Abstract
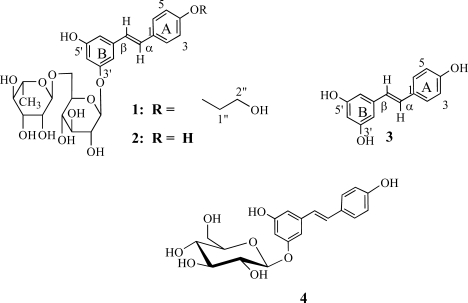
The ethanol extract of the root bark of Terminalia sericea yielded an unreported stilbene glycoside, 3′5′-dihydroxy-4-(2-hydroxy-ethoxy) resveratrol-3-O-β-rutinoside (1) together with known compounds resveratrol-3-β-rutinoside glycoside (2), 3′,4,5′-Trihydroxystilbene (resveratrol) (3), triterpenoic acid arjungenin and a mixture of β-sitosterol and stigmasterol. Structure determination of the isolated compounds was achieved on the basis of spectroscopic measurements.
Keywords: Terminalia sericea, stilbenes, combretastatin, resveratrol, glycosides
Introduction
Terminalia sericea Burch. Ex. DC (Combretaceae) is widely distributed in the tropical and warm temperate regions, especially in Africa (Watt and Breyer-Brandwijk, 1962; Hutchings et al., 1996). The plant has wide spread traditional uses in eastern and southern African countries (Arnold and Gulumian, 1984; Kokwaro, 1976; Msonthi and Magombo, 1983). Among some of its proven biological activities, include inhibition of topoisomerase II (Wall et al., 1996), antibacterial, and antifungal activity (Eloff, 1999; Fyhrquist et al., 2002; 2004). However, there is scanty information on compounds that are responsible for the exhibited biological activities of the plant. The stilbene glycoside, resveratrol-3-O-β-rutinoside (2) (Bombardelli et al., 1975), and pentacyclic triterpenes of the oleanane skeleton, together with their glucosides (Bombardelli et al., 1974; 1975) have been isolated. This includes also a triterpene, sericoside (Maeda and Fukuda, 1996). Skin lightening preparations containing sericoside have been patented in Japan (Maeda and Fukuda, 1996). A β-D-glucoside (4) of resveratrol was isolated from wine, a compound that is considered to be responsible for the protective ability of wines against coronary heart disease (Jeandet et al., 1991).
We hereby report the isolation of a an unreported stilbene glycoside 1, resveratrol-3-O-β-rutinoside (2), resveratrol (3) and other known compounds such as sericic acid, stigmasterol, β-sitosterol and triterpenoid acid were also isolated from the ethanol extract of the root bark of T. sericeae occurring in Tanzania.
Materials and Methods
Materials
Petroleum ether, dichloromethane, ethyl acetate (EtOAc), methanol (MeOH), chloroform (CHCl3), and ethanol were purchased from Fisher Scientific UK Ltd (Bishop Meadow Road, Loughborough, Leicestershire, LE 11 5RG, UK). Precoated silica gel plates (60 F254, 250 µm) were purchased from Merck and sephadex LH-20 from Pharmacia.
Collection of Plant materials
The root barks of T. sericea were collected from Iringa region, and identified by Mr. Frank Mbago. Voucher specimen no. IMPP 001-0144 is kept in the Herbarium of the Institute of Traditional Medicine, Muhimbili University College of Health Sciences, University of Dar es Salaam.
