Abstract
This study was designed to determine the effects of methanolic extracts of Annona muricata (Linn) on the blood glucose level of streptozotocin-induced diabetic Wistar rats. Thirty adult Wistar rats were randomly assigned into three groups (A, B and C) of ten rats each. Group A was the control, Group B was untreated hyperglycemic group and group C was A. muricata-treated group. Hyperglycemia was induced in groups B and C by a single intraperitoneal injection of 80mg/kg streptozotocin dissolved in 0.1M citrate buffer. The control group was intraperitoneally injected with equivalent volume of citrate buffer and all the animals were monitored for four weeks. Daily intra peritoneal injection of 100mg/kg A. muricata was administered to group C rats for two weeks and the animals were monitored for another two weeks. The data obtained were analyzed with descriptive and inferential statistics. The result showed a mean body weight of 206 ± 7.74g, 173.29±5.13g and 197 ± 5.62g respectively for the control, untreated diabetic and A. muricata-treated diabetic group, and a mean blood glucose concentration of 3.78 ± 0.190 mmol/L, 21.64 ± 2.229mmol/L and 4.22 ± 0.151mmol/L for the control, untreated diabetic and treated diabetic groups respectively. A significant difference exists between the blood glucose concentrations of treated and untreated hyperglycemic groups of rats. The result of this study demonstrated that A. muricata possesses anti-hyperglycemic activities.
Keywords: Annona muricata, Diabetes mellitus, Streptozotocin, Blood glucose level, hyperglycemia
Introduction
Diabetes mellitus, characterized by hyperglycemia, is a genetically and clinically heterogeneous group of disorders with common feature of glucose intolerance (WHO, 1994). Based on WHO recommendation, diabetes mellitus is classified into four major subtypes: type I (insulin dependent diabetes mellitus, IDDM), type II (non-insulin dependent diabetes mellitus, NIDDM) other specific types and gestational diabetes mellitus. IDDM or Juvenile-onset diabetes results from a cellular mediated autoimmune destruction of the β-cells of the pancreas (Atkinson and Maclaren, 1994, Takeshi et al., 2002). However, NIDDM or adult-onset diabetes results from the development of insulin resistance and the affected individuals usually have insulin deficiency (De Fronzo et al., 1997). In modern medicine, the beneficial effects of standard medications on glycemic levels are well documented; the preventive activity of medications against the progressive nature of diabetes and its complications was the modest but not always effective. Insulin therapy affords glycemic control in type 1 diabetes, yet its shortcomings such as ineffectiveness on oral administration, short shelf life, the requirement of constant refrigeration, fatal hypoglycemia in event of excess dosage, reluctance of patients to take insulin injection and above all the resistance due to prolonged administration limits its usage (Kasiviswanath et al., 2005). Similarly, treatment of type 2 diabetes patients with sulfonylureas and biguanides is always associated with side effects (Grandhipuram et al., 2006). Hence, search for a drug with low cost, more potential and without adverse side effect is being pursued in several laboratories around the world.
The bark, roots and leaves of Annona muricata has been reported to be used as anti-diabetes in the Peruvian Amazon (Vasquez, 1990). This investigation is therefore designed to confirm the effects of methanolic extracts of A. muricata leaves on glycemic control in streptozotocin-induced diabetic Wistar rats.
