Abstract
Cytotoxic, antioxidant, tyrosinase inhibitory, antimicrobial activities of the crude ethanol extract of dry powdered roots of Asparagus racemosus (Liliaceae) were investigated. The LC50 to brine shrimp was 2189.49 µg/ml; the EC50 for DPPH radical scavenging was 381.91 µg/ml; the IC50 for tyrosinase inhibition was 7.98 mg/ml. The extract was active at 5–20 mg/ml against various pathogenic microbial (16 species, 18 strains) using the agar dilution assay, with the minimum inhibitory concentration (MIC) between 10–20 mg/ml for enteropathogens, the MIC between 5–20 mg/ml for dermatopathogens, and MIC = 10 mg/ml for a pneumonia causing bacteria Klebsiella pneumoniae. TLC and HPLC finger printing showed the presence of steroids-terpenes, alkaloids and flavonoids.
Keywords: Asparagus racemosus, Antioxidant, Antityrosinase, Antimicrobial, Phytochemistry
Introduction
Asparagus racemosus Willd. (Liliaceae), locally known in Thailand as ‘Rag Samsib’, is a woody climber growing to 1–2 m in height. The Thai local name ‘Rag Samsib’ refers to its finger-like and clustered roots. The leaves are like pine-needles, small and uniform. The inflorescence has tiny white flowers, in small spikes (Vichien, 2003). The plant is common at low altitudes in shade and in tropical climates throughout Asia, Australia and Africa. In India, the plant is called Shatavari in Hindi. The root has long been used in Ayurveda as a tonic remedy to promote fertility and reducing menopausal symptoms. It is also used for dry coughs and gastric ulcers (Winston, 2004). Recent research indicates Shatavari enhances immune function, increases corticosteroid production, and promotes cell regeneration (Rege et al., 1999).
In Thailand the root is claimed as a galactagogue, antidysenteric, antipeptic, antipruritic, antirheumatic, tonic and longevity enhancer.
This study investigated various biological activities of the crude ethanol root extract of A. racemosus cultivated in Thailand. TLC fingerprints and HPLC fingerprint of the root powder were performed.
Materials and Methods
Plant material
Fresh roots of A. racemosus were collected in May 2003 from Nakhon Rachasima Province, Thailand. The sample was dried in a hot air oven at 40–50°C, and then pulverized into powder. The specimen (no. LRS-0110) was authenticated by the Research Botanist Officer and kept at the Lamtakhong Plant Research Station, TISTR.
Preparation of crude ethanol extract
The root powder was repeatedly macerated with 95% ethanol in a percolator. The combined filtrate was evaporated to dryness under reduced pressure at 40–50°C. The resulting crude ethanol extract was then stored at 10–15°C.
Cytotoxicity to brine-shrimp
The brine-shrimp micro-plate assay was a modified version of Solis et al. (1992) used to determine the inhibitory activity on Artemia sp. in 0.25% Tween 80-artificial seawater, as described by Potduang et al. (2007). The sample solution was added into 6 wells, each containing 5 newly hatched brine shrimps to make overall 30 brine shrimps in contact with the sample for 24hr. The dead organisms were counted under a binocular microscope (4x). Plot %Lethality vs Log concentration. Substituted y = 50 in the resulted linear equation to obtain the x value. The antilog x was then the LC50 (conc. of 50% lethality) value (Ballantyne et al., 1995). Thymol and kojic acid were used as reference standards.
Antioxidant activity Scavenging of DPPH radical
The DPPH radical scavenging micro-plate assay modified from Hatano et al. (1989), as described by Potduang et al. 2007, was used. Equal volumes of absolute ethanol solutions of the extract and 0.06 mM DPPH (2,2-diphenyl-1-picrylhydrazyl from Sigma, Germany) were mixed for 30 min in a micro-well plate, and absorbance measured at 517nm in a micro-plate reader (TECAN, Sunrise remote). All samples were run in triplicate. The % scavenging activity of test samples was determined as follows:
Where A, B and C represent the absorbances of DPPH in the reaction mixture, the blank, and the control respectively. Plot %Scavenging vs Log concentration. Substituted y = 50 in the resulted linear equation to obtain the x value. The antilog x was then the EC50 (conc. of 50% scavenging) value (Ballantyne et al., 1995). BHT, BHA and vitamin C were used as reference standards.
