Abstract
Hydrodistillation of berries and leaves of Juniperus phoenicea grown in Sinai yielded volatile oils in the yield of 0.36 and 1.96%, respectively. Using gas chromatography/mass spectrometry technique, fifty eight compounds were identified in berry oil representing 99.2% of the oil composition. α-Pinene was the major compound in berry oil (39.30%) followed by sabinene (24.29%). Berry oil composed mainly of monoterpenoids which amounted to 90.53%, of which 72.85% was monoterpene hydrocarbons. The sesquiterpenoids accounted for about 8% of the total oil composition. Leaf oil was composed of about 66 compounds representing 99.16% of the total composition of the oil. α-Pinene was the major constituent of leaf oil at concentration of 38.22%, followed by α -cedrol (31.23%). The monoterpene hydrocarbon was the predominant chemical group (41.29%) followed by the oxygenated sesquiterpenes (32.21%). Both oils showed very high cytotoxic activities against all cell line tested. They showed equal activities against brain (0.6 µg//ml) and cervix (5.0 µg//ml) human cell lines, while berry oil was slightly more active than leaf oil against lung (0.6 and 0.7 µ/ml, respectively), liver (0.7 and 0.9 µg//ml, respectively) and breast human cell lines (0.8 and 1. µg//ml, respectively).
The antimicrobial activity and minimum inhibitory concentration (MIC) of leaf and berry oils were also determined. The oils showed high activity against most of the tested strains.
Keywords: Juniperus phoenicea, Cupressaceae, essential oils, berries, leaves, antimicrobial, cytotoxic, GC-MS analysis
Introduction
The most famous of the oils of junipers is Juniperus communis L. They are used in aromatherapy, through inhalation, massage, bathing, or ingestion to create good health and beauty. Oil of juniper, distilled from the wood and leaves of several species, is used in perfumes. It is noted by German authorities that the tea is diuretic and urinary antiseptic (Hagar, 1979). The diuretic activity is thought to be largely due to the tea's content of terpinen-4-ol, a non irritating terpene. Juniper berry tea is listed in the German Pharmacopoeia as a digestive aid, both stimulating appetite as well as relieving flatulence. The oils also help increase the flow of digestive fluids, improve digestion and eliminate gas and stomach cramping (Uphof, 1968). Juniper has been used for centuries as a steam inhalant for bronchitis, and to control arthritis. The oil is also irritating to microbes, so much so that it kills many of them (Watt and Breyer-Brandwijk, 1962; Stassi et al., 1996). Traditional formulas are in combination with ginger and dong quai or with goldenseal or with Uva ursi. Juniper berry is probably best known as the unique flavouring agent of gin, an important component of the dry martini, a popular intoxicant and a putative calmative revered by western culture for over 300 years (Duke, 1980).
J. phoenicea L. (Fam. Cupressaceae) is the species found in Egypt. It is grown in Sinai (Yelleg, Halal and Maghara mountains) in rocky ridges. It extends to Central Arabia (Boulos, 1999). The leaf essential oil of J. phoenicea has been reported in varying details from Saudi Arabia (Dawidar et al., 1991), France (Tabacik and LaPorte, 1971; Tabacik and Poisson, 1971) and from Greece and Spain (Adams et al., 1996). Also, there are some reports on the analysis of fruit essential oils (Ramic and Murko, 1983; De Pascual et al., 1981; Delitala, 1980). Only one report dealt with composition of leaf and berry essential oils of J. phoenicea grown in Egypt (Afifi et al., 1992) in which 18 compounds in both oils were identified. In this paper, we report on the chemical composition of the essential oils of leaves and berries of J. phoenicea L grown in Egypt and their cytotoxic and antimicrobial activities.
Experimental
Plant materials
J. phoenicae leaves and berries were collected from Sinai, Egypt from fully grown plants when berries were at the ripening stage. They were identified in the Herbarium Department, Faculty of Sciences, Cairo University. A voucher specimen (voucher number J-3) was deposited in Pharmacognosy department, National Research Centre, Cairo.
Distillation of the volatile oils
The volatile oils of the dry leaves and berries were prepared by hydrodistillation for three hours according to Guenther (1961). The prepared volatile oils were dehydrated over anhydrous sodium sulphate and stored in refrigerator until analyzed.