Extraction and isolation
Air-dried and powdered plant material (2 Kg) was cold extracted sequentially in petroleum ether (2.0 L; 40–60°C), dichloromethane (2.0 L) and then ethanol (2.0 L). The amounts of both the petroleum ether and dichloromethane extracts were very small to the extent that these extracts could not be worked on further. The ethanol extract was then fractionated by vacuum liquid chromatography (VLC) eluting with a mixture of EtOAc/petroleum ether followed by MeOH/EtOAc to give 4 bulked fractions. Thin layer chromatography (TLC) was performed on pre-coated plates (silica gel 60 F254, 250 µm on polyester backing, Merck) eluting with mixtures consisting of petroleum ether (b.p. 40–60°C) and EtOAc; detection by UV and an anisaldehyde spray reagent (Jean, 1996). Column chromatography on silica gel 60 mesh (0.063–0.2 mm, Merck) eluting with mixtures of increasing polarity consisting of petroleum ether and EtOAc. Gel filtration was performed on Sephadex® LH-20 (Pharmacia) eluting with MeOH or a mixture of MeOH/CHCl3. VLC was performed on silica gel 60 (Merck), eluting with petroleum ether containing increasing amounts of EtOAc. Recrystallisation was achieved from MeOH/CHCl3, 9:1 v/v. Flash chromatography (FC) employed Merck silica gel 60 (0.063–0.2 mm, Merck) and petroleum ether (b.p. 40–60°C)-EtOAc mixtures. Repeated column chromatography on silica gel of fraction 1 yielded compound 2 and 3. Compound 1 was obtained from fraction 4 after repeated column chromatography followed by gel chromatography over Sephadex® LH-20 eluting with a mixture of methanol and water (1:1 v/v).
Spectra
1H NMR spectra were recorded on a Bruker AM 400 spectrometer operating at 400 MHz. CD3OD, CDCl3 or d6-DMSO were used as solvents. Chemical shifts are given in δ values relative to the internal standard TMS (δ = 0). Mass spectra were determined by direct inlet on a VG 7070 E instrument at 70 eV.
Results
The unreported stilbene glycoside, 3′5′-dihydroxy-4-(2 hydroxy-ethoxy) resveratrol-3-O-β-rutinoside (1), was isolated as a reddish gum. 1H-NMR (CDCl3): δ 7.49 (1H, d, J = 8.6 Hz, H-2 or H-6), 6.99 (1H, d, J = 16.3 Hz, H-α), 6.85 (1H, d, J = 16.3 Hz, H-β), 6.74 (1H, d, J = 2 Hz, H-2′), 6.66 (1H, d, J = 2 Hz, H-6′), 6.47 (1H, d, J = 2 Hz, H-4′), 4.90 (1H, d, H= 1‴), 4.75 (1H, d, H = 1″), 3.90 (1H, dd, J = 1.61 Hz, 3.4 Hz, H = 4″), 3.71 (1H, m, H = 3‴), 3.68 (2H, m, H-1″″), 3.58 (2H, m, H-2″″), 3.56 (1H, dd, J = 3.8 Hz, 5.4 Hz, H-3″), 3.46 (1H, d, J = 5.7 Hz, H-2″), 3.44 (1H, m, H-2‴), 3.35 ( 1H, m, H-4‴), 1.22 (d, d, J = 6.15 Hz, Me-6‴). 13C-NMR (CDCl3): δ 159.32 (C-3′), 158.48 (C-5′), 157.41 (C-4), 140.39 (C-1′), 129.29 (C-β), 127.98 (C-2/6), 125.64 (C-C-a), 115.58 (C-3/5), 107.16 (C-C-2′), 106.76 (C-C-6′), 103.10 (C-C-4′), 101.33 (C-1‴), 101.15 (C-1″), 76.93 (C-3‴), 75.85 (C-5″), 73.94 (C-2″), 73.08 (C-4‴), 72.49 (C-1″″), 71.35 (C-3‴), 71.08 (C-4″), 70.33 (C-2‴), 68.82 (C-5‴), 66.60 (C-6″), 61.24 (C-2″″), 16.93 (C-6‴). EIMS m/z (rel. int.): 580 [M]+ (C28H36O13), 564 (55), 537 (100), 391 (15), 375 (20), 229 (10). Compounds 2, 3 and 4 were also isolated. Compound 2, resveratrol-3-O-β-rutinoside, exhibited a weak antibacterial activity against Escherichia coli at 4 mg/ml. The compound was not tested on the other organisms due to its paucity.