Tyrosinase inhibition
The dopachrome micro-plate assay modified from Iida et al. (1995) was used to investigate the tyrosinase inhibition of the 20% ethanol derived extract, as described by Potduang et al. 2007. The 50 µl sample solution was mixed with 50 µl of mushroom tyrosinase buffer solution (314.8U/ml, Fluka) and 150 µl of 0.02 M sodium phosphate buffer (pH 6.8), and allowed to stand for 10 min. Added was 50 µl of 0.34 mM L-Dopa (Sigma Chemical) buffer solution as substrate, mixed and then incubated for 2 min. The absorbance was measured at 492nm by a micro-plate reader (TECAN, Sunrise remote). All samples were run in triplicate. The absorbance differences before and after the 2 min-incubation were used to calculate the percentage inhibition of tyrosinase as follows:
Where the absorbance difference A represents the control (L-Dopa mixed with enzyme in buffer); B represents the blank (L-Dopa in buffer); C represents the reaction mixture; and D represents the blank of C (L-Dopa mixed with test sample in buffer). Plot %Tyrosinase inhibition vs Log concentration. Substituted y = 50 in the resulted linear equation to obtain the x value. The antilog x was then the IC50 (conc. of 50% inhibition) value (Ballantyne et al., 1995). A well-known tyrosinase inhibitor, kojic acid, was used as the reference standard.
Anti-microbial activity
The agar dilution method (Washington and Sutter, 1980) was used to test the activities against pathogenic microorganisms, using specific assay media and broths as described by Potduang et al. 2007. The media were Mueller Hinton Agar (MHA; Difco Laboratories) for aerobes; WC Agar (Wilkins and Chalgren, 1976) for anaerobes; and Saboraud Dextrose Agar (SDA; Difco Laboratories) for yeasts. The isolates suspension was adjusted to McFarland 0.5 turbidity standard. Spot inoculated the 5–20 mg/ml dilution plates of the crude extract and incubated at 37°C (overnight for aerobes; 3 days for anaerobes; 48 hr for yeast). The minimum inhibitory concentrations (MICs) of the extract were determined.
TLC fingerprints
Thin-layer chromatography (TLC) of 3 different extracts containing steroids-terpenes, alkaloids or flavonoids from the root powder were performed on 0.25 mm thick TLC plates (Merck Silica gel 60 F254-precoated) using suitable developing solvents and special detection reagents (Merck, 1980; Wagner and Bladt, 1996). These 3 easily extracted groups possess various biological activities.
TLC fingerprint of the steroids-terpenes extract. The extract was prepared by stirring 5 g of the root powder with hexane (3x50 ml) for 30 min. The filtrate was concentrated to dryness under reduced pressure, and then dissolved in 0.5 ml chloroform. The extract (2 µl) was applied onto a TLC plate to perform 10 cm chromatography with suitable solvent system. The developed plate was sprayed with vanillin-sulfuric acid reagent, then heated until the spots attain maximum colour intensity of steroids-terpenes compared to ref. std. 1:1 w/v β-sitosterol (Sigma, USA) in chloroform.
