Abstract
The methanolic extract and conessine isolated from the stem bark of Holarrhena floribunda (Hf) were tested for their antibacterial activities on Bacillus: Bacillus cereus, Bacillus subtilis, Bacillus megaterium and Bacillus stearothermophilus using the disc diffusion method. Phytochemical analysis of the crude extract and fractions was also conducted. The inhibition parameters of the crude methanol extract and the total alkaloid fraction were determined using the macrodilution method. The results showed that the crude extract, the total alkaloid fraction and conessine exhibited a significant antibacterial effect against all the strains studied. The antibacterial effect of conessine is almost similar to that of chloramphenicol used as reference. The ratio of the minimal bactericidal concentration (MBC) over the minimal inhibitive concentration (MIC) indicates the bactericidal effect of the plant. These results support common use of stem bark of Hf and conessine isolated from Hf in the treatment of some infectious diseases.
Keywords: Antibacterial activities, Holarrhena floribunda, Bacillus, conessine
Introduction
Holarrhena floribunda, a plant belonging to the Apocynaceae family (Letouzey, 1972), is widely distributed in the Centre and West provinces of Cameroon. Its stem bark is used in Cameroon to treat various ailments such as abdominal pains, nausea, indigestion, diarrhoea (Berhaut, 1971). However, little experimental studies have been done on the biological properties of this plant. The stem bark of H. floribunda is febrifuge and could be a quinine substitute, since it showed remarkable inhibitory activity against drug-resistant strains of Plasmodium falciparum (Fotie et al., 2006). It contains conessine that is used for the destruction of amoeba without emetic effect. (Berhaut, 1971). However, there is no attempt to study the potential of H. floribunda's antibacterial activities against microorganisms that cause food poisoning and food alteration. The present study was conducted to investigate the antibacterial properties of the crude methanol extract and conessine from the stem bark of H. floribunda against four strains of Bacillus.
Materials and methods
Plant material
The stem bark of H. floribunda was collected in Ndikinimeki, in the Centre Province of Cameroon, in January 2003. The biological identification of the plant was done by the National Herbarium in Yaounde, where the voucher specimen is conserved under the reference number, 20147/SFR/CAM.
Extraction and purification
The stem bark of H. floribunda was air dried and pulverized. The resulting powder (5.4kg) was macerated in methanol for 72h. The concentrated methanolic extract (224g) was treated with hydrochloric acid (5%). The aqueous solution was made alkaline with ammonia and extracted with ethyl acetate. The methanolic extract (A), the ethyl acetate extract (B) and the extract remain (C) were screened for antibacterial activity. The active fraction B was subjected to a bio-guided fractionation by flash chromatography on aluminia column. Gradient elution using hexane (Hex) ethyl acetate (EA) to give eight fractions (F1–F8). The active fraction F1 (2.74g) obtained with Hex-EA (25–75%) was purified by flash chromatography on aluminia column gradienty eluted with hexane-ethyl acetate to afford ten fractions (FA1–FA10). The active fraction FA1 was crystallised from acetone to yield 106 mg of shiny pink crystals identified as conessine on the basis of spectroscopic data and comparison of them with the data reported (Goutarel, 1964).
Microorganisms
The test microorganisms: Bacillus cereus F3748, Bacillus subtilis NCTC 3610, Bacillus megaterium 8174 were obtained from the Microbiology Laboratory, Institute of Food Research Reading, UK and Bacillus stearothermophilus CNCH 5781 from the Institute Appert, France.
Phytochemical tests
Phytochemical tests were carried out as described by Odebiyi and Sofowara, 1978. Each of the test was qualitatively expressed as negative (−) or positive (+).
Antibacterial activity Disc diffusion method
The crude extract (A), total alkaloid fraction (B) and residue (C) were dissolved in the methanol to a final concentration of 10 mg/ml. Conessine was dissolved in methanol to a final concentration of 3 mg/ml. Antibacterial tests were then carried out by the disc diffusion method (Murray et al., 1995) using 100 µ l of suspension containing 108cfu/ml of bacteria spread on nutrient agar. Sterile paper discs (6mm in diameter) prepared from whatman number 1 filter paper were impregnated with A (1200 µ g/disc), B (500 µ g/disc), C (500 µ g/disc) and conessine (30 µ g/disc) placed on the inoculated agar. Negative control was prepared with methanol. Chloramphénicol (10 µ g/disc) was used as a positive reference standard for the susceptibility test. The inoculated plates were incubated at appropriate temperature for 24h. The antibacterial activity was evaluated by measuring the zone of inhibition against the test organisms. Each assay in this experiment was repeated twice. (Carbonnelle et al., 1987).
