1%) (Flowlab, Australia). HDFs derived from old donor (OF) were grown in Eagle's Minimum Essential Medium (EMEM) supplemented with 10% heat-inactivated fetal calf serum, 20 mM sodium carbonate (1M), 20 mM HEPES (1M), 100 IU/ml penicillin (1%), 100 IU/ml streptomycin (1%), 100 µg/ml non-essential amino acid, 1% sodium pyruvate and 0.25 µg/ml amphostate (1%) (Flowlab, Australia). Cells were maintained at 37°C in a 5% CO2/air incubator.
HDFs of both age categories were divided into five treatment groups viz; control cells without any treatment, cells supplemented with optimum dose of C. vulgaris, cells induced with oxidative stress by sub-lethal dose of H2O2 (100 µM/ml for YF and 700 µM/ml for OF), cells pre-treated with C. vulgaris before oxidative stress induction and cells induced with oxidative stress by sub-lethal dose of H2O2 with post-treatment of CVE.
Chlorella vulgaris extract (CVE) dose-response test
HDFs from both age categories were seeded at 2 × 104 in 96-well plates and treated with CVE supplemented in growth medium of various concentrations (0 – 400 µg/ml at 50 µg/ml intervals) for 24 hrs. Cell viability for each treatment concentration was then determined colorimetrically using the Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay kit (Promega). Treatment concentration of CVE promoting highest viability of cells was chosen as the effective dose for further tests.
Assesment of DNA Damage
Levels of DNA damage in cells were assessed by single cell gel electrophoresis (SCGE) assay also known as the Comet assay according to the method previously described by Singh et al. 1994 with slight modifications. Briefly, 1 × 104 cells in 10 µl medium was suspended in 75 µl warm 0.6% low melting point (LMA) agarose (Boehringer Mannheim, Germany) (DNAse free, RNAse free) and was quickly pipetted onto a prepared frosted slide coated with 85 µl of 0.6% normal melting (NMA) agarose. Slides with cell suspension were covered with glass coverslips and subsequently placed in a coplin jar containing chilled lysis solution (10% DMSO, 1% Triton-X in alkaline lysis buffer: 2.5 M NaCl, 10 mM Tris, 100 mM Na2EDTA, pH 10) for one hr to completely digest cellular proteins. Next, the slides were removed from lysis solution and placed in a horizontal gel electrophoresis unit filled with cold electrophoresis buffer (10 mM NaOH and 200 mM Na2EDTA at pH 13.2). Slides were allowed to sit in the alkaline buffer for 20 mins to allow unwinding of DNA strands and expression of alkali labile damage. Electrophoresis was performed for 20 mins at 300 mA and 24 V. Following drop wise neutralization (Tris-HCl, pH 7.5), cells were stained by applying 1X ethidium bromide. The slides were examined and the tail length was measured with a fluorescence microscope (Carl Zeiss, Germany). The DNA migration of 100 randomly selected cells was examined for each sample.
Estimation of telomere length
DNA was extracted from samples using DNA PUREGENE® DNA Purification kit (Gentra, USA) and isopropanol. Purity of extract was measured by UV-spectrophotometry whereby an absorbance range between 1.8 and 2.0 will only be used. Genomic DNA was processed to determine average telomere length following the method from the TAGGG Telomere Length Assay Telo Kit (Roche). DNA (2 ug) was completely digested by a cocktail of equal amounts of restriction enzyme, Hinf1 and Rsa1 and incubated for two hrs at 37°C to produce terminal restriction fragments (TRF). Separation of the digested DNA was done by gel electrophoresis on 0.8% agarose gel for two hrs followed by Southern blot transfer to a nylon membrane in 20X SSC transfer buffer. DNA was then fix transferred by UV-crosslinking (120 mJ) for 20 mins. Next, the membrane was prehybridized in a digoxigenin (DIG) containing hybridization solution for 30 mins followed by a hybridization process using a combination of hybridization solution with a telomere probe for 3 hrs. Telomere signal was subsequently detected using an anti-DIG antibody. A chemiluminescence signal was produced and exposed to an x-ray film. The amount of telomeric DNA was calculated by integrating the volume of each smear in the Image Master Total Lab software (Amersham Pharm. Biotech). The mean TRF length was defined as: ∑ (ODi)/ ∑ (Odi/Li), where ODi is the densitometer output and Li the length of the DNA at position i.
