Abstract
Acacia mellifera has been used widely in traditional African medicines against various diseases. Among the Kipsigis community of Kenya, water extracts from the plant is used for the treatment of skin diseases, coughs and gastrointestinal ailments. The aim of the study was to provide scientific rationale for the use of the plant in traditional medicine through bioassay-guided fractionation of A. mellifera stem bark. Bioactivity testing was done against selected microbes using disc diffusion technique as outlined in Clinical Laboratory Standard Institute (CLSI). Structure elucidation of the isolated compounds was based primarily on 1D and 2D NMR analyses, including HMQC, HMBC, and NOESY correlations. Fractionation yielded three triterpenoids; (20S)-oxolupane-30-al, (20R)-oxolupane-30-al, and betulinic acid. The three compounds were active against Staphylococcus aureus ATCC 25923 and only (20S)-oxolupane-30-al against clinical isolate of Microsporum gypseum. The three compounds had no activity against Escherichia coli ATCC 25922, Enterococcus feacalis, Candida albicans ATCC 90028, Cryptococcus neoformans, Trichophyton mentagrophyte, Candida krusei, Microsporum gypseum, and Sacharomyces cerevisiae. These results explain and support the use of A. mellifera stem barks for the treatment of infectious diseases in traditional Kenya medicine. It also shows that the antimicrobial activity is concentrated in the triterpenoid fractions.
Keywords: Acacia mellifera, Antimicrobial pentacyclic triterpenes, Kenya medicinal plant
Introduction
Acacia mellifera is a shrub or tree belonging to the family Leguminosae. It is widely distributed in Kenya and is widely used for medicinal purposes in both human and veterinary medicine in resource-poor rural and urban households. The decoction of its stem barks is used against diarrhea and eye problems in livestock, stomachache, malaria, coughs, primary infection of syphilis, sterility, and pneumonia in human being (Kokwaro, 1976). Plants in the family Leguminosae are known for their many sesquiterpenoids (Dastalik et al., 1991), diterpenes (Joshi et al., 1979), triterpenoids (Mutai et al., 2004) and flavanoids (Malan, 1995) which are responsible for various pharmacological properties including antimalarial activity (Rajic et al., 2000; Alves et al., 1997; Akihisa et al., 2002).
Nevertheless, no exhaustive phytochemical studies have been conducted on the A. mellifera species. The present paper reports on the chemical and antimicrobial properties of stem bark extracts of A. mellifera in an attempt to provide scientific justification of its use in Kenyan traditional medicine and to identify the bioactive compound.
Materials and methods
Plant material
The stem bark of A. mellifera was collected in January 2000 from Machakos, Kenya and identified by Mr. Onesmus Mwangangi of East Africa Herbarium-National Meseums, Nairobi. A voucher specimen (ChM-1) was deposited at the same institute.
Extraction and isolation
Dried powdered stem bark of A. mellifera (2.25kg) was successively extracted with dichloromethane (100%) and methanol (100%). The resulting extracts were filtered and concentrated under vaccum to afford a dark brown residue. The dichloromethane residue was subjected to column chromatography on silica gel, using cyclohexane, cyclo-hexane and EtOAc mixture of increasing polarity, and finally pure EtOAc, to yield 61 fractions. These fractions were further combined to fractions I-IX on the basis of TLC. Fraction III was eluted with cyclohexane-EtOAc (gradient, 98:2), and further purified on silica gel using cyclo-hexane EtOAc (9:1) to yield relatively pure triterpenoids. The triterpenoid extract was further purified by normal phase HPLC to obtain three pure triterpenoids identified by spectroscopic techniques.