Annona muricata is a plant which belongs to the family Annonaceae (Taylor, 1996). It is a medicinal plant that has been used as a natural remedy for a variety of illnesses. Several studies by different researchers demonstrated that the bark as well as the leaves had anti-hypertensive, vasodilator, anti-spasmodic (smooth muscle relaxant) and cardio depressant (slowing of heart rate) activities in animals (Feng, 1962). Researchers had re-verified A. muricata leaf's hypotensive properties in rats (Carbajal et al., 1991). Other properties and actions of A. muricata documented by traditional uses include its use as anti-cancerous, (Oberlies et al., 1995; Tormo et al., 2003), anti-diabetes (Vasquez, 1990); anti-bacterial, (Takahashi et al., 2006); anti-fungal (Heinrich et al., 1992); anti-malarial, anti-mutagenic (cellular protector), emetic (induce vomiting), anti-convulsant (N'gouemo, 1997), sedative, insecticidal and uterine stimulant. It is also believed to be a digestive stimulant, antiviral cardio tonic (tones, balances and strengthens the heart), febrifuge (cures fever), nerviness (balances/calms the nerves), vermifuge (expels worms), pediculocide (kills lice), and as an analgesic. Padma et al., (1998) confirmed the anti-viral activity of ethanolic extracts of A. muricata against Herpes simplex virus. Extracts of A. muricata have been shown to have anti-parasitic (Bories et al., 1991), anti-rheumatic, astringent, (dos Santos and Sant'Ana, 2000), anti-leishmanial and cytotoxic effects (Jaramillo et al., 2000; Liaw et.al. 2002). A. muricata has also been shown to be effective against multi-drug resistant (MDR) cancer cell lines (Oberlies et al., 1997; Liaw et al., 2002). Extracts of A. muricata were also shown to be effective against the cancer cell line U973 (Jaramillo et al., 2000), and hematoma cell lines in-vitro (Chen et al., 2000). Extracts were also shown to be lethal to the fresh water mollusk, Biomphalaria glabrata, which act as a host for the parasitic worm Schistosoma mansoni (dos Santos and Sant'Ana, 2000; Luna et al., 2006).
Materials and Methods
Plant Material
Annona muricata leaves were collected from Mowe, Ogun State, Nigeria in February 2006. The plant was identified by Dr. Folorunso of the Department of Botany, Obafemi Awolowo University, Ile Ife and a voucher specimen (IFE5) was deposited in the Herbarium of the Department
Preparation of Extracts
A. muricata leaves were air dried at room temperature for four weeks. The air-dried leaves were powdered in a warring blender (Christy and Norris - 47362, England) at the Department of Pharmacognosy Obafemi Awolowo University, Ile Ife. A 600g of the powdered leaf leaves was soaked in 5 litres of 70% methanol for 72 hours at room temperature. The mixture was filtered and the filtrate was evaporated at 60°C using a vacuum rotary evaporator (RE 100B, Bibby Sterilin, United Kingdom). The wet residue was freeze-dried using a vacuum freeze drier (FT33-Armfield, England) and was stored until ready to use.
Care and Management of Animals
Thirty healthy adult Wistar rats (Rattus norvegicus) of both sexes, weighing between 150g and 250g were used for the experiment. The rats were bred in the animal holding of department of Anatomy and Cell Biology Obafemi Awolowo University Ile Ife, were maintained on standard rat pellets (Ladokun feeds, Ibadan, Nigeria), and were given water ad libitum. The animals were randomly assigned into three groups A, B, and C of ten rats each. Group A was the control, non-diabetic group of rats, group B was the experimentally induced diabetic group without A. muricata treatment while group C was the experimentally induced diabetic group treated with methanolic extracts of A. muricata. There was a pre-experimental period of four weeks during which the body weight and blood glucose level were monitored in the animals before the commencement of the experiment. The rats receive humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
Acute toxicity test
Forty adult male Wistar rats (150 – 200g) were randomly assigned into six groups (T1, T2, T3, T4, T5 and T6) of eight animals in each group. T1 was treated with distilled water and is considered as control. The other five groups were treated with methanolic extract of A. muricata given intraperitoneally in increasing dosage of 25, 50, 100, 200, and 400mg/kg body weight. The extract was dissolved in distilled water and the average volume injected was 0.3ml. The control group was given equivalent volume of distilled water used in dissolving the extract. All the rats were returned to their cages and given free access to food and water. The mortality in each cage was assessed 24 hours, 48 hours and 72 hours after administration of extract. The percentage mortality in each group was calculated and plotted against the log10 of the extract dose. Regression line was fitted by method of least squares and confidence limits for the lethal dose (LD50) values were calculated by method of Abdel-Barry et al. (1997)
Administration of Streptozotocin and Annona muricata
Diabetes mellitus was experimentally induced in groups B and C by a single intraperitoneal injection of 80mg/kg b.w. streptozotocin (Sigma, St. Louis, USA) dissolved in 0.1M sodium citrate buffer pH 6.3. The control (group A animals) were injected intraperitoneally with equivalent volume of the citrate buffer. The rats were fasted overnight before STZ administration. The blood glucose level was monitored weekly in the animals for the next four weeks. After four weeks of experimental-induction of diabetes, group C rats were given daily intraperitoneal injection of 100mg/kg of extracts of A.muricata dissolved in distilled water for two weeks and the animals were monitored for another four weeks.
