Abstract
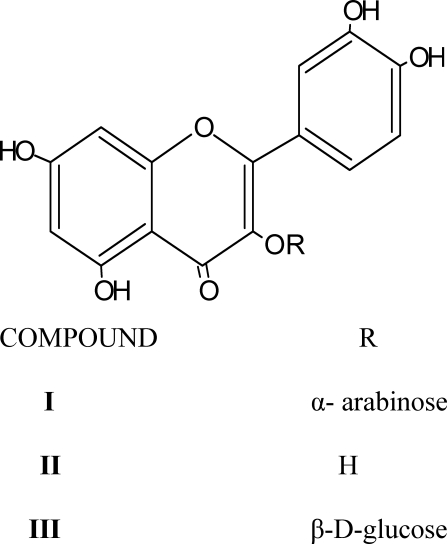
The bioactive ethyl acetate and N-butanol soluble parts of an ethanolic extract of Byrsocarpus coccineus leaves was subjected to column chromatography over silica gel G (60 – 120µ) and repeated purification of the flavonoid rich fraction over sephadex LH-20 eluted with methanol led to the isolation of three flavonoid glycosides identified as quercetin 3-O-α-arabinoside (I), quercetin(II) and quercetin 3-β-D-glucoside. Their structures were elucidated by 1H and 13C-NMR data and are reported here for the first time in this plant.
Keywords: Flavonoids Quercetin 3-O-α-arabinoside, Quercetin, Quercetin 3-O-β-D-glucoside
Introduction
The plant Byrsocarpus coccineus is indigenous to Africa especially Togo, Ghana and Nigeria (Dalziel, 1955). Folkloric uses of the plant include: The leaf decoction for venereal diseases and as antidote to arrow poisoning in Ghana; decoction of the leaves is applied to sore of mouth and skin, the Yorubas of Western Nigeria use the cold infusion of the bruised leaves for gonorrhoea, and the plant is considered as a urinary sedative (Irvine, 1961). A decoction of the leaves is drunk as remedy for pile, while the decoction of the whole plant is applied to swelling and tumours and also to arrest bleeding, the plant has also been reported as a remedy for diarrhoea (Akindele and Adeyemi, 2006). The uterotonic and molluscidal activities of the ethanol extract have been reported (Amos et al, 2002; Okunji and Iwu, 1988). We have found the antibacterial activity of the ethyl acetate and N-butanol soluble parts of the crude ethanol extract (Ahmadu et al, unpublished). A coumaroyl derivative have been isolated from the leaves of this plant Vickery and Vickery, (1980). To our knowledge, there had been no documented evidence in the isolation of flavonoids from this plant. In our continuous search for bioactive flavonoids from our medicinal plant, we report here the isolation of three flavonoids identified as quercetin 3-O-α-arabinoside, quercetin and quercetin 3-O-β-D-glucoside from the bioactive ethyl acetate and n-butanol soluble parts of ethanolic extract of Byrsocarpus coccineus.
Materials and Method
General Experimental Procedure
Column chromatography was performed using silica gel G (60 – 120µm) BDH, and Sephadex LH-20 (Sigma). 1H-and 13C NMR spectroscopy was carried out on Bruker DRX at 500MHz and 100MHz respectively. Chemical shifts values are given in ð-value (ppm) with tetramethylsilane (TMS) as internal standard.
The plant material consisting of the leaves where collected in the month of August 2005 in Samaru, Zaria, and authenticated at the Herbarium section, Biological Science Department, Ahmadu Bello University, Samaru-Zaria where a voucher specimen (864) was deposited.
Extraction and Isolation
The air-dried powdered leaves (220g) were extracted exhaustively with petroleum ether (60 – 80°C) using maceration. The combined extract was concentrated in vacuo to give a dark-green mass 6.5g (2.95%w/w). The marc (200g) was extracted to exhaustion with 95% ethanol to give a greenish mass 27.6g (12.95%w/w). 20g of the crude ethanolic extract was suspended in water and successively extracted with ethyl acetate (2 × 500ml) and N-butanol (3 × 500ml) and evaporation of the solvents at reduced pressure gave 1.4g of ethyl acetate (brownish - mass) and 1g of N-butanol (reddish-brown mass).