IR spectra were recorded on a Paragon 500 Perkin-Elmer infrared spectrophometer. EIMS data was obtained with a Hewlett Packard 5973 GC/MS spectrometer. The NMR spectra were recorded on a Bruker DRX 400 for 1H and Bruker AC 200, respectively, in CDCL3. Chemical shifts are given in δ (ppm) scale using TMS as internal standard. The 2D experiments (1H-1H COSY, HMQC, HSQC, HMBC) were performed using standard Bruker microprograms. EIMS data were recorded on a Hewlett Packard 5973 Mass Selective Detector. Column chromatography was performed with Kiesegel 60 (Merck), HPLC was conducted on an Agilent 1100 series with refractive index dectector, with Kromasil Sil 100, 5 um, 280x8mm column. TLC was performed with Kiesegel 60 F-254 (Merck aluminum support plates).
Antimicrobial assays
Antimicrobial activity was carried out at the Center for Microbiology Research using disc diffusion method (Vlientinck et al., 1995). Antibacterial activity was done on Mueller Hinton agar (Oxoid) using Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Enterococcus feacalis. Antifungal activity was determined on sabourauds dextrose agar (Oxoid) using Candida albicans ATCC 90028 and clinical isolates of Cryptococcus neoformans, Candida krusei, Microsporum gypseum, and Sacharomyces cerevisiae. Briefly, bacteria and yeast test strains were cultured on Mueller-Hinton gar for 24 hrs at 37°C and 35°C respectively and the molds on Sabouraud dextrose agar at 30°C for 72 hrs ambient air. A 0.5 McFarland standard suspension was prepared in normal saline. For molds, mycelia suspension was used. The suspension was spread uniformly on Muller Hinton agar for bacteria and sabouraud dextrose agar (SDA) for fungal strains. A 6 mm sterile paper disc was impregnated with 10 µl of the test extracts, dried in a clean bench before aseptically placing onto the surface of the inoculated media. The plates were then incubated at temperatures of 35 °C for yeast, 37°C for bacteria and 30°C for dermatophytes for 24 and 72 hrs respectively. The zones of inhibition were measured as indicators of activities. Extracts with activity were fractionated and retested for bioactivity as previously described. All the tests were done in triplicate. Chloramphenicol (30µg) and fluconazole (99.6% potency, Pfizer) were used as standards. Discs impregnated with extraction solvents were used as controls (Vlientink et al., 1995; National Committee for Clinical Laboratory Standards, 1997).
Data analysis
The diameter of inhibition zone around each disc was measured and recorded at the end of incubation period. The average of the triplicate tests was taken. The degree of activity of the extracts was expressed according to inhibition zone diameter as follows; no activity (<7 mm), 8–11 mm active, >12mm very active. The activities were compared using students‘t’-test with P < 0. 05.
Results
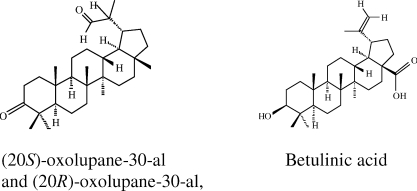
The antibacterial and antifungal activity-guided fractionation led to the isolation of three compounds. The three triterpenoids were (20S)-oxolupane-30-al, (20R)-oxolupane-30-al, and betulinic acid (Figure 1).
Figure 1.
Structures of the tritepenoids isolated from Acacia mellifera.