TLC fingerprint of the alkaloids extract. The extract was prepared by stirring 20 g of the root powder with 100 ml 0.1N sulphuric acid for 20 min. The filtrate was alkalinized to pH 8-9 with 5% ammonium hydroxide. The free alkaloids were extracted by partitioning with chloroform (3x80 ml). The combined chloroform extracts were dried over anhydrous sodium sulphate before evaporated to dryness under reduced pressure. The dried extract was dissolved in 0.2 ml methanol before applying 10 µl onto a TLC plate to perform 10 cm chromatography with a suitable solvent system. The developed plate was sprayed with Dragendroff's reagent to visualize orange-brown zones of alkaloids compared to ref. std. 1:1 w/v quinine sulphate (BDH, England) in methanol.
TLC fingerprint of the flavonoids extract. The extract was prepared by stirring 0.5 g of the root powder with 5 ml methanol on a dry block heat bath (60°C, 5 min), allowed to cool, filtered, evaporated to dryness under reduced pressure. Dissolved the dried extract in 0.2 ml methanol, then filtered through a PTFE syringe filter membrane (Orange Scientific, Belgium) before applying 5 µl onto a TLC plate to perform 10 cm chromatography with a suitable solvent system. The developed plate was sprayed with natural products-polyethylene glycol (NP/PEG) reagent to achieve fluorescing zones of flavonoids under UV-365nm compared to ref. std. 1:1 w/v rutin (Fluka, Switzerland) in methanol.
HPLC fingerprint
A methanol extract containing flavonoids was prepared by shaking 5 g of the root powder with 25 ml of methanol at 1,500 rpm for 2 min. The filtration was made through a Whatman paper no.41, and then added methanol to make the filtrate to 25 ml in a volumetric flask. The sample solution was then filtered through a 0.45 µ nylon syringe filter membrane before subjected to binary gradient RP-18, 30°C, 1 ml/min flow rate, HPLC analysis with 270nm UV detector. Solvent A was water with 0.1% TFA + 10% methanol, and solvent B was acetronitrile with 0.1% TFA. Standard addition of rutin (Merck, Germany) and quercetin (Fluka, Switzerland) was applied to HPLC chromatogram.
Results
The crude ethanol root extract of A. racemosus was 9.01%. The extract exhibited an LC50 of 2189.49 µg/ml on brine shrimp cytotoxicity, and gave EC50 of 381.91 µg/ml on DPPH radical scavenging. The derived 20% ethanol extract gave the IC50 of 7.98 mg/ml on mushroom tyrosinase inhibition (Tables 1–4).
Table 1.
Biological effects of the ethanol extract from the roots of A. racemosus
| Compound | Inhibitory effect on brine shrimp (LC50) |
DPPH radical scavenging effect (EC50) |
Anti-tyrosinase effect (IC50) |
| Crude ethanol extract | 2189.49 µg/ml | 381.91 µg/ml | - |
| 20%ethanol extract | - | - | 7.98 mg/ml |
| Kojic acid | 16.68 µg/ | - | 0.0023 mg/ml |
| Thymol | 13.59 µg/ml | - | - |
| BHT | - | 4.21 µg/ml | - |
| BHA | - | 4.23 µg/ml | - |
| Vitamin C | - | 1.22 µg/ml | - |
Table 4.
Inhibitory effect on mushroom tyrosinase of the 20% ethanol fraction from the ethanol extract from the roots of A. racemosus
| Compound | Concentration (mg/ml) |
Log concentration |
%Tyrosinase inhibition |
IC50 (mg/ml) |
| 20%ethanol | 2.5 | 0.3979 | 26.51 | 7.98 |
| extract | 5 | 0.6990 | 40.96 | |
| 10 | 1.0000 | 53.01 | ||
| 15 | 1.1761 | 63.86 | ||
| 20 | 1.3010 | 68.67 | ||
| Kojic acid | 1.42x10−5 | −4.8477 | 2.38 | 0.0023 |
| 7.1x10−4 | −3.1487 | 25.40 | ||
| 1.42x10−3 | −2.8477 | 38.89 | ||
| 1.42x10−2 | −1.8477 | 78.57 | ||
| 7.1x10−2 | −1.1487 | 88.10 |
Table 2.