Discussion
Compound 1 was obtained as a red gum. Its structure was established on the basis of extensive spectroscopic analysis, in particular H/H-COSY, HMQC, HMBC and MS measurements, as well as from comparison of the observed spectral data with those reported in the literature for the main skeleton (Bombardelli et al., 1975., Breitmaier and Voelter, 1989., Dorman et al., 1970). Thus, the aromatic region of the 1H and 13C NMR spectra exhibited features which were virtually the same as those observed for the stilbene moiety in resveratrol-3-O-β-rutinoside (2) and this indicated the presence of a resveratrolyl unit in structure 1, as was further also confirmed by the MS which consisted of a peak at m/z 228 due to the fragment ion corresponding to 3. Furthermore, the 1H NMR spectrum displayed two doublet at δ 4.94 and 4.75 which were assigned to protons at anomeric carbon atoms, thereby indicating the presence of a disaccharide unit that consisted of a rhamnoside and a glucoside unit, whereas the methyl carbon signal for the rhamnose unit was observed at δ 16.93 (Bombardelli et al., 1975., Breitmaier and Voelter, 1989., Dorman et al., 1970). The α-L-O-glycosidic linkage between the two sugar units was indicated from the observed J values as well as the 13C NMR resonances of the anomeric carbon atoms, which appeared at δ 101.18 and 101.33, respectively (Kasai et al., 1977; 1979). The 1H and 13C NMR spectral features for the disaccharide unit were virtually the same as those observed in the spectra of 2 and this indicated that, like in 2, glycoside 1 contained a rutinoside residue (Bombardelli et al., 1975., Breitmaier and Voelter, 1989., Dorman et al., 1970). The 13C NMR spectrum with DEPT 135 measurements of compound 1 indicated signals due to 14 carbon atoms in the region δ 16–101. Of these signals, 12 were characteristic resonances observed for the rutinoside sugar residue in compound 2 as reported in the preceeding discussion, also corresponding to literature data for the rutinoside 13C NMR resonances for compound 2 (Bombardelli et al., 1975., Breitmaier and Voelter, 1989. Dorman et al., 1970). The remaining two 13C NMR signals at δ 61.24 and 72.49 were attributed to a dioxymethylene group. These spectral features, as well as the positions of the 13C NMR resonances in the high field region being comparable to those observed for the resveratrolyl unit in compound 2 but having only slight differences led to the conclusion that compound 1 must be having structural features similar to those of 2, the former compound however bearing an additional hydroxyethane group. Addition of a two carbon atom in compound 1 as compared to compound 2 was also indicated by the MS which exhibited a molecular ion peak at m/z 580, which is 44 amu higher than the M+ peak for glycoside 2 (m/z 536). The additional 44 amu could be accounted for by considering the presence of an additional CH2CH2O unit in compound 1.
As for compound 2, the position of the rutinoside sugar residue in glycoside 1 was deduced based on the upfield shift of the 13C NMR resonances for C-3′ and C-5′ in ring A as compared to the corresponding signals in the spectrum of 3. The position of the rutinoside sugar residue was further corroborated by long range H/C interactions involving the anomeric proton at C-1″ as revealed in the HMBC spectrum.
Establishment of the position of the hydroxyethane group at C-4 was facilitated by considering the slight upfield shift of the 13C NMR resonance for C-4 in compound 3 as compared to the corresponding signals in the spectrum of glycoside 2. Apparently, the substitution could also be at C-5′ in ring B which makes structure 1 to be only tentative.
The MS of 1 consisted of the molecular ion peak at m/z 580 and a fragment ion peak at m/z 536 which corresponds to the M+ peak for compound 2, being formed through rearrangement of 1 followed by extrusion of acetaldehyde. Cleavage of the sugar residue yielded a fragment ion at m/z 229 due to the resveratrolyl unit. The MS fragmentation pattern of compound 1 is consistent with the proposed structure.
Acknowledgements
Financial support by Swedish International Development Agency (SIDA)/Department of Research Cooperation (SAREC) within an overall research support to the Faculty of Science at the University of Dar es Salaam is gratefully acknowledged. We also thank Prof. Berhanu M. Abegaz of the University of Botswana for availing spectral analytical facilities to us. One of the authors (IE) thanks the Vlaamse Interuniversitaire Raad-Flemish Interuniversity Council (VLIR) for fellowship.