Gas chromatography-Mass spectrometry
A Trace GC 2000 (produced by Thermo) was used. The Mass Spectrometer model SSQ 7000 produced by Finnigan equipped with library software Wiley 138 and NBS 75 was used. Capillary DB5 (Methylpolysiloxane containing 5% phenyl groups) column 25m X 0.25 mm i.d., Temp. program was; 2 min. at 60° C, 60-100° C (2° C/min) and 100–250° C (5° C /min). Carrier gas was helium at flow rate of 1.0 ml/min. Injection voltage 70 eV.; scan mass range 40–450.
Identification of compounds
Compounds were identified by matching their mass spectra with those recorded in the MS library and further confirmed by injecting the authentic samples of different available compounds with the volatile oil and by comparing the mass spectra with those of reference compounds or with the published data (Adams, 1995).
Measurement of potential cytotoxicity by SRB assay
Potential cytotoxicity of leaf and berry essential oils was tested using the method of Skehan and Storeng (1990). Cells were plated in 96-multiwell plate (104 cell/well) for 24 hours before treatment with the oils to allow attachment of cell to the wall of the plate. Different concentrations of the oils (0, 1, 2.5, 5 and 10 µg/ml) were added to the cell monolayer. Triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the oils for 48 hours at 37° C and in atmosphere of 5% CO2. After 48 h, cells were fixed, washed and stained with sulforhodamine B stain. Excess stain was washed with acetic acid and attached stain was recovered with Tris EDTA buffer. Color intensity was measured in an ELISA reader. The relation between surviving fraction and drug concentration was plotted to get the survival curve of the tumor cell line after the specified compound.
Antimicrobial activities
Microbial strains
The antimicrobial activity of the essential oils was tested against four bacterial strains, six fungal strains, and six yeast strains. Test organisms used were obtained from the Faculty of Agriculture, Cairo University.
Agar diffusion method
This method was carried out according to Collins and Lyne (1985). Nutrient agar (NA) was used for cultivation of bacteria and yeast, and Czapek-Dox's for cultivation of fungi. In this method, presterilized paper discs (0.5 mm in diameter) were impregnated with 100 µl of each oil and applied on the surface of agar plates freshly seeded with standard inoculums of young cultures, 24 h old bacteria and yeast, and 7 days old fungi. The plates of test organisms were then incubated at 27° C for 24 h for bacteria and yeast and 48 h for fungi. At the end of the incubation period, the inhibition zones were measured (the experiment were designed, the results are averages of triplicates).
Determination of minimum inhibitory concentration (MIC)
MIC values were determined by testing representative organisms in liquid NA (for bacteria and yeast) and Czapek-Dox's solutions, using the method reported by Gardener and Provine (1984). Stock solutions of known concentrations of the tested extracts were prepared and serial dilutions (ranging from 333.33 to 0.016 mg/ml) were made. The assay tubes were inoculated with standard inocula prepared from young cultures. The minimum dilution in which no visible growth was observed is the lowest inhibitory concentration of the extract. MIC were exhibited in terms of µg/ml media after 24 hrs incubation at 27±1° C for bacteria and yeast and 48–72 h for fungi.
Results and discussion
Chemical composition
Oils from leaves and berries of J. phoenicea were obtained in comparatively high yield (0.36 and 1.96% v/w, respectively). Berry oil was characterized by spicy aroma, while that of the leaves was characterized by pleasant odor. Results of GC/MS analysis of essential oils of leaves and berries are listed in Table 1. In contrast to Afifi et al. (1992) who stated that the major constituent of both leaf and berry oils of the Egyptian Juniperus was sabinene, it was found that α-pinene was the major constituent of both oils recording 39.3% for berry oil and 38.22% for leaf oil, while sabinene was the second major constituent in berry oil recording 24.29%. β-pinene was also found in considerable amounts in both oils (2.45% and 0.48% in berry and leaf oils, respectively). These results are in agreement with those reported on the analysis of other juniper oils (Adams et al., 1996) in which pinane derivatives are more common. On the other hand, this report agreed with Afifi et al. (1992) in that a-cedrol was the second major component in the leaf oil (31.23%) and its amount was far less in berry oil (0.47%).
Table 1.