Betulinic acid was the most abundant of the three triterpenoids identified. The (20S)-oxolupane-30-al and (20R)-oxolupane-30-al were eluded almost together. Spectroscopic analysis and direct comparison with authentic compounds identified the compounds on the basis of the following evidence. (20S) 3-oxolupane-30al - Colourless gum, [α] D20=5.5 (c 0.18, CHCl3); IR (CHCl3) vmax 2942, 2348, 1704, 1526, 1234 cm−1; 1H NMR (CDCL3, 400 MHz) and 13C NMR (CDCl3, 50.3 MHz), see Table 1 and Table 2, respectively; EIMS 70eV, m/z (rel.int. %): 382 (100), 163 (40), 205 (28), 81 (22), 55 (21), 107 (18), 189 (12), 135 (12), 425 (4), 273 (4); HRFAB-MS (m/z): 441.3735 [M+H]+ (calcd. for C30H49O2, [M+H]+: 441.37347). (20R) 3-oxolupane-30al- Colourless gum, [α] D20=−3.14 (c 0.64, CHCL3); IR (CHCl3) vmax 2942, 2348, 1704, 1526, 1234 cm−1; 1H NMR (CDCL3, 400 MHz) and 13C NMR (CDCl3, 50.3 MHz), see Table 1 and Table 2, respectively; EIMS 70eV, m/z (rel. int.%): 382 (100), 163 (40), 205 (28), 81 (22), 55 (21), 107 (18), 189 (12), 135 (12), 425 (4), 273 (4); HRFAB-MS (m/z): 439.3596 [M-H]+ (calcd. for C30H47O2, [M-H]+, 439.35781).
Table 1.
1 H NMR spectral data of compounds (20S)-oxolupane-30-al, (20R)-oxolupane-30-al, and betulinic acid
| Carbons | (20S) 3-oxolupane-30al PMR |
(20R) 3-oxolupane-30al PMR |
Betulinic acid PMR |
| 1 | α, 1.90 (ddd, 4.41, 7.64, 13.24 β, 1.38 (m) |
α, 1.40 (m), β, 1.91 (m) |
0.86, 1.64 (m) |
| 2 | α, 2.40 (m) β, 2.47 (ddd, 7.35, 9.70, 15.88) |
α, 2.40 (ddd, 4.76, 7.84, 15.68), β, 2.47 (ddd, 7.52, 9.56, 15.68) |
1.52 (m), 1.61(m) |
| 3 | - | 3.17 (m) | |
| 4 | - | ||
| 5 | 1.32 (m) | 1.33 (m) | 0.66 (m) |
| 6 | 1.41–1.53 (m) | α, 1.44 (m), β, 1.56 (m) |
1.50 (m) |
| 7 | 1.45 (m) | 1.44 (m) | 1.35 (m), 1.40 (m) |
| 8 | - | - | |
| 9 | 1.38 (m) | 1.38 (m) | 1.25 (m) |
| 10 | - | ||
| 11 | 1.06 (m), 1.52 (m) | β, 1.40 (m) α, 1.55 (m) |
1.37 (m) |
| 12 | 1.35 (m), 1.65 (m) | α, 1.38 (m) β, 1.56 (m) |
1.04, 1.68 (m) |
| 13 | 1.79 (ddd, 4.12, 11.75, 11.75) | 1.74 (m) | 2.20 (m) |
| 14 | - | - | |
| 15 | 1.03 (m), 1.69 (m) | α,1.05 (m) β, 1.69 (m) |
1.14, 1.49 (m) |
| 16 | 1.6 (m), 1.53 (m) | 2H, 1.47 (m) | 1.27 (m) |
| 17 | - | - | |
| 18 | 1.26 (m) | 1.43 (m) | 1.61 (m) |
| 19 | 2.35 (m) | 1.89 (m) | 3.00 (m) |
| 20 | 2.63 (dq, 2.94, 6.76) | 2.60 (m) | |
| 21 | 1.5 (m), 1.68 (m) | α, 1.51 (m) β, 1.88 (m) |
1.99, 1.44 (m) |
| 22 | 1.39 (m), 1.45 (m) | α, 1.36 (m) β, 1.47 (m) |
1.96, 1.55 (m) |
| 23 | 1.06 (s) | 1.06 (s) | 0.95 (s) |
| 24 | 1.01 (s) | 1.01 (s) | 0.73 (s) |
| 25 | 0.93 (s) | 0.91 (s) | 0.80 (s) |
| 26 | 1.07 (s) | 1.06 (s) | 0.91 (s) |
| 27 | 0.93 (s) | 0.92 (s) | 0.96 (s) |
| 28 | 0.78 (s) | 0.75 (s) | |
| 29 | 1.01 (d, 6.76) | 1.07 (d, 7.17 Hz) | 4.58 (s), 4.71 (s) |
| 30 | 9.60 (s) | 9.84 (d, 2.04 Hz) | 1.67 (s) |
Table 2.