1.3g of the ethyl acetate soluble part was packed with 50g of silica gel G in a column (75cm × 2.0cm) and eluted gradiently with chloroform; chloroform: methanol mixtures 9: 1, 8: 2, 7: 3 and methanol. The progress of separation was monitored by thin layer chromatography using solvent system I: ethyl acetate : methanol : water 100 : 16.5 : 13.5 and solvent system II : ethyl acetate : formic acid : water 10 : 2 : 3. Fractions eluted with chloroform: methanol (9: 1) homogeneous on TLC afforded the flavonoid mixture (52mg). This was subjected to repeated column chromatography (25cm × 1.1cm) over sephadex LH-20 eluted with methanol to afford a yellowish-brown powder 8mg, mp 235–237°C. TLC using solvent system I, and II gave a single spot with Rf (0.61, and 0.72) respectively. Compound I was identified by 1H-NMR (ID&2D), and 13C-NMR (ID&2D), HSQC, DEPT. The same procedure was repeated for n-butanol soluble part (1g) to give compound II as a yellowish powder (5mg) mp 162–164°C and III (3.5mg) identified as quercetin and quercetin 3-O-β-D-glucoside respectively by direct comparison of the 1H-NMR with that reported in literature (Mabry and Markham, 1968, Mabry et al, 1970).
Results and Discussion
Compound I was obtained as a yellowish-brown powder. The UV spectrum showed absorption bands at 300,360 and 400nm characteristic of a flavonoid nucleus. The 1H-NMR δ (CD3OD): 5.17 (1H, d, 3.5Hz) suggest a monoglycoside moiety with an α - linked sugar, absence of a methyl protons signals and the presence of 5 carbons in the region of the 13C-NMR spectra (68 – 77ppm) (table 1) suggested a pentose sugar probably arabinose (Young et al, 1996). A methylene signal (CH2) was due to the C-5 of the pentose unit. The 1H-NMR δ (CD3OD):6.20(1H,d,J=1.2Hz),6.39(1H,d,J=1.5Hz) are due to metacoupled protons of A-ring (H-6 and H-8) of a flavonoid nucleus. Signals at δ = 6.88 d= 8.5Hz, 7.74d, 1.5Hz and δ = 7.58d,d, 2Hz, 8.0Hz are assigned to H-5/, H-2/ and H-6/ of the ring B, these suggest a quercetin nucleus (Mabry et al, 1970), (Markham et al, 1978)). The 13C-NMR spectra (table 1) revealed 20 carbon signals typical of flavonoid monoglycoside nucleus (Mabry and Markham, 1968). The low field signal at 179.5ppm was due to the carbonyl group at C-4. The anomeric carbon of arabinose appeared at δ = 104.8ppm. Complete assignment was aided by DEPT, direct C-H correlation HSQC and HMBC. Thus compound I was identified as Quercetin 3-O-α-arabinose (guajavarin). The spectra compares very well with that reported in literature (Lozoya et al, 1994). Table 2 shows the 1H-NMR spectral data of compound II and III and compare very well with that reported in literature (Mabry et al, 1970; Markham et al, 1978). On the basis of that compounds II and III were identified as quercetin and quercetin 3-O-β-D-glucoside.
Table 1.