Inhibitory effect on brine-shrimp of the ethanol extract from the roots of A. racemosus
| Compound | Concentration (µg/ml) |
Log concentration |
% Lethality | LC50 (µg/ml) |
| Crude ethanol | 1,000 | 3.0000 | 0 | 2189.49 |
| root extract | 1,250 | 3.0969 | 20 | |
| 2,500 | 3.3979 | 56.66 | ||
| 5,000 | 3.6990 | 100 | ||
| Kojic acid | 1 | 0 | 0 | 16.68 |
| 10 | 1 | 16.67 | ||
| 100 | 2 | 100 | ||
| Thymol | 1 | 0 | 0 | 13.59 |
| 10 | 1 | 30 | ||
| 100 | 2 | 100 |
Table 3.
In vitro DPPH radical scavenging effect of the ethanol extract from the roots of A. racemosus
| Compound | Concentration (µg/ml) |
Log concentration |
% Scavenging | EC50 (µg/ml) |
| Crude ethanol | 50 | 1.6990 | 7.46 | 381.91 |
| root extract | 100 | 2.0000 | 17.16 | |
| 500 | 2.6990 | 50.75 | ||
| 1000 | 3.0000 | 73.88 | ||
| 2500 | 3.3979 | 94.78 | ||
| BHT | 0.25 | −0.6021 | 18.69 | 4.21 |
| 0.50 | −0.3010 | 23.53 | ||
| 1.25 | 0.0969 | 27.68 | ||
| 5 | 0.6990 | 57.44 | ||
| 50 | 1.6990 | 79.58 | ||
| BHA | 0.25 | −0.6021 | 9.53 | 4.23 |
| 0.50 | −0.3010 | 14.62 | ||
| 1.25 | 0.0969 | 27.31 | ||
| 2.50 | 0.3979 | 46.15 | ||
| 25 | 1.3979 | 76.92 | ||
| Vitamin C | 0.25 | −0.6021 | 22.77 | 1.22 |
| 0.50 | −0.3010 | 25.25 | ||
| 2.50 | 0.3979 | 67.33 | ||
| 3.75 | 0.5740 | 81.19 | ||
| 25 | 1.3979 | 95.54 |
The agar dilution assay indicated that the crude extract at 5-20 mg/ml, was active against various disease causing microorganisms (16 species, 18 strains). The minimum inhibitory concentrations (MICs) were 10–20 mg/ml against enteropathogens: Enterococcus faecalis, Salmonella velterans, Shigella dysenteriae, Staphylococcus aureus, Escherichia coli, Bacteroides spp., Clostridium spp., Peptococcus spp., Lactobacillus spp. and Streptococcus mutans. The MICs were 5–20 mg/ml against dermatopathogens as Staphylococcus epidermidis, Propionibacterium acnes, Candida albicans, Pseudomonas aeruginosa and Streptococcus spp. The MIC was 10 mg/ml against a pneumonia causing bacteria Klebsiella pneumoniae (Table 5).
Table 5.