Macrodilution assays
The minimal inhibitive concentration and the minimal bactericidal concentration values were also studied for the microorganisms that were determined as sensitive in the disc diffusion method.The bacterial strains were cultured overnight at appropriate temperature in nutrient agar. The test strains were suspended in nutrient broth to give a final density of 5 × 105cfu/ml and these were confirmed by viable count. The crude extract and the total alkaloid in 10% DMSO were first diluted to the highest concentrations (1200 µ g/ml and 600 µ g/ml respectively), and then serial two-fold dilutions were made with nutrient broth in the concentration range from 37.5 to 1200 µ g/ml for the crude extract and 19 to 600 µ g/ml for the total alkaloid fraction in 10 ml sterile test tubes containing 1 ml of the inocula. The final volume in each tube was 5 ml (Zgoda and Porter 2001). The last tube containing 4 ml of nutrient broth without extract and 1ml of the inocula was used as a negative control. Tubes containing the same concentration of extracts without microorganism were also used as sterility control. Chloramphénicol at the concentration range of 5 to 80 µ g/ml was prepared in nutrient broth and used as positive control. The contents of each tube were mixed on a plate shaker at 300 rpm for 20s and then incubated at appropriate temperature for 18h. Bacterial growth was determined by absorbance at 600 nm using the spectrophotometer UV-120-01. These values of absorbance were used to draw a concordance curve from which MIC, MBC and the dose that inhibits 50% growth after the incubation period (IC50) were determined (carbonnelle et al., 1987).
Results
The results of our assay on the crude extract, the total alkaloid fraction and the residue are shown on Table 1. The crude extract showed the presence of alkaloids, saponins, tannins, polyphenols, cardiac glucosides, triterpenes, leucoanthocyans and sugars; with the absence of phlobatannins, flavonoids, lipids, anthraquinones, sterols and glucosides. Saponins, tannins, polyphenols, cardiac glucosides, triterpenes, leucoanthocyans, and sugars were detected in the residue.
Table 1.
Phytochemical screening of the crude methanol extract, the total alkaloid fraction and the residue of Holarrhena floribunda.
| Extracts | Chemical Groups | |||||||||||||
| Alk | Sap | Tan | Glu | Pph | Cgl | Tri | Leu | Su | Phl | Fla | Lip | Anth | Ster | |
| Crude Extract |
+ | + | + | − | + | + | + | + | + | − | − | − | − | − |
| Total alkaloid |
+ | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Residue | − | + | + | − | + | + | + | + | + | − | - | − | − | − |
Alk: Alkaloids Pph: Polyphenols Su: Sugars Anth: Anthraquinones Sap: Saponins Cgl: Cardiac glucosides Phl: Phlobatannins Ster: Setrols Tan: Tannins Tri: Triterpenes Fla: Flavonoids Glu: Glucosides Leu: Leucoanthocyans Lip: Lipids
The bio-guided purification of the total alkaloid fraction from the crude extract led to the isolation of the active compound. This compound was identified as conessine.
Conessine
The results in Table 2 indicated that the crude extract, the total alkaloid fraction and conessine possessed significant antibacterial activities against all the microorganisms tested. The residue was inactive.
Table 2.
Antibacterial activity of Holarrhena floribunda extracts from the disc diffusion method.
| Extracts and compound tested |
Inhibition zone diameters (mm) | |||
| Bacterial stains | ||||
| B. cereus | B. subtilis | B. megaterium |
B. stearothermophilus |
|
| Chloramphenicol (10 µ g) |
22 | 26 | 21 | 29 |
| Crude extract (1200 µ g) |
17 | 18 | 19 | 22 |
| Total alkaloid fraction (500 µ g) |
18 | 22 | 23 | 23.5 |
| Conessine (30 µ g) | 18 | 23 | 23 | 24.5 |
| Residue (500 µ g) | - | - | - | - |
| Negative control | - | - | - | - |
(-): absence of inhibition zones
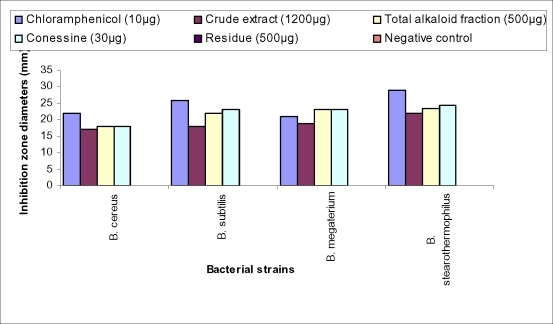
Figure 1 illustrates the zone of lysis by the disc diffusion method using the crude methanol extract, the total alkaloid fraction, the residue and conessine against four Bacillus species, compared to chloramphenicol. The antibacterial effect of these extracts and conessine was found to be comparable to the antibacterial activity exhibited by chloramphenicol.
Figure 1.