Estimation of telomerase activity
Telomerase activity was assessed using a PCR-based TRAP assay kit; TRAPeze® Telomerase Detection kit (Chemicon, USA). Harvested cells (1.5 × 106 cells) were lysed in 200 µl CHAPS reagent and centrifuged at 13 000 g. Supernatant containing protein extract was analyzed using Bradford's reagent to estimate total protein concentration. The telomeric sequence substrate (TS primer) was allowed to extend by cell extract (0.75 µg of total protein) containing telomerase activity using TRAP reaction master mixture containing 2.5 µl 10X TRAP reaction buffer, 0.5 µl 50X dNTP mix, 0.5 µl TS primer, 0.5 µl TRAP primer mix, 0.4 µl Taq DNA polymerase and 19.8 µl PCR grade water for each sample. PCR amplication in a thermocycler was performed for 35 cycles (94°C for 30 seconds, 59°C for 30 seconds and 72°C for 1 min). PCR product was then resolved in 12.5% polyacrylamide gel in TBE buffer. Gel was then stained by silver staining and intensity of the DNA ladder 6 bp bands formed were quantified using the Image Master Total Lab software (Amersham Pharm. Biotech). Total telomerase activity was determined in Total Product Generated, TPG units whereby TPG values for each group were based on the various control sets and negative controls for each sample (heat treated).
Statistical Analysis
Each experiment was carried out in triplicates with at least 3 independent cultures with comparable results. Data are reported as mean ± SD of at least three experiments. All statistical analysis was done by the SPSS 11.0 statistical analysis software using the unpaired Student's t-test whereby p<0.05 was regarded as significant.
Results
Dose response of Chlorella vulgaris extract (CVE)
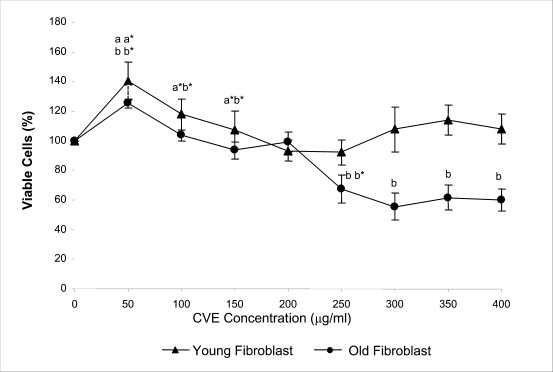
Incubation of fibroblast cells derived from young and old individuals with CVE for 24 hrs resulted in an increase in the number of viable cells (Figure 1). The effective dose for the algal hot water extracts that improved cell viability was at 50 µg/ml in fibroblasts derived from both young and old donors. An increase in the dosage of the algal extract of more than 50 µg/ml did not exhibit any added increase in the viability of the cells. Interestingly, a high dosage of CVE was found to give a toxic effect in fibroblasts derived from old donor whereby a decline in the cells' survivability was observed. In contrast, this toxic effect was not detected in fibroblast cells derived from young donor in which raising the concentration of CVE did not impair cell proliferation.
Figure 1.
Effect of CVE hot water extract on fibroblasts derived from young and old aged human as assessed by MTS assay. Percent MTS reduction corresponds to the viable cell number. Fibroblasts were incubated with increasing concentrations of CVE for 24 hours at 37oC. Incubation with CVE caused a significant increase in the number of cell viable. The percentage of the viable cell was highest at CVE concentration of 50 ug/ml for fibroblasts from both young and old donors. The number of cell viable decreased with high concentration of CVE incubation. a,b Denotes p<0.05 compared to control, a*,b* p<0.05 compared to previous concentration for fibroblasts derived from both young and old donors respectively. Data is presented as means ± SD, n = 3.