Composition of the volatile oils of berries and leaves of Juniperus Phoenicea L grown in Egypt.
| Compound | KI | berries | leaves |
| Area% | Area% | ||
| Terpenoids | |||
| (a) Monoterpene hydrocarbons | |||
| α-Pinene | 939 | 39.30 | 38.22 |
| Camphene | 953 | - | 0.02 |
| Sabinene | 976 | 24.29 | - |
| 2,4 (10)Thujadiene | 957 | 0.14 | - |
| β-Pinene | 980 | 2.45 | 0.48 |
| Myrcene | 991 | - | 0.45 |
| α-Phellandrene | 1005 | 1.01 | - |
| δ-3-Carene | 1011 | 1.25 | 0.99 |
| α-Terpinene | 1018 | 0.05 | 0.03 |
| Limonene | 1031 | - | 0.74 |
| β-Phellandrene | 1031 | 4.13 | - |
| Cis-Ocimene | 1040 | - | 0.01 |
| γ-Terpinene | 1062 | 0.07 | 0.14 |
| Terpinolene | 1088 | 0.16 | 0.21 |
| (b) Oxygenated monoterpenes | |||
| Camphenone | 1093 | 0.50 | - |
| α-Pinene oxide | 1095 | - | 0.14 |
| Linalool | 1098 | - | 0.28 |
| Camphenol | 1109 | 0.06 | - |
| β-Campholene aldehyde | 1125 | 1.34 | 0.24 |
| Trans-pinocarveol | 1139 | 4.22 | - |
| Camphor | 1143 | 1.93 | 0.54 |
| Trans-verbenol | 1144 | 0.04 | - |
| p-Mentha-1,5-diene-8-ol | 1166 | 2.88 | - |
| Isopinocamphone | 1173 | - | 0.34 |
| Terpineol | 1177 | - | 0.53 |
| Myrtenal | 1193 | 0.30 | - |
| Cis-pipretol | 1193 | 1.10 | 0.55 |
| Myrtenol | 1194 | 0.22 | - |
| Verbenone | 1204 | 0.86 | 0.17 |
| Myrtenyl acetate | 1235 | - | 0.07 |
| Carvacrol methyl ether | 1244 | 0.27 | - |
| Carvotanacetone | 1246 | 0.07 | - |
| Piperitone | 1252 | 0.28 | - |
| Linalyl acetate | 1257 | - | 0.51 |
| Methyl citronellate | 1261 | - | 0.06 |
| Isopulegol acetate | 1273 | - | 1.46 |
| Bornyl acetate | 1285 | 0.05 | 0.59 |
| Perilla alcohol | 1295 | 0.03 | 0.01 |
| Trans pinocarvyl acetate | 1297 | 0.12 | - |
| Dehydro-elsholtzione | 1298 | - | 0.01 |
| Isothujyl acetate | 1301 | - | 0.03 |
| Trans-carvyl acetate | 1337 | 0.15 | 0.26 |
| α-Terpinyl acetate | 1350 | 3.36 | 1.01 |
| (c) Sesquiterpene hydrocarbons | |||
| δ--Elemene | 1339 | 0.15 | - |
| α-Copaene | 1376 | 0.09 | 0.24 |
| β-Elemene | 1391 | 0.58 | - |
| Italicene | 1401 | - | 0.65 |
| Junipene | 1402 | 0.02 | 0.24 |
| Cedr-8-ene | 1409 | - | 5.11 |
| β--Caryophyllene | 1418 | 0.50 | - |
| Widderene | 1429 | - | 0.61 |
| α-Himachalene | 1447 | 0.30 | - |
| α-Humulene | 1454 | 0.30 | 1.11 |
| Cis muurola-4 (14),5-diene | 1460 | - | 0.95 |
| Germacrene D isomer | 1462 | - | 0.63 |
| β-Cadinene | 1473 | 0.80 | 2.59 |
| β-Chamigrene | 1475 | - | 0.01 |
| γ-Muurolene | 1477 | - | 1.24 |
| α-Selinene | 1494 | 0.28 | - |
| α-Muurolene | 1499 | 0.50 | - |
| δ-Cadinene | 1524 | - | 3.70 |
| Cadina-1,4-diene | 1532 | 0.20 | 0.41 |
| α-Calacorene | 1542 | 0.11 | 0.14 |
| Selina-3,7(11)1diene | 1542 | 0.18 | 0.06 |
| Germacrene B | 1556 | - | 0.14 |
| (d) Oxygenated sesquiterpenes | |||
| α-Agarofuran | 1545 | 0.30 | - |
| Occidentalol | 1549 | 0.11 | - |