13C NMR spectral data of compounds (20S)-oxolupane-30-al, (20R)-oxolupane-30-al, and betulinic acid (Spectra recorded at 50.3 MHz in CDCl3 at 25°C)
| Carbons | (20S) 3-oxolupane-30al CMR |
(20R) 3-oxolupane-30al CMR |
Betulinic acid CMR |
| 1 | 39.5 | 39.4 | 38.7 |
| 2 | 34.1 | 34.0 | 27.4 |
| 3 | 217.8 | 218.5 | 78.9 |
| 4 | 47.3 | 47.3 | 38.8 |
| 5 | 54.8 | 54.7 | 55.3 |
| 6 | 19.6 | 19.6 | 18.2 |
| 7 | 33.6 | 33.4 | 34.2 |
| 8 | 40.8 | 40.6 | 40.7 |
| 9 | 49.3 | 49.2 | 50.5 |
| 10 | 36.8 | 36.7 | 37.2 |
| 11 | 21.2 | 21.3 | 20.8 |
| 12 | 26.5 | 27.4 | 25.5 |
| 13 | 37.8 | 37.8 | 38.3 |
| 14 | 43.1 | 42.9 | 42.4 |
| 15 | 27.2 | 27.1 | 29.6 |
| 16 | 35.2 | 35.1 | 32.1 |
| 17 | 42.9 | 43.0 | 56.2 |
| 18 | 47.0 | 48.9 | 49.2 |
| 19 | 37.3 | 42.6 | 46.9 |
| 20 | 49.7 | 48.9 | 150.4 |
| 21 | 23.6 | 25.0 | 30.5 |
| 22 | 40.4 | 39.9 | 37.0 |
| 23 | 26.6 | 26.6 | 28.0 |
| 24 | 21.1 | 21.0 | 15.3 |
| 25 | 15.9 | 15.7 | 16.1 |
| 26 | 15.8 | 15.7 | 16.0 |
| 27 | 14.3 | 14.2 | 14.7 |
| 28 | 17.9 | 17.5 | 181.4 |
| 29 | 7.4 | 14.4 | 109.7 |
| 30 | 205.1 | 207.1 | 19.3 |
Bioactivity testing of (20S)-oxolupane-30-al (IZD=10 mm), (20R)-oxolupane-30-al (IZD=10 mm) and betulinic acid (IZD =9 mm) demonstrated antibacterial activity against S. aureus ATCC 25923 (Table 3) at a concentration of 1mg/ml. However, no significant activity was observed against Escherichia coli ATCC 25922, Candida albicans ATTC 90028 and clinical isolates of Enterococcus feacalis and Cryptococcus neoformans common opportunistic pathogens.
Table 3.
Inhibition zone diameter IZD (mm) of tritepenes against the bacterial strains and fungal strains
| Bacterial strains and fungal strains |
Oxolupane-30-al | Betulinic acid |
Chloramphenical | Fluconazole | |
| (20S) | (20R) | ||||
|
Staphylococcus aureus (ATCC 25923) |
10 | 10 | 9 | 19 | N/A |
| Microsporum gypseum | 21 | - | - | - | 0.25* |
N.B there was no activity against Escherichia coli ATCC 25922, Enterococcus feacali, Candida albicans ATCC 90028, Cryptococcus neoformans, Trichophyton mentagrophyte, Candida krusei, Microsporum gypseum, and Sacharomyces cerevisiae
Determined by MIC method
The (20R)-oxolupane-30-al and betulinic acid did not demonstrate any in-vitro antifungal activity against a wide spectrum of pathogenic fungi namely; Candida albicans, Trichophyton mentagrophyte, Candida krusei, Microsporum gypseum and Sacharomyces cerevisiae. However, (20S)-oxolupane-30-al (IZD=21 mm) was active against Microsporum gypseum a common agent of dermatophytoses.