Determination of Body Weight and Blood Glucose Level
The body weights of the animals were measured using a top loader weighing balance. Blood sample was obtained from the tail vein of the animals and their fasting blood glucose level was determined in mmol/L using a digital glucometer (Accu-chek® Advantage, Roche Diagnostic, Germany). The animals were fasted for a period of 16 hours before their blood glucose level was measured.
Statistical Analysis
The data were analysed using descriptive and inferential statistics. All values are presented as mean ± standard error of mean (SEM) for ten rats in each of the three group of rats. The significance of difference in the means of all parameters reported for the three groups of animals was determined using paired sample student t - test and a p - value of < 0.05 (two tailed) was considered as significant.
Results
Effects of Annona muricata on the Weight
Prior to STZ administration, there was no significant (p < 0.05) difference in the average weights of the three groups of experimental animals. By the end of the first week after diabetes mellitus was experimentally induced, the weights of Groups B and C animals was significantly (p < 0.05) reduced despite the increase in food and fluid intake in these animals. This weight loss continued for four week after STZ administration (Figure 1). However, the weight of animals in group C gradually increased on treatment with extracts of A. muricata over the period of five weeks as shown in Figure 1. At the end of the experiment, there was a significant (p < 0.05) difference in the weights of groups B and C animals while there was no statistically significant (p < 0.05) difference between weights of animals in groups A and C (Figure 1, Table 1.)
Figure 1.
Weekly changes in the body weight of the rats after STZ and A. muricata Administration
Table 1.
Changes in the Body Weight and Blood Glucose Level of Control and Experimental Group of Rats after STZ and Annona muricata Administration
| Body Weight | Blood glucose level | |
| Group | (g) | (mmol/L) |
| Group A (initial) | 207.65 ± 9.31a | 3.60 ± 0.127a |
| Group A ± Citrate buffer | 206.00 ± 7.74a | 3.78 ± 0.190a |
| Group B (initial) | 203.46 ± 7.99a | 3.28 ± 0.182a |
| Group B ± STZ | 173.29 ± 5.13b | 21.64 ± 2.229b |
| Group C (initial) | 201.00 ± 7.26a | 3.57 ± 0.121a |
| Group C ± STZ | 170.60 ± 5.23b | 24.74 ± 2.923b |
| Group C ± STZ ± A. muricata | 197.70 ± 5.62a | 4.22 ± 0.151a |
Values are given as mean ± SEM for ten rats in each group. a, b within column signifies that means with different letters differs significantly at P < 0.05 (two tailed T-test) while means with the same letters does not differ significantly at P <0.05. (two tailed T-test)
Figure 1 show that, in contrast to control rats, there was a significant (p < 0.05) weight loss among the group B and C rats, one week after STZ administration. However, A. muricata -treated rats showed sign of recovery in body weight gains four weeks after treatments (197.70 ± 5.62g) compared to the diabetic control rats (173.29 ± 5.13 g).
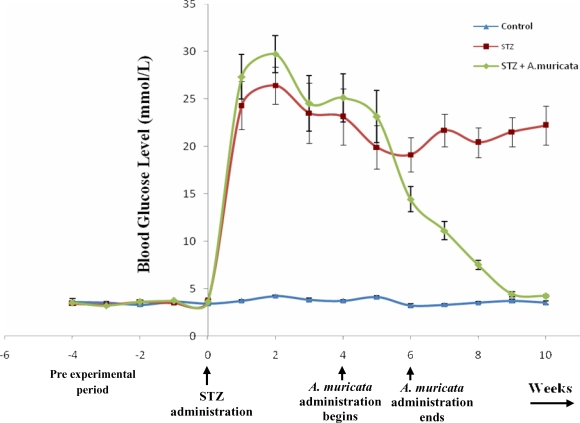
Effects of Annona Muricata on the Blood Glucose Level
Prior to STZ administration, the fasting blood glucose level did not differ significantly (p < 0.05) between the three groups of experimental animals. 24 hours after administration of STZ, the blood glucose level was significantly (p < 0.05) higher in groups B and C animals. The blood glucose level of animals in group C gradually decreased on treatment with extracts of A. muricata over the period of five weeks (fig. 2). Control rats treated with citrate buffer were euglycemic throughout the period of experiment. At the end of the experiment, there was a significant (p < 0.05) reduction in the blood glucose level of groups C rats compared to that of group B rats. In addition, there exist no significant (p < 0.05) difference between blood glucose level of rats in groups A and C (Fig. 2, Table 1) This shows that extracts of A. muricata demonstrate a potent antihyperglycemic activity.