| Cis-isoeugenol acetate | 1563 | - | 0.04 |
| β-Copen-4-α-ol | 1584 | 0.35 | - |
| Guaiol | 1595 | - | 0.43 |
| α-Cedrol | 1596 | 0.47 | 31.23 |
| 1, 10-Di-epi-cubenol | 1614 | 0.30 | 0.11 |
| Trans-isolongifolanone | 1618 | 0.41 | - |
| γ-Eudesmol | 1630 | - | 0.31 |
| Trans dihydro-occidentalol | 1645 | - | 0.01 |
| β-Eudesmol | 1649 | - | 0.17 |
| α-Eudesmol | 1652 | 0.32 | - |
| Khusinol | 1674 | 0.06 | 0.21 |
| Methyl epi-jasmonate | 1676 | - | 0.01 |
| Acorenone | 1685 | - | 0.01 |
| α-Epi-bis alolol | 1686 | - | 0.01 |
| Cedr-8-en-4-ol | 1688 | 0.07 | 0.01 |
| Juniper camphor | 1691 | - | 0.01 |
| Cedryl acetate | 1762 | - | 0.01 |
| Cedr-8(15)-ene-α-ol acetate | 1739 | 0.01 | - |
| 14, α-Hydroxy muurolene | 1775 | 0.01 | - |
| 14, Hydroxy δ-cadinene | 1799 | 0.01 | - |
| Oblopanonyl acetate | 1881 | 0.01 | - |
| (e) Diterpene hydrocarbons | |||
| Abietatriene | 2054 | - | 0.03 |
| Abeitadiene | 2080 | - | 0.03 |
| (f) Oxygenated diterpenes | |||
| Manool | 1961 | - | 0.01 |
| Manoyl oxide | 1989 | - | 0.28 |
| Sclareol | 2220 | 0.08 | - |
| Miscellaneous compounds | |||
| a)Hydrocarbons Tricycline |
926 | - | 0.01 |
| a)Oxygenated | |||
| Hexanal | 800 | - | 0.01 |
| Diacetone alcohol | 840 | 0.17 | 0.06 |
| Oroselone | 2146 | 0.33 | - |
| Total | |||
| No of compounds | 58 | 65 | |
| Monoterpene hydrocarbons | 72.85 | 41.29 | |
| Oxygenated monoterpenes | 17.78 | 6.80 | |
| Sesquiterpene hydrocarbons | 5.56 | 18.33 | |
| Oxygenated sesquiterpenes | 2.43 | 32.21 | |
| Diterpene hydrocarbons | - | 0.06 | |
| Oxygenated diterpenes | 0.08 | 0.29 | |
| Miscellaneous compounds | |||
| a) Hydrocarbon | - | 0.07 | |
| b) Oxygenated | 0.50 | 0.06 | |
| KI: Kovat index | |||
Fifty eight compounds were identified in berry oil representing 99.20% of the composition of the oil while sixty five compounds were identified in leaf oil representing 99.12 % of the oil. Berry oil composed mainly of monoterpenoid which amounted to 90.53% of which 72.85% were monoterpene hydrocarbons. β-phellandrene, β-pinene and δ-3-carene were the most abundant monoterpene hydrocarbons in the berry oil after α-pinene and sabinene, while trans-pinocarveol, α-terpinyl acetate, p-mentha-1, 5-diene-8-ol, camphor, α-campholene aldehyde, cis-pipretol and verbenone accounted for most of the oxygenated monoterpenes. Although the highest group in the leaf oil was monoterpene hydrocarbons (41.29%) predominated by α-pinene, δ-3-carene, limonene, β-pinene and myrcene, yet the sesquiterpenes predominated in leaf oil (50.54%) of which 32.21% was oxygenated sesquiterpenes (mostly α-cedrol) and 18.33% was sesquiterpene hydrocarbones, the most abundant were cedr-8-ene,δ-cadenene, β-cadenene, γ-muurolene and α-humulene. Very low amounts of diterpenes were found in both berry and leaf oils (0.08% and 0.35%, respectively).