Effect of Chlorella vulgaris extract (CVE) on H2O2-induced DNA damage
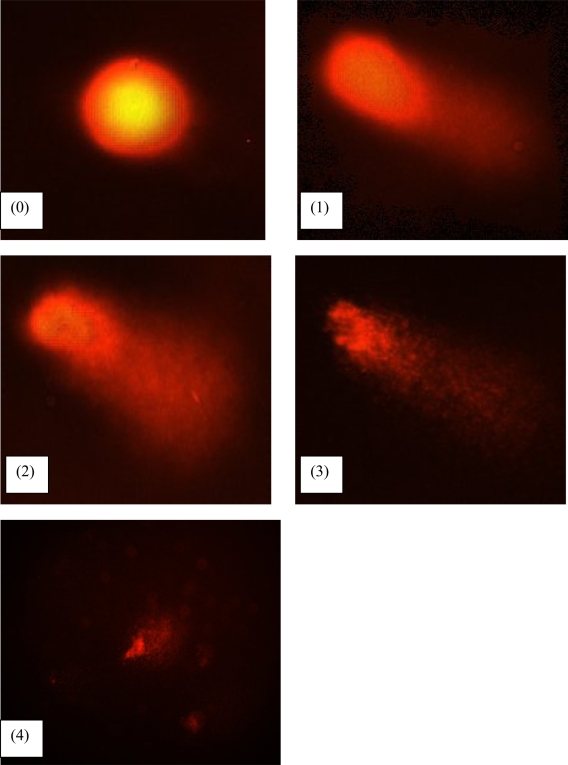
DNA damage was scored according to Heaton et al. (2002). Randomly selected cells (100 cells) were scored and categorized using an arbitrary scale (0–4) (Figure 2). Score 0 was given to cells with no DNA damage where the nucleus appeared round and intact without a comet tail. Score 1 was given to cells with slight damage whereby nuclei were present with comet tails up to two fold longer than nucleus diameter. Score 2 was given to cells with minimum damage to the DNA, breaks in the nucleus were observed whereby the nuclei were present with comet tails two to three fold longer than nucleus diameter and score 3 was given to cells with extensive damage to the nucleus with comet tails three fold longer than nucleus diameter. Score 4 was given to cells with extreme DNA damage, the cell nucleus was almost not visible with long and dispersed comet tail. Comet scores obtained was converted to arbitrary units using the equation; arbitrary unit = (0 × n0) + (1 × n1) + (2 × n2) + (3 × n3) + (4 × n4)
Figure 2.
Comets analysis by fluorescence microscopy with visual inspection of tail length of nuclei. The cell nuclei were classified into five categories: (0) undamaged (nuclei without comet tail); (1) low damaged (nuclei with comet tails up to two fold longer than nucleus diameter); (2) damaged (nuclei with comet tail two to three fold longer than nucleus diameter), (3) highly damaged (nuclei with comet tails three fold longer than nucleus diameter), and (4) severely damaged, respectively.
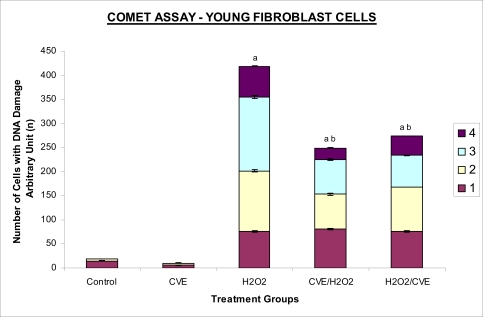
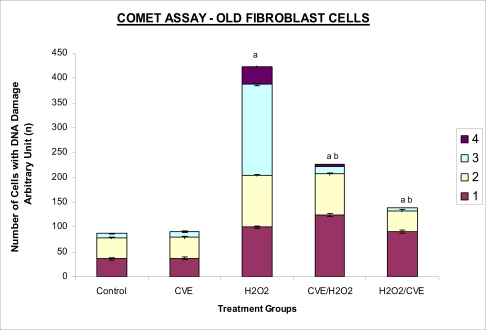
Treatment of fibroblasts with IC50 dose of H2O2 resulted in an increased number of cells with damaged DNA (p<0.05) (Figures 3a and 3b). In fibroblasts derived from both young and old donors, minumum DNA damage was observed in control with scores in the low range (score 1 and 2). Fibroblasts treated with CVE alone did not show any significant change in DNA damage while a significant reduction of damaged DNA was observed in cells induced with oxidative stress with pre- or post-treatment of CVE (p<0.05). In cells derived from both young and old donors, pre- and post-treatment with the algal extract coupled with an induction of oxidative stress resulted in protection as indicated by a reduction in the number of damaged DNA. It was found that fibroblasts derived from old donor responded better to a post-treatment of CVE as opposed to the young fibroblasts which fared better with a pre-treatment of CVE.
Figure 3a.
Protective effects of CVE against H2O2-induced DNA damage in fibroblasts cultures. Fibroblasts derived from young aged human were treated with optimum concentration of CVE for 24 hours at 37oC before or after H2O2 exposure (IC50) for 2 hours. DNA damage was assessed using Comet assay. Protective effect of CVE against H2O2-induced DNA damage was seen in fibroblast cells derived from young aged human. a Denotes p<0.05 compared to control, b p<0.05 compared to H2O2. Data is presented as mean ± SD, n=2.