Figure 2.
Weekly changes in the blood glucose level of the rats after STZ and A. muricata Administration
Figure 2 shows that prior to the extract administration, there was no significant (p < 0.05) difference between the blood glucose levels of the control and the two diabetic groups of animals. The blood glucose level of the two diabetic groups of rats significantly (p < 0.05) increased twenty-four hours after STZ administration, and there was no significant (p < 0.05) difference between the blood glucose levels of these two groups of diabetic rats for a period of four weeks. After 4 weeks of treatment with extracts of A. muricata, the blood glucose levels of the treated diabetic rats does not differ significantly (p < 0.05) from that of the control (4.22 ± 0.151 and 3.78 ± 0.190 mmol/L, respectively). In contrast, the blood glucose level of the untreated diabetic rat remained elevated throughout the experimental period (21.64 ± 2.229 mmol/L). The blood glucose level of the control remained unchanged throughout the period of the investigation.
Discussion
Streptozotocin-induced hyperglycemia in rats is considered a good model for the preliminary screening of agents active against type II diabetes (Ivorra et al., 1989) and is widely used. Generally, destruction of β-cells starts three days after STZ administration and reaches its peak at three to four weeks in rats (Adeghate and Ponery, 2002). Streptozotocin-induced diabetes in laboratory animals has been widely used for research on diabetes and its long-term complications. Control animals in these studies are usually injected with citrate buffer solution. However, STZ is known to possess pharmacological effects other than its diabetogenic properties (Schein et al., 1974), and extra pancreatic actions of streptozotocin cannot be excluded. The presence of GLUT2 in liver and kidney might explain the long-term complications seen with hepatic and renal tumors in rats treated with streptozotocin (Delahunt et al., 1995). Because of the extra pancreatic effects of streptozotocin, it may be difficult to distinguish effect secondary to diabetes from those secondary to streptozotocin per se.
Diabetes mellitus is a chronic metabolic disorder characterized by a high blood glucose concentration caused by insulin deficiency, often combined with insulin resistance. Diabetes mellitus is also major cause of disability and hospitalization and it results in significant financial burden (Vats et al., 2002). By the year 2010, the total number of people worldwide with diabetes mellitus is projected to reach 239 million. Region with greatest interest are Asia and Africa, where diabetes rates could rise to 2–3 folds than the present rates (American Diabetes Association, 1997).
Many traditional plant treatments for diabetes mellitus are used throughout the world (Marles and Farnswort, 1995). Management of diabetes without any side effect is still a challenge to the medical system. This has led to an increasing demand for natural products with anti-diabetic activity and fewer side effects (Kameswara et al., 1999). Many herbs and plant products have been shown to have hypoglycemic action. Various morphological parts of Annona muricata have been reported to be useful as effective remedies against diabetes, hypertension, headache, dizziness, constipation, catarrh, liver problems, neuralgia, rheumatism and arthritis pain (de Almeida, 1993).
It has been suggested that bioactive compounds from plants sources having anti-hyperglycemic activities might act by several mechanisms such as stimulating insulin secretion, increasing repair or proliferation of β-cells and enhancing the effects of insulin and adrenalin (Fayed et al., 1998). The result of this present study indicated that there was a significant reduction in the blood glucose concentration of diabetic rats by A. muricata treatment. The present study also shows that daily intraperitoneal administration of 100mg/kg of extracts of A. muricata to diabetic rats for 15 consecutive days caused a statistically significant increase in the body weight of diabetic animals despite the decrease in food and fluid intake observed in these animals. This could be the result of improved glycemic control produced by A. muricata extracts.