Zone of lysis measured in mm by disc diffusion method for the methanol crude extract, the total alkaloid fraction, the residue and conessine from Holarrhena floribunda on four Bacillus species compared to chloramphenicol.
The MIC, MBC, and IC50 were determined for the microorganisms that were identified as being sensitive to the extracts in the disc diffusion method. The results are shown in Table 3.
Table 3.
MIC, MBC and IC50 values (µ g/ml) of Holarrhena floribunda extracts in the macrodilution assays compared to chloramphenicol.
| Crude extract | Total alkaloid fraction | chloramphenicol | ||||||||||
| Inhibition parameters Bacterial strains |
MIC | MBC | IC50 | MIC | MBC | IC50 | MIC | MBC | IC50 | |||
| B. cereus | 215 | 390 | 110 | 1.8 | 150 | 280 | 70 | 1.8 | 18.5 | 33 | 9.5 | 1.8 |
| B. subtilis | 185 | 320 | 100 | 1.7 | 95 | 170 | 45 | 1.8 | 9 | 16 | 4.5 | 1.8 |
| B. megaterium | 175 | 317 | 90 | 1.8 | 90 | 160 | 40 | 1.8 | 36 | 70.5 | 18 | 1.9 |
|
B. stearother mophilus |
115 | 210 | 55 | 1.8 | 50 | 105 | 30 | 2 | 8.5 | 14.5 | 4.5 | 1.7 |
Discussion
The extracts of H. floribunda have significant antibacterial properties against all the microorganisms tested. However, greater and remarkable antibacterial activities of the crude extract, the total alkaloid fraction and conessine were observed on Bacillus stearothermophilus. The inhibition zone values were in the range of 22 mm, 23.5 mm and 24.5 mm respectively at a concentration of 10 mg/ml (Table 2). Maximum MIC values were recorded with Bacillus cereus and were 215 µ g/ml for the crude extract and 150 µ g/ml for the total alkaloid fraction (Table 3). In the diffusion method in solid medium, 30 µg of conessine and 10 µg of chloramphenicol showed almost similar antibacterial effects.
These results together with the phytochemical analysis, suggest that the biological activity obtained is attributed to conessine. The antibacterial activity of this compound is due to the formation of pores in the cell walls and the leakage of cytoplasmic constituents by this active compound present in this plant (Gnanamani et al., 2003). In all the cases, the ratio suggested that H. floribunda has a bactericidal effect.
Conclusion
Bacillus stearothermophilus, B. cereus, B. subtilis and B. megaterium were found to be sensitive to H. floribunda extracts. The results therefore, suggest that H. floribunda extracts possess antibacterial activity. The bio-guided purification of this plant showed that the antibacterial activity is attributed to conessine. This active compound can be exploited as an antibacterial agent for the production of new drugs against food poisoning.
References
- 1.Berhaut Jean. Flore illustrée du Sénégal. Gouvernement du Sénégal, Ministère du développement rural, Direction des eaux et forets Dakar; 1971. p. 385.p. 386. [Google Scholar]
- 2.Carbonnelle B, Denis F, Marmonier A, Pinon G, Vague R. Bactériologie médicale. Edition SIMED SA; 1987. Techniques usuell; p. 9.p. 15.p. 227.p. 232. [Google Scholar]
- 3.Fotie J, Bohle Scott D, Leimanis Mara L, Elias G, Geoffrey R, Nkenfack E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J Nat Prod. 2006;69(1):62–67. doi: 10.1021/np050315y. [DOI] [PubMed] [Google Scholar]
- 4.Gnanamani A, Priya Shanmuga K, Radhakrishnan N, Babu Mary. Antibacterial activity of two plants extracts on eight burn pathogens. J Ethnopharmacol. 2003;86(1):59–61. doi: 10.1016/s0378-8741(03)00044-8. [DOI] [PubMed] [Google Scholar]
- 5.Goutarel R. Les alcaloïdes stéroidiques des Apocynaceae, Herman, Paris. 1964. pp. 74–76. [Google Scholar]
- 6.Letouzey R. Manuel de botanique forestière Afrique Tropicale, Tome 2B. 45 bis Avenue de la Belle Gabrielle, 94 Nogent S/Marne: Centre technique forestière tropicale; 1972. p. 304.p. 306. [Google Scholar]
- 7.Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolke R H. Manual of Clinical Microbiology. 6th edition. Washington, DC: ASM; 1995. [Google Scholar]
- 8.Odebiyi O, Sofowara E A. Phytochemical screening. Nigeria medical plants. Loydia. 1978;41:231–234. [PubMed] [Google Scholar]
- 9.Zgoda J R, Porter J R. A convenient microdilution method for screening natural products against bacteria and fungi. Pharmaceutical Biol. 2001;39:221–225. [Google Scholar]