Figure 3b.
Protective effects of CVE against H2O2-induced DNA damage in fibroblasts cultures. Fibroblasts derived from old aged human were treated with optimum concentration of CVE for 24 hours at 37oC before or after H2O2 exposure (IC50) for 2 hours. DNA damage was assessed using Comet assay. Protective effect of CVE against H2O2-induced DNA damage was seen in fibroblast cells derived from old aged human. a Denotes p<0.05 compared to control, b p<0.05 compared to H2O2. Data is presented as mean ± SD, n=2.
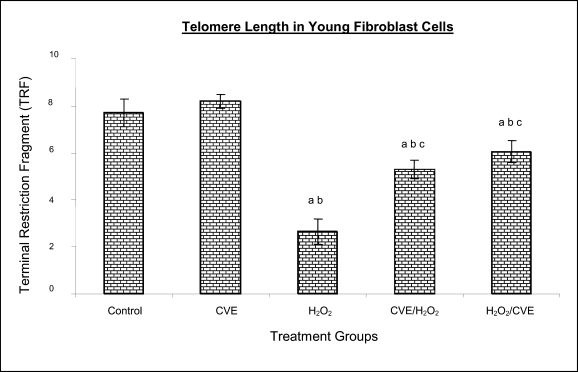
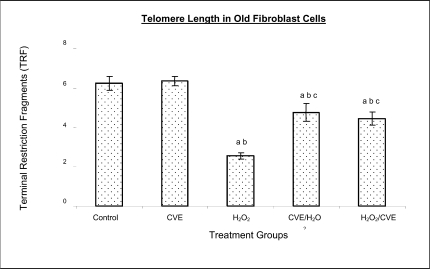
Effect of Chlorella vulgaris extract (CVE) on telomere length
Control cells and cells treated with CVE exhibited higher terminal restriction fragment (TRF) values compared to H2O2-treated cells (Figures 4a and 4b). Induction of oxidative stress decreased the TRF values but a pre-treatment or a post-treatment of C. vulgaris to the stressed cells caused a rise in TRF values to a significant degree when compared to the stressed cells (p<0.05). There was no significant difference observed between treating the cells prior or post induction of oxidative stress.
Figure 4a.
Effect of CVE on telomere length (TRF length) of human skin fibroblast cell line derived from young aged human. Cells were treated with optimum dose of CVE prior or after H2O2 exposure. Protective effects of CVE against H2O2-induced telomere shortening was observed in fibroblast cells derived from young aged human. a Denotes p<0.05 compared to control, b p<0.05 compared to CVE, c p<0.05 compared to H2O2. Data is presented as means ± SD, n = 3.
Figure 4b.
Effect of CVE on telomere length (TRF length) of human skin fibroblast cell line derived from old aged human. Cells were treated with optimum dose of CVE prior or after H2O2 exposure. Protective effects of CVE against H2O2-induced telomere shortening was observed in fibroblast cells derived from old aged human. a Denotes p<0.05 compared to control, b p<0.05 compared to CVE, c p<0.05 compared to H2O2. Data is presented as means ± SD, n = 3
In fibroblasts derived from both young and old donors, cells telomere attrition was significantly reduced when given a prior treatment of CVE before induction of oxidative stress as compared to cells induced with oxidative stress only (p<0.05). Cells from both age groups that were treated with CVE after induction of oxidative stress also exhibited reduced telomere erosion as compared to cells induced with oxidative stress only (p<0.05).
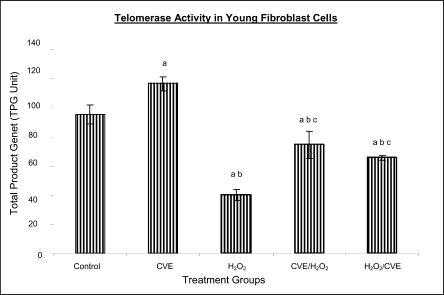
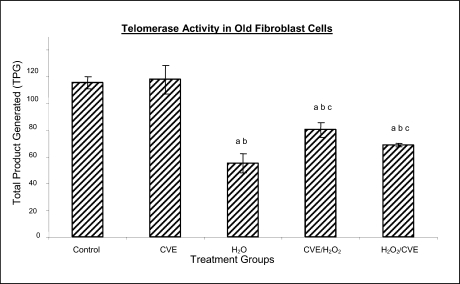
Effect of Chlorella vulgaris extract (CVE) on telomerase activity
Telomerase activity in fibroblast cells derived from young donor were significantly increased after treatment with CVE alone compared to control while fibroblasts derived from old donor were unaffected (Figures 5a and 5b).