References
- 1.Abdel-Barry JA, Al-Hakiem MHH, Abdel-Hassan IA. Acute intraperitoneal toxicity (LD50) and target organ effects of aqueous extract of Trigonella foenumgraceum leaf in the mouse. Basrah J Sci. 1997;58:C58–C65. [Google Scholar]
- 2.Adeghate E, Ponery A S. GABA in the endocrine pancreas: cellular localization and function in normal and diabetic rats. Tissue Cell. 2002;34:1–6. doi: 10.1054/tice.2002.0217. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association, author. Clinical practice recommendations. Diabetes Care. 1997;(Suppl 1):S1–S70. [PubMed] [Google Scholar]
- 4.Atkinson MA, Maclaren NK. The Pathogenesis of insulin dependent diabetes. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 5.Bories C, Loiseau P, Cortes D, Myint SH, Hocquemiller R, Gayral P, Cave A, Laurens A. Anti-parasitic activity of Annona muricata and Annona cherimolia seeds. Planta Med. 1991;57(5):434–436. doi: 10.1055/s-2006-960143. [DOI] [PubMed] [Google Scholar]
- 6.Carbajal D, Casaco A, Arruzazabala L, Gonzalez R, Fuentes V. Pharmacological Screening of Plant Decoctions Commonly Used in Cuban Folk Medicine. J Ethnopharmacol. 1991;33(1/2):21–24. doi: 10.1016/0378-8741(91)90155-7. [DOI] [PubMed] [Google Scholar]
- 7.Chen JC, Tsai CC, Chen NN, Wang WC. Therapeutic, effect of gypenoside on chronic liver injury and fibrosis induced by CC14 in rat. Am J Clin Med. 2000;28:175–185. doi: 10.1142/S0192415X00000222. [DOI] [PubMed] [Google Scholar]
- 8.De Fronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM, International Text book of Diabetes mellitus. 2nd ed. England: Chichester, John Wiley; 1997. pp. 635–712. [Google Scholar]
- 9.deAlmeida ER. Plantas Medicinais Brasileiras, Conhecimentos Populares E Cientificos. Sau Paulo, Brazil: Hemus Editora Ltda; 1993. [Google Scholar]
- 10.Delahunt B, Cartwright PR, Thornton A, Dady PJ. Proliferation kinetics of streptozotocin-induced renal tumours in mice. Virchows Arch. 1995;425:577–582. doi: 10.1007/BF00199345. [DOI] [PubMed] [Google Scholar]
- 11.dos Santos AF, Sant'Ana AE. Molluscicidal properties of some species of Annona. Phytomedicine. 2000;8:115–120. doi: 10.1078/0944-7113-00008. [DOI] [PubMed] [Google Scholar]
- 12.Fayed T, El-Missiry MA, Emara H, El-Sayaad N. Effect of Nigella sativa or fish oil supplementation in alloxan diabetic rats. J Union Arab Biol. 1998;9:237–250. [Google Scholar]
- 13.Feng PC. Pharmacological screening of some West Indian medicinal plants. J Pharm Pharmacol. 1962;14:556–561. doi: 10.1111/j.2042-7158.1962.tb11139.x. [DOI] [PubMed] [Google Scholar]
- 14.Gandhipuram PSK, Palanisamy A, Durairaj SK, Sorimuthu PS. Anti diabetic activity of fruits of Terminalia chebula on streptozotocin-induced diabetic rats. J Hlth Sci. 2006;52:283–291. [Google Scholar]
- 15.Heinrich M, Kuhnt M, Wright CW, Rimpler H, Phillipson JD, Schandelmaier A, Warhurst DC. Parasitological and Microbiological Evaluation of Mixe Indian Medicinal Plants (Mexico) J Ethnopharmacol. 1992;36(1):81–85. doi: 10.1016/0378-8741(92)90063-w. [DOI] [PubMed] [Google Scholar]
- 16.Ivorra MD, Paya M, Villar A. A review of natural products and plants as potential antidiabetic drugs. J Ethnopharmacol. 1989;27:243–275. doi: 10.1016/0378-8741(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 17.Jaramillo M C, Arango G J, Gonzalex MC, Robledo SM, Velez ID. Cytoxicity and antileishmanial activity of Annona muricata pericarp. Fitoterapia. 2000;71:183–186. doi: 10.1016/s0367-326x(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 18.Kameswara RB, Kesavulu MM, Guiri R, Apparao CH. Hepatic key enzyme in experimental diabetes. J Ethnopharmacol. 1999;91(1):109–113. doi: 10.1016/j.jep.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Kasiviswanath R, Ramesh A, Kumar K E. Hypoglycemic and antihyperglycemic effect of Gmelina asiatica LINN. in normal and in alloxan induced diabetic rats. Biol Pharm Bull. 2005;28:729–732. doi: 10.1248/bpb.28.729. [DOI] [PubMed] [Google Scholar]
