Abstract
Four plants (Amphimas pterocaroides, Harungana madagascariensis, Myrianthus arboreus, and Cussonia barteri) that are commonly used in Cameroon for the management or reversal of anaemia were screened for their phytochemical content and antioxidant potential. Four extracts (methanolic, hydro-ethanolic, aqueous, and hydrolysed) from each of the plants were prepared and analysed. Qualitative phytochemical tests were used to detect the presence of alkaloids, tannins, saponins, flavonoids, glycosides and phenols, while three quantitative methods; Folin, Ferric Reducing Antioxidant Power (FRAP) and diphenyl -1, 2-picryl hydrazyl (DPPH) were used to determine the antioxidant potential of these extracts. With the exception of the extracts of Cussonia barteri (negative for triterpenes and phenols) and the aqueous extract of Harungana madagascariensis (negative result for cardiac glycosides and glycosides), all other extracts contained the phytochemicals tested. The highest antioxidant activities were observed in the hydrolysed extracts of each plant, while the aqueous extract showed the least activity irrespective of the method used. The presence of active phytochemical substances with antioxidant activities may provide substantial basis for the use of these plants in ethnomedicine.
Keywords: Phytochemicals, Antioxidant, Folin, FRAP, DPPH
Introduction
Medicinal plants are plants that have at least one of their parts (leaves, stem, barks or roots) used for therapeutic purposes (Bruneton, 1993). Recently, medicinal plants have become important for the treatment of different disease conditions, such as diabetes, malaria, anaemia (Fola, 1993). The availability and relatively cheaper cost of medicinal plants in sub-Saharan Africa, makes them more attractive as therapeutic agents when compared to ‘modern’ medicines (Agbor and Ngogang, 2005; Agbor et al., 2005a). The importance of medicinal plants, and the contribution of phytomedicine to the well-being of a significant number of the world's population, has attracted interest from a variety of disciplines.
Many medicinal plants contain large amounts of antioxidants other than vitamin C, E and carotinoids (Javanmardi et al., 2003). Antioxidants are molecules that can delay or prevent an oxidative reaction (Velioglu et al., 1998) catalysed by free radicals. This antioxidant effect is mainly due to the presence of phenolic components such as flavonoids (Pietta, 1998), phenolic acids and phenolic diterpenes (Shahidi et al., 1992). Antioxidants such as BHA (Butylated Hydroxy-Anisol), BHT (Butylated Hydroxy-Toluene) protect plants against oxidative assault (Dziezak, 1986) either by binding to metallic ions, eliminating free radicals or by decomposing peroxides (Matook, 2005). Despite the availability of synthetic antioxidants, present research seeks at discovering new natural antioxidant compounds that may play a role in oxidative stress related disorders (Agbor et al, 2005b). Epidemiological studies (Urquiaga and Leighton, 2000; Halliwell, 1994) have showed that decreases in the incidence of chronic diseases in some populations were related to the consumption of fruits and vegetables.
Four medicinal plants used for the treatment of anaemia are Harungana madagascariensis, Cussonia barteri and Myrianthus arboreus. Earlier studies have shown that Harungana madagascariensis have antibacterial, antifungal, and antiviral activities (Moulari and al., 2006). Phytochemical studies of Cussonia barteri methanol extract led to the isolation of 1-O-chlorogenoylchlorgenic acid and 1-O- chlorogenoylneochlorogenic acid, a new type of quinic acid esters, in addition to six known quinic acid esters, rutin, and a mixture of saponins (Papajewski et al., 2001). Arjunolic acid and a new triterpene acid, myriantic acid, have also been isolated from the root, wood of Myrianthus arboreus (Ojinnaka et al., 1984).
The present study investigates the phytochemical and antioxidant properties of four medicinal plants (Amphimas pterocaroides, Harungana madagascariensis, Myrianthus arboreus, and Cussonia barteri) traditionally used in the treatment of anaemia.