Cytotoxic activities
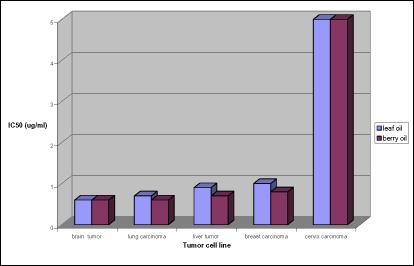
Both oils possess very high activities against all tumor cell lines tested. Activities of leaf and berry oils are shown in Table 2 and Figure 1. Berry oil possess the highest activities against brain tumor and lung carcinoma cell line (IC50= 0.6 µg/ml), followed by liver carcinoma cell line and breast carcinoma cell line (IC50= 0.7 and 0.8 µg/ml, respectively), while the least activity was recorded against cervix carcinoma cell line (5.0 µg/ml). The activities of leaf oil resemble that of berry oil against both brain tumor and cervix carcinoma cell lines, while the activities of the leaf oil showed an ascending order from lung carcinoma, liver tumor and breast carcinoma cell lines (IC50= 0.7, 0.9 and 1.0 µg/ml, respectively). Figure 1 showed that the activities of the berry oil against lung carcinoma, liver tumor and breast carcinoma cell lines were higher than those of leaf oil, while both oils had the same activities against brain tumor and cervix carcinoma cell lines.
Table 2.
Percent cell survival of different human tumor cell lines against different concentrations of essential oils of berries and leaves of J. phoenicea L grown in Egypt.
| Tumor cell line |
Oil conc. µg/ml | % cell survival ±SEM | ||
| Leaf oil (100µl/disc) |
Berry oil (100µl/disc) |
Cisplatin | ||
| bU251 | 0 | 100 ± 0.06 | 100 ± 0.10 | 100 ± 0.07 |
| 1 | 13.5 ± 0.03 | 17.2 ± 0.07 | 60.9 ± 0.07 | |
| 2.5 | 14.0 ± 0.03 | 7.7 ± 0.06 | 56.4 ± 0.05 | |
| 5 | 3.3 ± 0.01 | 5.2 ± 0.07 | 55.5± 0.07 | |
| 10 | 7.5 ± 0.04 | 7.0 ± 0.04 | 49.4± 0.05 | |
| cH460 | 0 | 100 ± 0.08 | 100 ± 0.08 | 100 ± 0.07 |
| 1 | 34.0 ± 0.05 | 23.5 ± 0.04 | 79.6 ± 0.02 | |
| 2.5 | 21.6 ± 0.06 | 27.1 ± 0.03 | 66.5 ± 0.05 | |
| 5 | 16.7 ± 0.02 | 19.9 ± 0.06 | 48.8 ± 0.03 | |
| 10 | 9.8 ± 0.03 | 18.0 ± 0.02 | 40.1 ± 0.0 | |
| dHepg2 | 0 | 100 ± 0.09 | 100 ± 0.07 | 100 ± 0.10 |
| 1 | 44.2 ± 0.06 | 24.7 ± 0.0 | 60.1 ± 0.04 | |
| 2.5 | 32.8 ± 0.05 | 29.9 ± 0.03 | 59.3 ± 0.06 | |
| 5 | 22.0 ± 1.0 | 27.0 ± 0.05 | 52.4 ± 0.04 | |
| 10 | 29.6 ± 0.0 | 37.9 ± 0.09 | 51.8 ± 0.05 | |
| eMCF7 | 0 | 100 ± 0.10 | 100 ± 0.10 | |
| 1 | 52.1 ± 0.04 | 34.6 ± 0.04 | ||
| 2.5 | 42.5 ± 0.07 | 33.8 ± 0.03 | NT | |
| 5 | 37.1 ± 0.06 | 33.5 ± 0.04 | ||
| 10 | 30.7 ± 0.01 | 20.6 ± 0.01 | ||
| fHela | 0 | 100 ± 0.09 | 100 ± 0.08 | 100 ± 0.08 |
| 1 | 73.4 ± 0.08 | 83.5 ± 0.07 | 90.3 ± 0.06 | |
| 2.5 | 70.9 ± 0.04 | 70.6 ± 0.08 | 42.4 ± 0.02 | |
| 5 | 51.5 ± 0.02 | 48.7 ± 0.06 | 17.0 ± 0.04 | |
| 10 | 11.0 ± 0.0 | 38.2 ± 0.02 | 5.9 ± 0.02 | |
aOil concentration (µg/ml) required to reduce cell survival by 50%, bbrain tumor cell line, clung carcinoma cell line, dliver carcinoma cell line, ebreast carcinoma cell line, fcervix carcinoma cell line. NT: not testes.