Figure 5a.
Effect of CVE on telomerase activity (Total Product Generated, TPG) of human skin fibroblast cell line derived from young aged human. Cells were treated with optimum dose of CVE prior or after H2O2 exposure. Pretreatment with optimum dose of CVE protected against H2O2-induced decreased telomerase activity in fibroblast cells derived from young aged human. a Denotes p<0.05 compared to control, b p<0.05 compared to CVE, c p<0.05 compared to H2O2. Data is presented as means ± SD, n = 3.
Figure 5b.
Effect of CVE on telomerase activity (Total Product Generated, TPG) of human skin fibroblast cell line derived from old aged human. Cells were treated with optimum dose of CVE prior or after H2O2 exposure. Pretreatment with optimum dose of CVE protected against H2O2-induced decreased telomerase activity in fibroblast cells derived from old aged human. a Denotes p<0.05 compared to control, b p<0.05 compared to CVE, c p<0.05 compared to H2O2. Data is presented as means ± SD, n = 3.
Treatment of fibroblast cells with IC50 dose of H2O2 resulted in a reduction of telomerase activity (p<0.05). An increase in telomerase activity in fibroblasts derived from both young and old donors was observed when cells were treated with CVE prior to induction of oxidative stress as compared to cells induced with oxidative stress alone (p<0.05). Post-treatment of cells from both age groups with the algal extract after induction of oxidative stress increased the telomerase activity as compared to cells induced with oxidative stress alone (p<0.05).
Discussion
The present study evaluated the possible modulatory effects of Chlorella vulgaris against oxidative stress-induced DNA damage and telomere shortening of fibroblasts obtained from young and old donors. Extracts of C. vulgaris has previously been reported to show antitumour effect in fibrobsarcomas (Konishi et al., 1985; Hasegawa et al., 2002). Furthermore, the algal extract has also been shown to exhibit possible anticancer properties in hepatocellular carcinoma cell lines, HepG2 (Llovet et al., 2003; Md. Saad et al., 2006). There has also been reports of using C.vulgaris as a potential therapeutic agent against advanced glycation end product (AGE), in which AGE has long been recognized and implicated in the development of age related disorders such as artherosclerosis and diabetes (Yamagishi et al., 2005).
Our data showed that maximum viability was achieved when fibroblasts derived from both young and old donors were given a treatment dose of 50 µg/ml C. vulgaris extract. In the fibroblasts derived from young donor, increasing the algal extract dosage of up to 400 ug/ml did not affect overall cell viability which suggests that a high dose of C. vulgaris will not cause any detrimental effect on the cell survivability. Inversely, raising the extract dosage to 250 ug/ml and above did cause a decrease in the cell viability of fibroblast cells derived from old individual. However, the decline in cell viability of fibroblasts derived from old individual did not reach the 50% inhibitory concentration (IC50). This dwindling proliferative capacity may in fact represent a cytostatic but not cytotoxic nature of C. vulgaris on cells whereby the cells were arrested during the G0 phase in the cell cycle. Generally, aging cells accumulate DNA damage throughout its lifespan which may consequently lead to an irreversible growth arrest (Lim, 2006). In order to maintain its genetic integrity and viability, growth of cells with extensive DNA damage are retarded at the G1-S dan G2-M point in the cell cycle in order to proceed for cellular repair before entering either the mitosis phase or the apoptotic pathway which will inevitably lead to cell death.
The chemical compositon of C.vulgaris and its beneficial potential have not yet been fully recognized. Therefore, the decrease in cell viability of fibroblasts derived from old individual may be attributed to the various bioactive compounds in C.vulgaris reacting to inhibit growth in the cells that could not be repaired. Alternatively, it could also induce cell death (apoptosis or necrosis) in order to maintain normal cell homeostasis. This observation was in line with the findings of Md. Saad et al., (2006) who reported anticancer properties in HepG2 liver cancer cell line.