Minimal inhibitory concentrations (MICs) of the ethanol extract from the roots of A. racemosus on various pathogenic microorganisms
| Cultured strains | MIC (mg/ml) | |
| Aerobes | ||
| Gram negative aerobic/microaerophilic rods and cocci | ||
| Pseudomonas aeruginosa ATCC 27853 | 20 | |
| Pseudomonas vulgaris | >20 | |
| Gram negative, facultative anaerobic rods | ||
| Escherichia coli ATCC 25922 | 20 | |
| Salmonella choleraesuis subsp. choleraesuis ATCC10708 | >20 | |
| Salmonella typhimurium ATCC 13311 | >20 | |
| Salmonella velterans | 10 | |
| Shigella dysenteriae D 2137 | 10 | |
| Klebsiella pneumoniae | 10 | |
| Gram positive cocci | ||
| Enterococcus faecalis ATCC 29212 | 10 | |
| Staphylococcus aureus ATCC 6538 | 10 | |
| Staphylococcus aureus ATCC 25923 | 20 | |
| Staphylococcus epidermidis ATCC 14990 | 5 | |
| Streptococcus spp. | 20 | |
| Anaerobes | ||
| Gram negative non-sporing rods | ||
| Bacteroides spp. | 10 | |
| Gram positive non-sporing rods | ||
| Lactobacillus spp. | 10 | |
| Propionibacterium acnes | 10 | |
| Gram positive spore-forming rods | ||
| Clostridium spp. | 10 | |
| Gram positive cocci | ||
| Peptococcus spp. | 10 | |
| Streptococcus mutans | 10 | |
| Yeast | ||
| Candida albicans ATCC 10231 | 10 | |
| Candida albicans ATCC 90028 | 20 | |
| 1positive control | + | |
| 2negative control | + | |
+ = cultured growth
assay media with acetone
assay media
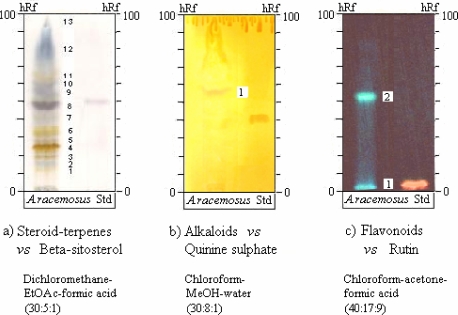
TLC investigation showed the presence of steroids-terpenes, alkaloids and flavonoids (Table 6, Figure 1).
Table 6.
hRf values of chief constituents detected on TLC of 3 different extracts from the root powder of A. racemosus.
| Zone | Steroids-terpenes | Alkaloids | Flavonoids | |||
| hRf value |
visible colour | hRf value |
visible colour | hRf value |
UV-365nm fluorescence |
|
| 1 | 13–15 | yellow | 55–57 | orange-brown | 0–3 | sky blue |
| 2 | 15–17 | sky blue | 52–56 blue-green | |||
| 3 | 19–21 | grayish brown | ||||
| 4 | 25–27 | yellow brown | ||||
| 5 | 29–30 | brown | ||||
| 6 | 33–35 | yellow | ||||
| 7 | 39–42 | light gray | ||||
| 8 | 46–51 | grayish purple | ||||
| 9 | 55–57 | violet | ||||
| 10 | 58–62 | violet | ||||
| 11 | 62–67 | yellow | ||||
| 12 | 67–69 | grayish blue | ||||
| 13 | 86–88 | grayish yellow | ||||
Figure 1.
TLC fingerprints of 3 different extracts from the root powder of A. racemosus
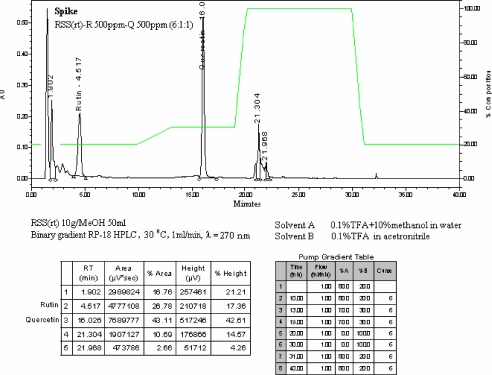
RP-HPLC fingerprint, under a suitable 40min-program linear gradient, showed 1 peak more polar than ref. std. rutin (4.517 min RT) and 2 peaks more non-polar than ref. std. quercetin (16.026 min RT) at 270nm, as shown in Figure 2.
Figure 2.
RP-HPLC chromatogram of the flavonoids extracted in methanol from the root powder of A. racemosus showing polar and non-polar peaks compared with ref. std. peaks of rutin (4.517 min RT) and quercetin (16.026 min RT) at 270nm.