Cisplatin: standard antitumor drug.
Figure 1.
Comparison between the cytotoxic activities of both leaf and berry essential oils of Juniperus Phoenicea L on human tumor cell lines.
The high activities of these oils may be accounted for by the presence of high ratio of monoterpenes in their composition ((Buhagaiar et al., 1999). Setzer et al. (1999) referred the activity of the essential oil of Myrcianthes sp. against liver tumor cell line to the presence of α-pinene, β-pinene and limonene. This may explain the higher activities of berries oils than leaf oil against lung carcinoma, liver tumor and breast carcinoma cell lines since the former contained higher percentages of total monoterpenes, α-pinene and β-pinene. Sylvestre et al. (2006) attributed the activity of Croton flavens leaf oil against lung carcinoma cell line to its contents of elemene and α-humulene which are constituents of both tested oils. Elemene was found to inhibit proliferation, stimulate apoptosis and induce cell cycle arrest in malignant cell. Limonene and perilla alcohol have been shown to inhibit protein prenylation in cultured cells (Crowell et al., 1992). A minor metabolite of both limonene and perillyl alcohol, perillic acid methyl ester, is a potent inhibitor of both protein farnesyl transferase (PFT) and protein geranylgeranyl transferase (PGGT) in vivo (Gelb et al., 1995). Limonene and perilla alcohol inhibited chemically induced carcinogenesis from chemical carcinogens (Elegbede et al., 1984; Elegbede et al., 1986; Elson et al., 1988; Maltzman et al., 1989).
Antimicrobial activity
Results of antimicrobial activity of Juniperus leaf and berry oils are shown in Tables 3, 4. By comparing the activities of oils, leaf oil was found to have higher activity than berry oil. Berry oil was inactive against two bacterial strains; S. aureus and B. cereus, while it showed highest activity against B. subtilis and E. coli. In case of fungi, the highest activity of berry oil was against T. verdius, while it was inactive against F. oxysporium and A. niger. Leaf oil showed high activity against Candida spp., the least activity against R. minuta and no activity against C. albicans and S. chevallii. Similarly, leaf oil showed highest activity against B. subtilis and inactive against S. aureus, but in contrast to berry oil, it was active against B. cereus. The activity of leaf oil against fungi was higher than that of berry oil and the leaf oil was also active against A. niger. In general, the highest activities of leaf oil were against yeasts (lowest MIC value) except for C. carlspergensis and S. chevallii, which were insensitive to leaf oil. In contrast to Stassi et al. (1996), who stated that the antimicrobial activity of J. oxycedrus berry oil was due mainly to its content of terpineol, J. phoenicea berry oil did not contain terpineol, yet it had a high antimicro-bial activity.
Table 3.
Antimicrobial activities of volatile oils of leaves and berries of Juniperus Phoenicea L grown in Egypt.
| Test organism | Berry v. oil (100µl/disc) |
leaf v. oil (100µl/disc) |
Control (100µg/disc) |
| Inhibition zone in mm ± SE | |||
| Bacteria | |||
| (Gram negative) | |||
| Escherichia coli | 20± 0.3 | 15± 0.3 | 16± 0.6 |
| (Gram positive) | |||
| Staphylococcus aureus | R | R | 22± 0.8 |
| Bacillus subtilis | 25± 11 | 28± 0.3 | 24± 0.5 |
| Bacillus cereus | 0 | 20± 0.9 | 10± 0.0 |
| Fungi | |||
| Fusarium oxysporium | R | R | 18± 0.5 |
| Aspergillus niger | R | 20± 0.8 | 9± 0.3 |
| Aspergillus flavus | 25± 1.2 | 18± 0.7 | 20± 0.5 |
| Maccrophomina phasioli | 10± 0.5 | 20± 0.3 | 21± 0.7 |
| Botryties allii | 18± 0.3 | 20± 0.0 | NT |
| Trichoderma veridus | 30± 1.3 | 30± 0.9 | NT |
| Yeast | |||
| Candida pseudotropicalis | 20± 1.0 | 40± 1.3 | NT |
| Candida albicans | R | 28± 1.1 | NT |
| Candida carlspergensis | 30± 0.8 | R | NT |
| Saccharomyces cerevisiae | 20± 0.8 | 25± 0.9 | 11± 0.2 |
| Saccharomyces chevallii | R | R | 12± 0.1 |
| Rhodotorula minuta | 11± 0.5 | 22± 1.2 | NT - |
R: resistant. NT: not tested. Control: amoxycillin for bacteri and canestin for fungi and yeast.