Discussion
The 20S and 20R configurations are usually difficult to be distinguished and separated and have only been previously isolated as a mixture (Corbett et al., 1987; Fang et al., 1984). But this is the first time these two compounds have been isolated as separate compounds elucidated and tested for antimicrobial activity. However, betulinic acid has been isolated from various plants (Weinges and Schick, 1995). The (20S)-oxolupane-30-al, (20R)-oxolupane-30-al, and betulinic acid showed antibacterial activity against S. aureus. However, there were no significant difference in the activity of the three compounds against S. aureus (p>0.05). Like other triterpenoids such as Imberbic acid, which have been shown to have potent bactericidal activity against Mycobacterium fortuitum and S. aureus (Katerere et al., 2003), the three compounds were active on S. aureus. Apart from antimicrobial and cytotoxic properties, other biological properties of betulinic acid have been reported (Bringmann et al., 1997; Pisha et al., 1995). Betulinic acid derivatives applied topically have been shown to selectively treat or inhibit melanoma (Pezzuto et al., 1999). Investigations of butulinic acid derivatives have been shown to be a specific HIV-1 inhibitor and a potential candidate for anti-HIV drug (Kashiwada et al., 1998).
Previously we demonstrated cytotoxic activity of lupane types of triterpinoids against the NSCLC-N6 cell line, derived from a human non-small-cell bronchopulmonary carcinom (CI50 15–30 µg/ml) (Mutai et al., 2004). However, the two triterpenoids; (20S)-oxolupane-30-al and (20R)-oxolupane-30-al were found to have no cytotoxic effect on the cells (Mutai et al., 2007). Therefore, the lack of cytotoxicity exhibited by (20S)-oxolupane-30-al and (20R)-oxolupane-30-al against NSCLC-N6 cell line suggests a good antimicrobial product.
In conclusion, the activity shown in bactericidal and fungicidal activities of (20S)-oxolupane-30-al, (20R)-oxolupane-30-al and betulinic acid indicate that they are a major active constituent of the stem bark extract of A. mellifera. Therefore, it partly explains and supports the use of the decoction of the stem bark of A. mellifera in traditional medicine for the treatment of infectious diseases and human mycoses in Kenyan population. Studies with (20S)-oxolupane-30-al showed good antifungal potency and lack of cytotoxicity make it an important subject for structure-activity studies aimed at development of novel antifungal agents with an improved therapeutic index.
Acknowledgements
This work was supported by state scholarships foundation of Greece awarded to C. Mutai. I wish to thank the Director of Kenya Medical Research Institute for granting me study leave.