1H-NMR of Compound I (Lozoya et al., 1994, Markham et al., 1978)
| Position C/H |
DEPT | δaH | δbC |
| 2 | C | 158.7 | |
| 3 | C | 135.7 | |
| 4 | C | 179.5 | |
| 5 | C | 163.2 | |
| 6 | CH | 6.20(d, 1.6Hz) | 100.1 |
| 7 | C | 166.8 | |
| 8 | CH | 6.39(d, 1.2Hz) | 94.90 |
| 9 | C | 158.60 | |
| 10 | C | 105.7 | |
| 1/ | C | 123.0 | |
| 2/ | CH | 7.74(d, 1.5Hz) | 117.6 |
| 3/ | C | 146.1 | |
| 4/ | C | 150.1 | |
| 5/ | CH | 6.88(d, 8.5Hz) | 116.3 |
| 6/ | CH | 7.58(d,d, 2.0Hz, 8.0Hz) | 123.16 |
| α-arabinose | |||
| 1// | CH | 5.17 (d, 3.5Hz) | 104.8 |
| 2// | CH | 73.02 | |
| 3// | CH | 69.29 | |
| 4// | CH | 3.65 – 3.62(d,d, 1.5, 7Hz) | 74.28 |
| 5// | CH2 | 3.46 – 3.48 (d, J = 7.0Hz) | 67.1 |
Table 2.
1H-NMR of Compound II and III
| H-Position | δaH | δbH |
| 6 | 6.18d(2Hz) | 6.21d(2Hz) |
| 8 | 6.38d(2Hz) | 6.41d(2Hz) |
| 2/ | 7.73d(2Hz) | 7.76d(2Hz) |
| 5/ | 6.87d(8Hz) | 6.87d(2Hz) |
| 6/ | 7.62d,d(2,8Hz) | 7.58d,d(2,8Hz) |
| β-glucose | ||
| 1 | 5.20d(7Hz) | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | ||
| 3.64d,d(1,7Hz) |
a = compound II
b = compound III
Acknowledgement
The authors wish to acknowledge the assistance of Dr. Simon Gibbons of the Centre for Phytotherapy Research, University of London for the NMR analysis.
References
- 1.Akindele A J, Adeyemi O O. Evaluation of antidiarrhoeal activity of Byrsocarpus coccineus. J Ethnopharmacol. 2006;108(1):20–25. doi: 10.1016/j.jep.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Amos S, Binda L, Kunle OF, Wambebe C, Gamaniel K. Uterotonic Properties of the ethanol extract of Byrsocarpus coccineus. Pharml Biol. 2002;40(1):33–38. [Google Scholar]
- 3.Dalziel J M. The useful plants of West Tropical Africa. London: A Crown Agent for Oversea Publication; 1955. pp. 568–570. [Google Scholar]
- 4.Irvine F R. Woody Plants of Ghana. London: An Oxford University Press Publication; 1961. pp. 568–570. [Google Scholar]
- 5.Lozoya X, Meckes M, Abou-Zaid M, Turtoiello J, Nozzolillio C, Arnson J. Quercetin glycosides in Psidium guajava L. leaves and determination of spasmolytic principle. Arch Med Res. 1994;25(1):11–16. [PubMed] [Google Scholar]
- 6.Mabry T J, Markham K R, Thomas M B. The Systematic Identification of Flavonoids. New York: Springer-Verlag Publication; 1970. pp. 261–266.pp. 294 [Google Scholar]
- 7.Mabry T J, Markham K R. Flavonoids from Bauhinia species. Tetrahedron. 1968;24:823–826. [Google Scholar]
- 8.Markham K R, Ternai B, Stanley R, Geiger H, Mabry T J. 13C-NMR Studies of Flavonoids II. Tetrahedron. 1978;34:1391–1397. [Google Scholar]
- 9.Okunji C O, Iwu M M. Control of Schistosomiasis using Nigerian Medicinal plants as molluscicides. Int J crude Drug Res. 1988;26(4):246–252. [Google Scholar]
- 10.Vickery M, Vickery B. Coumarins and related compounds in members of the Connaraceae. Toxicol Letter. 1980;5:115–118. doi: 10.1016/0378-4274(80)90159-9. [DOI] [PubMed] [Google Scholar]
- 11.Young H C, Young HL, Hosup Y, Jingwood K. A Flavonoid diglycoside from Lepisorus ussuriensis. Phytochemistry. 1996;43(5):1111–1113. doi: 10.1016/s0031-9422(96)00417-7. [DOI] [PubMed] [Google Scholar]