Table 4.
Minimal inhibitory concentration (MIC) of volatile oils of leaves and berries of Juniperus Phoenicea L grown in Egypt.
| Test organism | Berry v. oil | leaf v. oil |
| mg/ml | ||
| Bacteria | ||
| Escherichia coli | 12.34 | 37.03 |
| Staphylococcus aureus | - | - |
| Bacillus subtilis | 12.34 | 0.46 |
| Bacillus cereus | - | 12.34 |
| Fungi | ||
| Fusarium oxysporium | - | - |
| Aspergillus niger | - | 4.11 |
| Aspergillus flavus | 37.03 | 4.11 |
| Maccrophomina phasioli | 12.34 | 4.11 |
| Botryties allii | 12.34 | 4.11 |
| Trichoderma veridus | 12.34 | 12.34 |
| Yeast | ||
| Candida pseudotropicalis | 37.03 | 0.15 |
| Candida albicans | - | 0.46 |
| Candida carlspergensis | 37.03 | - |
| Saccharomyces cerevisiae | 4.11 | 4.11 |
| Saccharomyces chevallii | - | - |
| Rhodotorula minuta | 111.11 | 0.46 |
IC50= extract concentration (µg/ml) required to reduce cell survival by 50%.
References
- 1.Adams R P, Barrero A F, Lara A. Comparisons of the leaf essential oils of Juniperus phoenicea, Juniperus phoenicea subsp eu-mediterranea Lebr & Thiv and J. phoenicea var turbinaata (Guss) Parl J Essential Oil Res. 1996;8(4):367–371. [Google Scholar]
- 2.Adams R P. Identification of Essential Oils Components by Gas Chromatography/Mass Spectrometry. Carol Stream, Illinois: Allured Publ; 1995. [Google Scholar]
- 3.Afifi M S, El-Sharkawy S H, Maatoog G T, Sohly M, Rosazza J P N. Essential oils of Thuja occidentalis, Thuja orientalis, Cupressus sempervirena and Juniperus phoenicea. Mansoura J Pharmaceu Sci. 1992;8:37–46. [Google Scholar]
- 4.Boulos L. Flora of Egypt. Vol. 1. Cairo, Egypt: Alhadara Publishing; 1999. p. 10. [Google Scholar]
- 5.Buhagaiar J A, Podesta M T, Wilson A P, Micallef M J, Ali S. The induction of apoptosis in human melanoma, breast and ovarian cancer cell lines using an essential oil extract from the conifer Tetraclinis articulata. Anticancer Res. 1999;19(6B):5435–5443. [PubMed] [Google Scholar]
- 6.Collins C T, Lyne P M. Micrbiological Methods. 5th ed. London and Toronto: Butterworth and Co Pub Ltd; 1985. pp. 167–181. [Google Scholar]
- 7.Crowell P L, Lin S, Vedej E, Gould M N. Identification of metabolites and of the antitumor agent d -limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother Pharmacol. 1992;31:205–212. doi: 10.1007/BF00685549. [DOI] [PubMed] [Google Scholar]
- 8.Dawidar A M, Ezmirly S T, Abdel-Mogib M. Sesquiterpenes and diterpenes from Juniperus phoeniciea L. Pharmazie. 1991;46:472–473. [Google Scholar]
- 9.De Pascual Teresa, J Barrero A F, Caballero M C, Ramos M A, San Feliciano A. Components of the essential oil of Juniperus Phoenicia L berries. Riv Ital EPPOS. 1981;63:353–354. [Google Scholar]
- 10.Delitala L F. Essential oil extracted from the berries of Juniperus phoenicea L 1. Terepene fraction. Riv Ital EPPOS. 1980;62:303–309. [Google Scholar]
- 11.Duke J A. Handbook of Medicinal Herbs. Boca Raton FL: CRC Press; 1980. p. 356. [Google Scholar]