Hot water extracts of C.vulgaris in this study were not found to exert pro-oxidant capacity whereby its supplementation to fibroblast cells did not cause a rise in DNA damage compared to normal untreated cells. However, we had observed that oxidative stress induced by H2O2 resulted in an increased number of cells with damaged DNA in skin fibroblasts obtained from both the young and old individual donors. This is in complete agreement with numerous observations reporting DNA damage as a detrimental consequence of oxidative stress (Kawanishi et al. 2001). ROS are fundamental to any biochemical processes. Under normal conditions, equilibrium exists between the amounts of free radicals being generated and antioxidants available to quench or scavenge them thus protecting the organism against deleterious effects of oxidative stress (Sorg, 2004). Adversely, irrepressible increase in stress owing to free radical attacks causes a cessation of DNA replication and eventual senescence or cell mortality in cultured fibroblasts as was demonstrated when H2O2 was observed to directly stimulate endonuclease activity in renal tubular epithelial cells, leading to DNA fragmentation and cell death via apoptosis (Ueda and Shah, 1992).
C.vulgaris extract supplementation was found to provide protection against H2O2-induced DNA damage. When fibroblast cells were given a pre-treatment of C.vulgaris before induction of oxidative stress, we observed a significant decrease in DNA damage suggesting a bioprotective effect against free radical attacks. The same was also observed in oxidatively stressed cells post-treated with the algal extract whereby a decline in DNA damage ensued proving C.vulgaris to exhibit bioremediative properties against free radicals. The protective and remediative nature of C.vulgaris may be attributed to its chemical composition. During the algal growth process, as with other photosynthetic organisms, it is exposed to light and high concentration of oxygen which in turn forms various free radicals and oxidative agents (Dykens et al., 1992). However, absence of oxidative damage in its cell structure suggests an innate antioxidative protection system (Jimenez-Escrig et al., 2001). The high levels of antoxidants found in C.vulgaris such as carotenoids, astaxanthin, canthaxanthin, flavoxanthin, loraxanthin, neoxanthin, violaxanthin and also various phenolics such as salycylic, trans-cinnamic, synaptic and cafeic acid may prove to be a potent cocktail to prevent damage from occuring to DNA.
Both telomere erosion and decreased telomerase activity have been generally accepted as contributing factors in cellular ageing (Hayflick 2000). The shortening of telomere with H2O2 treatment in our study was consistent with previous accounts stating telomeres as particularly sensitive to oxidative stress (von Zglinicki, 2002). We have shown here that C.vulgaris treatment exhibited protection against H2O2-induced telomere shortening and reduction of telomerase activity in both young and old donors given either a pre-treatment or a post treatment of the algal extract. Therefore, the anti ageing effect of C.vulgaris may not only be due to its potent antioxidant property but through its role in maintaining telomere length possibly mediated by telomerase. Consistent with the telomere theory of ageing, activation of telomerase activity has been shown to sufficiently bypass replicative senescence in normal human cells (Bodnar et al., 1998). Telomerase influenced the stress response indirectly by elongating telomere length (Rubio et al., 2004). Our findings showed similar results whereby increased telomerase activity induced by C.vulgaris significantly increased telomere length in skin fibroblast obtained from young and old donors.
The progressive shortening of telomere has been touted a cell division counter in proliferating fibroblasts, or ‘replicometer’ and its shortening down to a threshold length seems to be the best known predictor of senescence (Hayflick, 2000). However, a recent study reported that telomere shortening was largely dependent on the interplay of oxidative stress and antioxidant defense rather than to the counter clock of numbers of cell divisions (von Zglinicki, 2002). Unlike cells that have undergone necrosis or apoptosis caused by cytotoxic doses of H2O2, sublethal H2O2 treatment induces senescence-like growth arrest of human fibroblast (Dimri et al., 1995). The sublethal dose (IC50) of H2O2 used in this study induced shortening of telomere length and decreased the telomerase activity in skin fibroblast cells derived from young and old donors.
Results from this present study on telomerase activity in fibroblast cells suplemented with C.vulgaris alone showed that the enzyme did increase in fibroblasts derived from young individual but remained unchanged in fibroblasts derived from old individual. This proves that C.vulgaris does not cause any negative effect to telomerase but might actually improve overall telomerase activity. It is known that inhibiting telomerase may cause arrested proliferation and changes in the cell cycle which could in turn lead to apoptosis (Boklan et al., 2002).
We have observed oxidatively stressed fibroblast cells given a pre- or post-treatment of C.vulgaris raised telomerase activity significantly compared to non treated stressed cells. Being a form of protein, telomerase itself is open to any attacks from free radicals leading to modification of the enzyme such as cross linking, peptide fragmentation or changing amino acid constituents. Changes in secondary and tertiary structures of the protein may also cause the telomerase mechanism to alter when the enzyme integrity is compromised (Gracy et al., 1994).