Materials and Methods
Collection of plant material and identification
All the plants (Amphimas pterocaroides, Harungana madagascariensis, Myrianthus arboreus, and Cussonia barteri) used in this study were harvested in June 2005, from their natural habitat in Okola, situated in the outskirts of Yaoundé. Identification of the plants was confirmed in the National Herbium of Yaoundé.
Extraction of plants
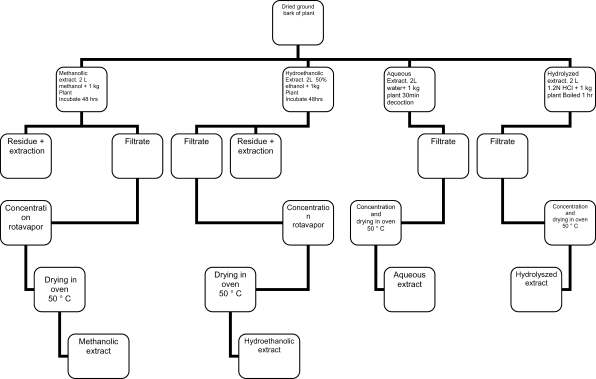
After harvesting, the bark of each plant was chopped and air-dried in the laboratory for two months. These were later ground into a powder with the aid of a mill blender. The powdered plant material was then extracted as outlined in the schematic presentation (Figure 1).
Figure 1.
Schematic drawing of the extraction of the plants studied
Phytochemical screening of plants
A qualitative phytochemical test to detect the presence of alkaloids, tannins, saponins, flavonoids, glycosides and phenols was carried out using the method of Trease and Evans, (1983). The intensity of the coloration determines the abundance of the compound present.
Antioxidant potential
Three methods were used for the determination of antioxidant potential: Folin, FRAP (ferric reducing antioxidant power), DPPH (diphenyl -1, 2-picryl hydrazyl).
Folin Ciocalteu reagent (sigma chemical Co., St Louis, MO) is used to determine the concentration of polyphenol as a measure of antioxidant potential. The reagent was diluted 10 times before used as described by Singleton et al, (1999). The absorbance was measured at 750 nm with the aid of a spectrophotometer (Spectronic 20. Genesys).
FRAP was measured as earlier described by Benzie and Strain, (1996). In brief, 2000 µl of freshly prepared FRAP reagent (10 parts of 300 mM acetate buffer (pH 3.6), 1 part of 10 mM TPTZ (Sigma, in 400 mM HCL), and 1 part of 20 mM ferric chloride) was mixed with 75 µl of sample, methanol as appropriate for the reagent blank. The absorbance was read at 593 nm using a spectrophotometer (Spectronic 20. Genesys), after six minutes of incubation.
DPPH measured the ability of the extracts to scavenge free radical. This was estimated using the method described by Hatano et al, (1988).
In all the measurement of antioxidant potential, catechin was used as standard.
Statistical analysis
Measurements were done in triplicates and the results were presented as mean ± standard deviation. The results were analysed using ANOVA one way with Student Newman-Keuls (p<0.05). Correlations between antioxidant capacities as determined by different methods were established using the Pearson product moment correlation. Sigmastat 3.01 was used for this analysis.
Result and Discussion
The results showed that all the extracts studied contain the bioactive compounds glycosides, phenols, alkaloids, flavonoïds, tannins, saponins, except aqueous extracts of H. madagascariensis which lack cardiac glycoside and glycosides, and C. barteri which lack phenols, triterpenes and sterol (Table 1). Phenols, flavonoids and tannins are good antioxidant substances which have been reported to have anti-diarrhoeal activity (Agbor et al., 2004), and prevent or control oxidative stress related disorders (Vinson et al., 1995a&b).
Table 1.