References
- 1.Arnold HJ, Gulumian M. Pharmacopoeia of Traditional Medicine in Venda. J Ethnopharmacol. 1984;12:35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 2.Bombardelli E, Bonati A, Gabetta B, Mustich G. Phytochemistry. 1974;13:2559–2562. [Google Scholar]
- 3.Bombardelli E, Martinelli EM, Mustich G. A new hydroxystilbene glycoside from Terminalia sericea. Fitoter. 1975;46:199–201. [Google Scholar]
- 4.Breitmaier E, Voelter W. Carbon-13 NMR Spectroscopy: High Resolution Methods and Applications in Organic Chemistry and Biochemistry. 3rd Edn. New York, USA: VCH publisher; 1989. p. 450. [Google Scholar]
- 5.Dorman DE, Angyal SJ, Robert JD. J Amer Chem Soc. 1970;92:1351–1354. [Google Scholar]
- 6.Eloff JN. The antibacterial activity of 27 Southern African members of the Combretaceae. S Afri J Sci. 1999;95:148–152. [Google Scholar]
- 7.Fyhrquist P, Mwasumbi L, Haeggstrom CA, Vuorela H, Hiltunen R, Vuorela P. Ethnobotanical and antimicrobial investigation on some species of Terminalia and Combretum (Combretaceae) growing in Tanzania. J Ethnopharmacol. 2002;79:169–177. doi: 10.1016/s0378-8741(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 8.Fyhrquist P, Mwasumbi L, Haeggstrom CA, Vuorela H, Hiltunen R, Vuorela P. Antifungal activity of selected species of Terminalia, Pteleopsis and Combretum (Combretaceae) in Tanzania. Pharmaceut Biol. 2004;42:308–317. [Google Scholar]
- 9.Hutchings A, Scott AH, Lewis G, Cunninghham A. Zulu medicinal plants, An inventory. Scottsville 3209, South Africa: University of Natal Press; 1996. p. 151. [Google Scholar]
- 10.Jean IMR. Tenth Science in Africa Symposium. Baltimore, Maryland: 1996. p. 83. [Google Scholar]
- 11.Jeandet P, Bessis R, Gautheron B. The production of resveratrol (3,5,4′-trihydroxystilbene) by grape berries in different developmental stages. Amer J Enol Viticult. 1991;42:41–46. [Google Scholar]
- 12.Kasai R, Suzuo M, Asakawa J, Tanaka O. C-13 chemical-shifts of isoprenoid-β-D-glucopyranosides and isoprenoid-β-D-mannopyranosides - stereochemical influences of aglycone alcohols. Tetrahedron Letters. 1977;2:175–178. [Google Scholar]
- 13.Kasai R, Okihara M, Asakawa J, Mizutani K, Tanaka O. 13C NMR study of α- and β-anomeric pairs of D-mannopyranosides and L-rhamnopyranosides. Tetrahedron. 1979;35:1427–1432. [Google Scholar]
- 14.Kokwaro JO. Medicinal plants of East Africa. Nairobi: East African Literature Bureau; 1976. p. 57. [Google Scholar]
- 15.Maeda N, Fukuda M. Skin lightening preparations containing sericoside. 1996:6. Patent-Japan Kokai Tokkyo Koho-08133, 951.
- 16.Msonthi JD, Magombo D. Medicinal herbs in Malawi and their uses. Hamdard. 1983;26:94–100. [Google Scholar]
- 17.Rogers CB, Verotta L. First International IOCD-Symposium. Victoria Falls, Zimbabwe: 1996. p. 121. [Google Scholar]
- 18.Wall ME, Wani MC, Brown DM, Fullas F, Olwald JB, Josephson FF, Thornton NM, Pezzuto JM, Beecher CWW, Farnsworth NR, Cordell GA, Kinghorn AD. Effect of tannins on screening of plant extracts for enzyme inhibitory activity and techniques for their removal. Phytomedicine. 1996;3:281–285. doi: 10.1016/S0944-7113(96)80067-5. [DOI] [PubMed] [Google Scholar]
- 19.Watt MJ, Breyer-Brandwijk GM. Medicinal and poisonous plants of Southern and Eastern Africa. Edinburgh and London: E and S Livingstone Ltd; 1962. p. 196. [Google Scholar]