Generally, elevated telomerase levels will result in increases in its replicative capacity due to the ensuing lengthening of telomeric sequence through the specific action of the enzyme. Zou et al., (2002) noted successful immortalization of Indian Muntjac deer cells (mammalian cells with lowest number of diploid chromosomes) after introducing exogenous telomerase catalytic subunit (hTERT). However, the higher level of telomerase in young fibroblast cells in comparison to old fibroblast cells indirectly corresponds to the analysis of its telomere length. A closer comparative examination of figures 4a and 5a indicates that despite an upregulation of telomerase activity by C.vulgaris extract treatment; there is no significant disparity in its telomere length compared to the untreated control young fibroblast cells. This phenomenon can be explained through the negative feedback mechanism of telomerase enzyme. Longer telomeres i.e. in young normal viable cells; contain a lot more negative regulators to suppress telomerase enzyme. It has been proposed that this may be a safe check system in the cells to prevent the cells from overproliferating (Hemann et al. 2001). Fibroblast cells are also known to only secrete telomerase during the S-phase of cell cycle. Therefore, telomere is believed to only elongate during this phase (Masutomi et al., 2003). Furthermore, there has also been reports whereby normal human fibroblast cells expressing exogenous hTERT maintains telomerase activity although elongation did not occur during quiescent phase suggesting that telomerase only exerts effect on telomere during a specific cell cycle phase.
Conclusion
Our results showed that C.vulgaris increased the viability of skin fibroblasts obtained from young and old individual donors. H2O2 induced DNA damage, telomere length shortening and reduction in telomerase activity which was alleviated by C.vulgaris pre- and post-treatment in fibroblasts obtained from both young and old donors.
Acknowledgement
This study was funded by the Ministry of Science, Technology and Innovation under the E-Science Fund 02.01.02. SF0027 and Universiti Kebangsaan Malaysia.
References
- 1.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 2.Boklan J, Nanjangud G, Mackenzie KL, May C, Sadelain M, Moore MA. Limited proliferation and telomere dysfunction following telomerase inhibition in immortal murine fibroblasts. Cancer Res. 2002;62:2104–2114. [PubMed] [Google Scholar]
- 3.Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith OM, Peacocke M, Campisi J. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dykens J A, Shick J M, Benoit C, Buettner G R, Winston GW. Oxygen radical production in the sea anemone Anthopleura elegantissima and its endosymbiotic algae. J Exp Biol. 1992;168:219–241. [Google Scholar]
- 5.Endo H, Fukuchi S, Nakano I. Effect of culture condition on tocopherol formation in Chlorella Regularis. Annual Report of the Yakult Institute of Microbiology Research. 1974;5:91–98. [Google Scholar]
- 6.Euralimsky JD, Kurz DJ. Cellular senescence in vivo: Its relevance in ageing and cardiovascular diseases. Exp Gerontol. 2005;40:634–642. doi: 10.1016/j.exger.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Fukuchi S, Endo H. Effect of culture conditions on ubiquinone formation in Chlorella Regularis. Annual Report of the Yakult Institute of Microbiology Research. 1975;6:1–8. [Google Scholar]
- 8.Gracy RW, Talent JM, Kong Y, Conrad CC. Reactive oxygen species: the unavoidable environmental insult? Mut Res. 1999;428:17–22. doi: 10.1016/s1383-5742(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 9.Gracy RW, Yuksel KU, Schnackerz KD. ISOZYMES: Organization and Roles in Evolution, Genetics and Physiology. River Edge: World Scientific Publishing; 1994. Isozymes and aging: how do enzymes wear out? pp. 127–146. [Google Scholar]