Phytochemical study of medicinal plants extracts
| Family | Sc. name | Extract | G.C | G | TR | P | S | A | F | TA |
| Cesalpiniaceae |
Amphimas pterocaroides |
M H A HCl/M |
+++ +++ ++ +++ |
++ ++ ++ +++ |
++ ++ ++ +++ |
++ ++ ++ +++ |
+++ +++ ++ +++ |
++ ++ + +++ |
+++ +++ ++ +++ |
++ ++ ++ +++ |
| Hypericaceae |
Harungana madagascariensis |
M H A HCl/M |
++ ++ − +++ |
++ ++ − +++ |
+++ +++ +++ +++ |
++ ++ + +++ |
+++ +++ +++ +++ |
++ ++ ++ +++ |
+++ +++ ++ +++ |
+++ +++ +++ +++ |
| Moraceae |
Myrianthus arboreus |
M H A HCl/M |
+ + + ++ |
+ + + ++ |
++ ++ ++ ++ |
+ + + ++ |
+ + + ++ |
+ + + ++ |
+ + + ++ |
++ ++ ++ ++ |
| Araliaceae |
Cussonia barteri |
M H A HCl/M |
+ + + ++ |
+ + + ++ |
− − − ++ |
− − − ++ |
+ + + ++ |
+ + + ++ |
+ + + ++ |
++ ++ ++ +++ |
+= Present, ++=abundant, +++= most abundant, − = absent Sc= Scientific M= methanol, H = hydroethanolic, G= Glycosides, A= aqueous, HCl/M= hydrolyzed, TA= Tannins, TR = Triterpenes and sterols, P= Phenols, F = Flavonoides, A= Alkaloides, S= Saponins, GC= cardiac Glycosides
Table 1 also shows that methanolic, hydroethanolic and hydrolysed (1.2N HCL/Methanol) extracts of H. madagascariensis and A. pterocarpoïdes qualitatively have more dense concentration of alkaloïdes, cardiac glycosides, flavonoids and soponnins than the aqueous extract. M. arboreus and C. barteri have lower concentrations of all compounds tested. The presence of bioactive compounds in the bark of these plants is an indication of their many possible therapeutic uses.
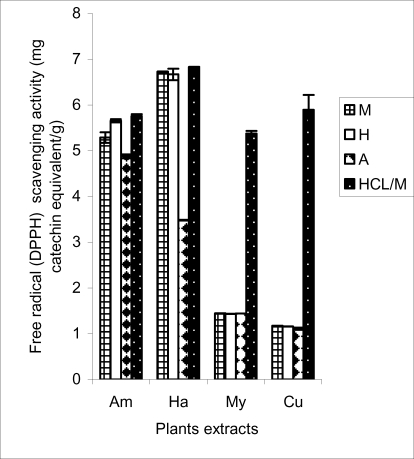
The antioxidant capacities of the plant extracts as measured by the three different methods are presented in Figures 2, 3 and 4. Generally, the hydrolysed extracts had the highest antioxidant capacity irrespective of the method of assay, followed by the hydroethanolic and the methanolic extracts. This confirms that hydrolysis liberate bound antioxidant substances such as phenols which are bound to sugars as earlier reported (Agbor et al., 2005a; Vinson et al, 1998, 2001). In the antioxidant potential determined by DPPH reagent, H. madagascariensis had the highest activity followed by A. pterocarpoïdes. No significant difference (p>0.05) was observed between M. arboreus and C. barteri extracts irrespective of the medium of extraction (Figure 2). DPPH is a free radical that forms a stable molecule on accepting an electron or a hydrogen atom. Free radicals induce oxidative stress in vivo that may lead to oxidative modification or damage of some biological structures such as lipids, proteins, DNA and may give rise to degenerative diseases. There is need for antioxidant intervention which one of the plants studied may be of importance. The in vitro study sounds encouraging as all the plants studied have some radical scavenging effect.
Figure 2.
Free radical (DPPH) scavenging activity of plants extracts
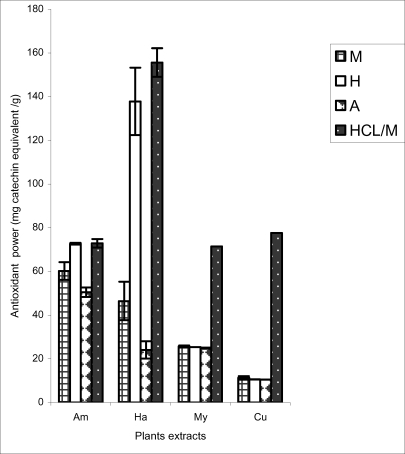
Figure 3.