- 20.Liaw C C, Change FR, Lin C Y, Chon CJ, Chiu HF, Wu M J, Wu Y C. New cytotoxic monotetrahydrofuran annonaceous acetogenins from Annona muricata. J Nat Prod. 2002;65:470–475. doi: 10.1021/np0105578. [DOI] [PubMed] [Google Scholar]
- 21.Luna J S, DeCarvalho JM, DeLima MR, Bieber LW, Bento ES, Franck X, Sant'ana AE. Acetogenins in Annona muricata L (Annonaceae) leaves are potent mollusicides. Nat Prod Res. 2006;20(3):253–257. doi: 10.1080/14786410500161445. [DOI] [PubMed] [Google Scholar]
- 22.Marles J R, Farnsworth N R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2(2):123–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 23.N'gouemo P. Effects of ethanol extract of Annona muricata on pentylenetetrazol-induced convulsive seizures in mice. Phytother Res. 1997;11(3):243–245. [Google Scholar]
- 24.Oberlies N, Chang CJ, McLaughlin JL. Structure-activity relationships of diverse Annonaceous acetogenins against multidrug resistant human mammary adenocarcinoma (MCF - 7/Adr) cells. J Med Chem. 1997;40(13):2102–2106. doi: 10.1021/jm9700169. [DOI] [PubMed] [Google Scholar]
- 25.Oberlies NH, Jones J L, Corbett TH, Fotopoulous SS, McLaughlin JL. Tumor cell growth inhibition by several Annonaceous acetogenins, in an in vitro disk diffusion assay. Cancer Lett. 1995;96:55–62. doi: 10.1016/0304-3835(95)92759-7. [DOI] [PubMed] [Google Scholar]
- 26.Padma P, Pramod NP, Thyagarajan SP, Khosa RL. Effect of the extract of Annona muricata and Petunia nyctaginiflora on Herpes simplex virus. J Ethnopharmacol. 1998;61:81–83. doi: 10.1016/s0378-8741(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 27.Schein PS, Connell MJO, Blom J, Hubbard S, Magrath IT, Bergevin P, Wiernik PH, Ziegler JL, DeVita VT. Clinical antitumor activity and toxicity of streptozotocin. Cancer. 1974;34:993–1000. doi: 10.1002/1097-0142(197410)34:4<993::aid-cncr2820340404>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi JA, Pereira CR, Pimenta LP, Boaventura MA, Silva LG. Antibacterial activity of eight Brazilian Annonaceae plants. Nat Prod Res. 2006;20(1):21–26. doi: 10.1080/14786410412331280087. [DOI] [PubMed] [Google Scholar]
- 29.Takeshi K, Shoichi N, Yasunori K, Yasuhiko I. Report of the committee on the classification and diagnostic criteria of diabetes mellitus (The Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus) Diabetes Res Clinical Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 30.Taylor L. Healing Power of Rainforest Herbs: A Guide to Understanding and Using Herbal Medicine. Carson City Nevada: Raintree Nutrition Inc.; 1996. [Google Scholar]
- 31.Tormo JR, Royo I, Gallardo T, Zafra-Polo MC, Hernandez P, Cortes D, Pelaez F. In vitro-antitumor structure activity relationships of threo/trans/three mono-tetrahydrofuranic acetogenins: correlations with their inhibition of mitochondial complex 1. Oncol Res. 2003;14(3):147–154. doi: 10.3727/000000003771013099. [DOI] [PubMed] [Google Scholar]
- 32.Vasquez M R. Useful Plants of Amazonian Peru Second Draft. USA: Filed with USDA's National Agricultural Library; 1990. [Google Scholar]
- 33.Vats V, Grover JK, Rathi SS. Evaluation of anti-hyperglycaemic and hypoglycaemic effect of Trigonella foenum-graecum Linn., Ocimum sanctum Linn and Pterocarpus marsupium Linn in normal and alloxanised diabetic rats. J Ethnopharmacol. 2002;79:95–100. doi: 10.1016/s0378-8741(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization, author. Prevention of diabetes mellitus. Technical Report Series. 1994;844:11–15. [PubMed] [Google Scholar]