- 10.Greider CW, Blackburn EH. Identification of a specific lelomere terminal transferase acivity in Telrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Free radicals and antioxidants: A personal view. Nutr Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 12.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 13.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Ho K, Ueno S, Kumammoto S, Ando Y, Yamada A, Nomoto K, Yasunobu Y. Oral administration of hot water extracts of Chlorella vulgaris reduces IgE production against milk casein in mice. Int J Immunopharmacol. 1999;21(5):311–323. doi: 10.1016/s0192-0561(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa T, Matsuguchi T, Noda L, Tanaka K, Kumamoto S, Shoyama Y, Yoshikai Y. Toll-like receptor 2 is at least partly involved in the anti tumor activity of glycoprotein from Chlorella vulgaris. Int J Immunopharmacol. 2002;2(4):579–589. doi: 10.1016/s1567-5769(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 16.Hayflick L. How and why we age. Exp Gerontol. 1998;33(7/8):639–653. doi: 10.1016/s0531-5565(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 17.Hayflick L. The future of ageing. Nature. 2000;408:267–269. doi: 10.1038/35041709. [DOI] [PubMed] [Google Scholar]
- 18.Heaton PR, Ransley R, Charlton CJ, Mann SJ, Stevenson J, Smith HE, Rawlings JM, Harper EJ. Application of Single-Cell Gel Electrophoresis (Comet) Assay for Assessing Levels of DNA Damage in Canine and Feline Leukocytes. J Nutr. 2002;132:1598–1603. doi: 10.1093/jn/132.6.1598S. [DOI] [PubMed] [Google Scholar]
- 19.Hemann MT, Strong MA, Hao L-Y, Grieder CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 20.Ichioka M, Endo H. Effect of light on cellular carotenoids formation in Chlorella regularis S-50 grown on glucose. Annual Report of the Yakult Institute of Microbiology Research. 1973;4:91–99. [Google Scholar]
- 21.Jimenez-Escrig A, Jimenez-Jimenez I, Pulido R, Saura-Calixto F. Antioxidant activity of fresh and processed edible seaweeds. J Science Food Agric. 2001;81:530–534. [Google Scholar]
- 22.Kawanishi S, Hiraku Y, Oikawa S. Mechanism of guanine-specific DNA damage by oxidative stress and its role in carcinogenesis and aging. Mutat Res. 2001;488:65–76. doi: 10.1016/s1383-5742(00)00059-4. [DOI] [PubMed] [Google Scholar]
- 23.Konishi F, Tanaka K, Himeno K, Taniguchi K, Nomoto K. Antitumor effect induced by a hot water extract of Chlorella vulgaris (CE): Resistance to Meth-A tumor growth mediated by CE-induced polymorphonuclear leukocytes. Cancer Immunol Immunothe. 1985;19:73–78. doi: 10.1007/BF00199712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim CS. Cellular senescence, cancer mortality, and organismal aging: A paradigm shift. Biochem Biophys Res Comm. 2006;344:1–2. doi: 10.1016/j.bbrc.2006.03.161. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 26.Masutomi K, Yu EY, Khurts S, Ben-Porath I, Currier JL, Metz GB, Brooks MW, Kaneko S, Murakami S, DeCaprio JA, Weinberg RA, Stewart SA, Hahn WC. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 27.Md Saad S, Mohd Yusof YA, Wan Ngah WZ. Comparison between locally produced Chlorella vulgaris and Chlorella vulgaris from Japan on proliferation and apoptosis of liver cancer cell line, HepG2. Mal J Biochem Mol Biol. 2006;13:32–36. [Google Scholar]
- 28.Rubio M, Davalos A, Campisi J. Telomere length regulates the effects of telomerase on the cellular response to genotoxic stress. Exp Cell Res. 2004;298:17–27. doi: 10.1016/j.yexcr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Singh WP, Stephens RE, Schneider EL. Modification of alkaline microgel electrophoresis for sensitive detection of DNA damage. Int J Radiant Biol. 1994;66:23–28. doi: 10.1080/09553009414550911. [DOI] [PubMed] [Google Scholar]
- 30.Sorg O. Oxidative stress: a theoretical model or a biological reality. CR Biol. 2004;327:649–662. doi: 10.1016/j.crvi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Tamiya H. Mass culture of algae. Ann Rev Plant Physiol. 1957;8:309–334. [Google Scholar]
- 32.Ueda N, Shah SV. Endonuclease-induced DNA damage and cell death in oxidant injury to renal tubular epithelial cells. J Clin Invest. 1992;90(6):2593–2597. doi: 10.1172/JCI116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 34.Wickens AP. Aging and the free radical theory. Resp Physiol. 2001;128:379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 35.Yamagishi S, Nakamura K, Inoue H. Therapeutic potentials of unicellular green alga Chlorella in advanced glycation end product (AGE)-related disorders. Med Hypot. 2005;65:953–955. doi: 10.1016/j.mehy.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Yuan YV, Walsh NA. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol. 2006;44:1144–1150. doi: 10.1016/j.fct.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Zou Y, Yi X, Wright WE, Shay JW. Human telomerase can immortalize Indian Muntjac cells. Exp Cell Res. 2002;281:63–76. doi: 10.1006/excr.2002.5645. [DOI] [PubMed] [Google Scholar]