Antioxidant power of plants extracts as determined by FRAP
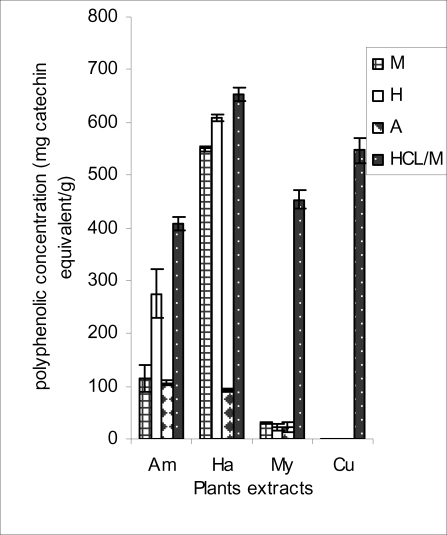
Figure 4.
Polyphenolic concentration of plants extracts as determined using Folin reagent
In the FRAP method, it was the hydrolyzed and the hydroethanolic extracts of H. madagascariensis that had the highest antioxidant activity though its methanolic and aqueous extracts were significantly lower (p<0.001) than corresponding extracts of A. pterocarpoïdes (Figure 3).
In the Folin method, the hydrolyzed, hydroethanolic and methanolic extract of H. madagascariensis were significantly higher than the corresponding extracts of the other samples. This makes H. madagascariensis the overall best antioxidant source of the four plants studied and 1.2N HCL/M (hydrolyzed extract) the best extraction medium for polyphenols (Figure 4). Folin measures the polyphenolic concentration of the extracts. Polyphenols are the principal antioxidant agents in natural products (Kähkönen et al., 1999). They are potent free radical terminators (Shahidi et al., 1992).
They donate hydrogen to free radicals, and hence, break the reaction of lipid oxidation at the initiation step (Gülçin et al., 2004). Thus, high polyphenolic content will mean a strong antioxidant power and a strong radical scavenging activity. However, this is not always the case since plant tissues are often made up of different matrix that may react differently with change of chemicals/reagent or reaction mechanism.
Of the plants studied, H. madagascariensis and A. pterocarpoides had significant higher (p<0.001) antioxidant potential than M. arboreus and C. barteri. These two plants may play an important role in preventing cell destruction and other diseases mediated by oxidative stress. An in vivo antioxidant study of these plants extracts is needed to justify these claims.
References
- 1.Agbor AG, Ngogang YJ. Toxicity of herbal preparations. Cam J Ethnobot. 2005;1:23–28. [Google Scholar]
- 2.Agbor AG, Oben JE, Ngogang JY. Haematinic activity of Hibiscus cannabinus. Afr J Biotechnol. 2005a;4(8):833–837. [Google Scholar]
- 3.Agbor AG, Oben JE, Ngogang JY, Xinxing C, Vinson JA. Antioxidant Capacity of Some Herbs/spices from Cameroon. A Comparative study of two Methods. J Agri Food Chem. 2005b;53(17):6819–6824. doi: 10.1021/jf050445c. [DOI] [PubMed] [Google Scholar]
- 4.Agbor AG, Talla L, Ngogang JY. The Antidiarrhoeal activity of Alchornea cordifolia leaf extract. Phytother Res. 2004;18(11):873–876. doi: 10.1002/ptr.1446. [DOI] [PubMed] [Google Scholar]
- 5.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 6.Bruneton J. Plantes Médicinales: Phytochimie, Pharmacognosie. 2 éme. New York: Lavoisier; 1993. p. 914. (Fre). [Google Scholar]
- 7.Ojinnaka CM, Okogun JI, Okorie DA. Myrianthic acid: a triterpene acid from the rootwood of Myrianthus arboreus. Phytochemistry. 1984;23:1125–1127. [Google Scholar]
- 8.Dziezak JD. Antioxidants. Food Technol J. 1986;40:94. [Google Scholar]
- 9.Fola A. Local medecinal plants and the health of the consumers. A paper delivered at the PSN/CF PCON organization workshop: in Clinical Pharmacy and Herbal Medicine. 1993;9:28–31. [Google Scholar]