- 12.Elegbede J A, Elson C E, Qureshi A, Tanner M A, Gould M N, Qureshi A. Regression of rat primary mammary tumors following dietary d -limonene. J Natl Cancer Institute. 1986;76:323–325. [PubMed] [Google Scholar]
- 13.Elegbede J A, Elson C E, Qureshi A, Tanner M A, Gould M N. Inhibition of DMBA-induced mammary cancer by the monoterpene d -limonene. Carcinogenesis. 1984;5:661–664. doi: 10.1093/carcin/5.5.661. [DOI] [PubMed] [Google Scholar]
- 14.Elson C E, Maltzman T H, Bostion J L, Tanner M A, Gould M N. Anti-carcinogenic activity of d -limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis. 1988;9:331–332. doi: 10.1093/carcin/9.2.331. [DOI] [PubMed] [Google Scholar]
- 15.Gardner P, Provine A T. Manual of Acute Bacterial Infections. 2nd ed. Boston and Toronto: Little, Brown and Co (Inc); 1984. p. 328. [Google Scholar]
- 16.Gelb M H, Tamanoi F, Yokoyama K, Ghomashchi F, Esson K, Could M N. The inhibition of protien prenyltransferases by oxygenatd metabolites of limonene and preillyl alcohol. Cancer Letters. 1995;91(2):169–175. doi: 10.1016/0304-3835(95)03747-k. [DOI] [PubMed] [Google Scholar]
- 17.Guenther E. The Essential Oils. VI. New York: Van Nostrand; 1961. p. 353. [Google Scholar]
- 18.Hagar H H J. Hagers Handbuch des pharmazeutischen Praxis. Vol. 5. Berlin: Springer-Verlag; 1979. p. 333. (Ger). [Google Scholar]
- 19.Maltzman T H, Hurt L M, Elson C E, Tanner M A, Gould M N. The prevention of nitrosomethylurea-induced mammary tumors by d -limonene and orange oil. Carcinogenesis. 1989;10:781–783. doi: 10.1093/carcin/10.4.781. [DOI] [PubMed] [Google Scholar]
- 20.Ramic S, Murko D. Chemical composition of Juniperus species. Arh Farm. 1983;33:15–20. [Google Scholar]
- 21.Setzer W N, Setzer M C, Moriarity D M, Bates R B, Haber W A. Biological activity of the essential oil of Myrcianthes sp. “black fruit” from Monteverde, Costa Rica. Planta Medica. 1999;65(5):468–469. doi: 10.1055/s-2006-960816. [DOI] [PubMed] [Google Scholar]
- 22.Skehan P, Storeng R. New colorimetric cytotoxicity assay for anticancer drug screening. J Natl cancer Institute. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 23.Stassi V, Verykokidou E, Loukis A, Harvala C, Philianos S. The antimicrobial activity of the essential oils of four Juniperus species growing wild in Greece. Flavour and Fragrance Journal. 1996;11:71–74. [Google Scholar]
- 24.Sylvestre M, Pichette A, Longtin A, Nagau F, Legault J. Journal of Ethnopharmacology. 2006;103:99–102. doi: 10.1016/j.jep.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Tabacik C, LaPorte Y. Diterpenes of Juniperus phoenicea. Major constituents. Phytochemistry. 1971;10:2147–2153. [Google Scholar]
- 26.Tabacik C, Poison C. Diterpenes of Juniperus phoenicea. Minor constituents. Phytochemistry. 1971;10:1639–1645. [Google Scholar]
- 27.Uphof J C T. Dictionary of Economic Plants. Germany: Verlag von Cramer; 1968. p. 290. [Google Scholar]
- 28.Watt O M, Breyer-Brandwijk M G. The Medicinal and Poisonus Plants of Southern and Eastern Africa. Edinburgh & London: E & S Livingstone LTD; 1962. p. 841. [Google Scholar]