Discussion
The concentration of 50% activity of the A. racemosus root extract were calculated from the following computerized linear equations: y = 138.86x – 413.84 on brine shrimp cytotoxicity; y = 52.359x – 85.189 on DPPH radical scavenging; and y = 46.767x + 7.8191 on tyrosinase inhibition. Where x was obtained by substituting y = 50, the antilog x gave the value of either the LC50, EC50 or IC50, respectively. The roots had mild cytotoxicity (brine shrimp inhibition approx.0.8% of kojic acid and 0.6% of thymol), mild DPPH radical scavenging activity (approx.1% of BHT, BHA and 0.3% of vitamin C), and non-significance melanin biosynthesis inhibitors (anti-tyrosinase activity approximately 0.03% of kojic acid).
The MICs of 5–20 mg/ml against various pathogenic microbial (16 species, 18 strains) indicated that A. racemosus root extract has a wide spectrum activity.
RP-HPLC chromatogram of the flavonoid extract and zoning patterns of steroids-terpenes, alkaloids and flavonoids on the TLC fingerprints were specific enough to be used for the identification of A. racemosus root powder.
Conclusion
The root of A. racemosus is a potential broad spectrum antibiotic.
Acknowledgements
We thank the Pharmaceuticals and Natural Products Department Thailand Institute of Scientific and Technological Research (TISTR) for providing the fund and good laboratory facilities, and to Mr. Parinya Wilairatana, Ex-Director of the Lamtakhong Plant Research Station TISTR for providing the plant materials.
References
- 1.Ballantyne B, Marrs T, Turner P. General & applied toxicology. A bridged ed. London: Macmillan press; 1995. [Google Scholar]
- 2.Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fugita Y, Yasuhara T, Yoshida T, Okuda T. Effect of the interaction of tannin with co-existing substance VI Effect of tannins and related poly-phenols on superoxide anion radical, and on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:2016–2012. [Google Scholar]
- 3.Iida K, Hase K, Shimomura K, Sudo S, Katota S, Namba T. Potent inhibitors of tyrosinase activity and melanin biosynthesis from Rheum officinale. Planta Med. 1995;61:425–428. doi: 10.1055/s-2006-958129. [DOI] [PubMed] [Google Scholar]
- 4.Merck E. Dyeing reagents for thin-layer and paper chromatography. Damstadt: E Merck; 1980. [Google Scholar]
- 5.Potduang B, Chongsiriroeg C, Benmart Y, Giwanon R, Supatanakul W, Tanpanich S. Biological Activities of Schefflera leucantha. Afr J Trad CAM. 2007;4(2):157–164. [PMC free article] [PubMed] [Google Scholar]
- 6.Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six rasayana herbs used in ayurvedic medicine. Phytother Res. 1999;13(4):275–291. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Solis PN, Wright CW, Anderson MM, Gupta MP, Phillipson JD. A microwell cytotoxicity assay using Artemia salina (Brine Shrimp) Planta Med. 1992;59:250–252. doi: 10.1055/s-2006-959661. [DOI] [PubMed] [Google Scholar]
- 8.Wagner H, Bladt S. Plant Drug Analysis. Berlin: Springer-Verlag; 1996. [Google Scholar]
- 9.Vichien 2003. http://wwwpharmchulaacth/vichien/crude-45/cardiac/asparghtm.
- 10.Washington JA, II, Sutter VL. Mannual of Clinical Microbiology. 3rd ed. Washington D.C.: American Society for Microbiology; 1980. Dilution susceptibility test: agar and macro-broth dilution procedure. [Google Scholar]
- 11.Wilkins TD, Chalgren S. Medium for use in antibiotic susceptibility testing of anaerobic bacteria. Antimicrobial Agents Chemotherapy. 1976;10(6):9265–9328. doi: 10.1128/aac.10.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winston D. Harmony Remedies: An Overview of Adaptogens. 2004. http://www.herbaltherapeutics.net/HarmonyRemedies.pdf.