- 10.Gülçin I, Beydemir S, Alici HA, Elmastaş M, Büyükokuroğlu ME. In vitro antioxidant properties of morphine. Pharmacol Res. 2004;49:59–66. doi: 10.1016/j.phrs.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B. Antioxidants sense or speculation. Nutrition today. 1994;29:15–19. [Google Scholar]
- 12.Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoïds and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull. 1988;36:2090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- 13.Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003;83:547–550. [Google Scholar]
- 14.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heininen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 15.Matook S M. Antioxidant activities of water-soluble polysaccharides from Buncan (citrus grandis osbeck) fruit flavedo tissues. Pak J Biol Sci. 2005;8:1472–1477. [Google Scholar]
- 16.Moulari B, Lboutounne H, Chaumont JP, Guillaume Y, Millet J, Pellequer Y. Potentiation of the bactericidal activity of Harungana madagascariensis Lam. ex Poir. (Hypericaceae) leaf extract against oral bacteria using poly (D, L-lactide-co-glycolide) nanoparticles: in vitro study. Acta Odontol Scand. 2006;64:53–158. doi: 10.1080/00016350500483152. [DOI] [PubMed] [Google Scholar]
- 17.Papajewski S, Vogler B, Conrad J, Klaiber I, Roos G, Walter CU, Suessmuth R, Kraus W. Isolation from Cussonia barteri of 1′-O-chlorogenoylchlorogenic acid and 1′-O-chlorogenoylneochlorogenic acid, a new type of quinic acid esters. Planta Med. 2001;67(8):732–736. doi: 10.1055/s-2001-18338. [DOI] [PubMed] [Google Scholar]
- 18.Pietta PG. Flavonoids in medicinal plants. In: Rice-Evans CA, Packer L, editors. Flavonoids in health and disease. New York: Dekker; 1998. pp. 61–110. [Google Scholar]
- 19.Shahidi F, Janitha PK, Wanasundara PKJPD. Phenolic antioxidants. Crit Rev Food Sci Nutrit. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 20.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciacolteu reagent. Meth Enzymol. 1999;299:152–178. [Google Scholar]
- 21.Trease GE, Evans WC. Pharmacognosy. 12th Ed. London: Bailliere Tindal; 1983. p. 622. [Google Scholar]
- 22.Urquiaga I, Leighton F. Plant polyphenol antioxidants and oxidative stress. Biol Res. 2000;33:9716–9760. doi: 10.4067/s0716-97602000000200004. [DOI] [PubMed] [Google Scholar]
- 23.Vilioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. [Google Scholar]
- 24.Vinson JA, Dabbagh YA, Serry MM, Jang J. Plant flavonoids, especially tea flavonoids, are powerful antioxidants using an in vitro oxidation model for heart disease. J Agric Food Chem. 1995b;43:2800–2802. [Google Scholar]
- 25.Vinson JA, Hao Y, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001;49:5315–5321. doi: 10.1021/jf0009293. [DOI] [PubMed] [Google Scholar]
- 26.Vinson JA, Hao Y, Su X, Zubik L. Phenol antioxidant quantity and quality in foods: vegetables. J Agric Food Chem. 1998;46:3630–3634. [Google Scholar]
- 27.Vinson JA, Jang J, Dabbagh YA, Serry MM, Cai S. Plant phenols exhibit lipoprotein-bound antioxidant activity using an in vitro model for heart disease. J Agric Food Chem. 1995a;43:2798–2799. [Google Scholar]