References
- 1.Akihisa T, Takamine Y, Yoshizumi K, Tokuda H, Kimura Y, Ukiya M, Nakahara T, Yokochi T, Ichiishi E, Nishino H. Microbial transformations of two lupane-type triterpenes and antitumor-promoting effects of the transformation products. J Nat Prods. 2002;65:278–282. doi: 10.1021/np010424m. [DOI] [PubMed] [Google Scholar]
- 2.Avles TM, Nagem TJ, De Carvalho LH, Krettli AU, Zani CL. Antiplasmodial triterpene from Vernonia brasiliana. Planta Medica. 1997;63:554–557. doi: 10.1055/s-2006-957764. [DOI] [PubMed] [Google Scholar]
- 3.Bringmann G, Saeb W, Assi L A, Francois G, Narayanan A S S, Peters K, Peters E M. Betulinic Acid. J Ethnopharmacol. 1997;46:255–257. doi: 10.1055/s-2006-957666. [DOI] [PubMed] [Google Scholar]
- 4.Corbett R E, Cong ANT, Holland P T, Wilkins A L. Extractives from pseudocyphellaria rubella. Ausral J Chem. 1987;40:461–468. [Google Scholar]
- 5.Dastlik KA, Ghisalberti EL, Skelton BW, White AH. Structural study of (-)-8-Epi-11-nordriman-9-one. Austral J Chem. 1991;44:123–127. [Google Scholar]
- 6.Fangs S, Berry D E, Lynn D G, Hecht S M, Campbell J, Lynn W S. The chemistry of toxic principles from Maytenus numerosa. Phytochemistry. 1984;23:631–633. [Google Scholar]
- 7.Joshi KC, Bansal T, Murray R D H, Forbes I T, Cameron A F, Maltz A. Two novel cassane diterpenoids from Acacia jacquemontii. Tetrahedron. 1979;35:1449–1453. [Google Scholar]
- 8.Kashiwada Y, Wang H K, Nagao T, Kitanaka S, Yasuda I, Fujioka T, Yamagishi T, Consentino L M, Kozuka M, Okabe H, Ikeshiro Y, Hu C Q, Lee K H. J Nat Prods. 1998;61:1090–1095. doi: 10.1021/np9800710. [DOI] [PubMed] [Google Scholar]
- 9.Katerere DR, Gray AI, Nash RJ, Waigh RD. Pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry. 2003;63:81–88. doi: 10.1016/s0031-9422(02)00726-4. [DOI] [PubMed] [Google Scholar]
- 10.Kokwaro O. Medicinal plant of East Africa. Nairobi, Dar es Salaam: East African Literature Bureau Kampala; 1976. [Google Scholar]
- 11.Malan E, Sireeparsad A. The structure and synthesis of the first dimeric proteracacadins from Acacia galpinii. Phytochemistry. 1995;38:237–239. [Google Scholar]
- 12.Mutai C, Abatis D, Vagias C, Moreau D, Roussakis C, Roussis V. Cytotoxic lupane-type triterpenoids from Acacia mellifera. Phytochemistry. 2004;65:1159–1164. doi: 10.1016/j.phytochem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Mutai C, Abatis D, Vagias C, Moreau D, Roussakis C, Roussis V. Lupane triterpenoids from Acacia mellifera with cytotoxic activity. Molecules. 2007;12:1035–1044. doi: 10.3390/12051035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards, author. performance Standards for Antimicrobial Disk Susceptibility Tests. Approved standards M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 15.Pezzuto J M, Dasguta tapas, Kim K, Darrick S H L. 1999;19:5–68. U. S. Patent. [Google Scholar]
- 16.Pisha E, Chai H, Lee I S, Chagwedra T E, Fransworth NR, Cordell G A, Beecher C E W, Fong H H S, Kinghorn A D, Brown D M, Wani M C, Wall M E, Hieken T J, Gupta T K D G, Pezzuto J M. Discovery of betulinic acid as a selective inhibitor of human melanoma. Natural Medicine. 1995;1:1046–1050. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 17.Rajic A, Kweifio-Okai G, Macrides T, Sandeman RM, Chandler DS, Polya GM. Inhibition of serine proteases by anti-inflammatory triterpenoids. Planta Medica. 2000;66:206–210. doi: 10.1055/s-2000-8657. [DOI] [PubMed] [Google Scholar]
- 18.Vlientink J, Van Hoof L, Lasure A, Vanden Berghe D, Rwangabo PC, Mwakiyumwani J. Screening of a hundred Rwandese medicinal plants for antibacterial and antiviral properties. J Ethnopharmacol. 1995;46:31–47. doi: 10.1016/0378-8741(95)01226-4. [DOI] [PubMed] [Google Scholar]
- 19.Weinges K, Schick H. Dodecaacetylprodelphinidin B3 from the dried leaves of Ziziphus spinachristi. Phytochemistry. 1995;38(2):505–507. [Google Scholar]