Abstract
Ampicillin and Norfloxacin are used to treat variety of bacterial infections. These two drugs have low oral bioavailability. Co-administration of Piperine (20mg/kg), an alkaloid from Piper nigrum L. enhanced oral bioavailability of Ampicillin and Norfloxacin in animal model. This is reflected in various pharmacokinetic measurements like Cmax, Tmax, AUC and t½ of the above antibiotics in animal model.
Keywords: Ampicillin, Bioavailability, Norfloxacin, Phamacokinetics, Piperine, Piper nigrum
Introduction
Piperine(1-piperoyl piperdine) is a major component of the Piper species. This species has been used widely as spices and in various systems of Indian medicine (Atal et. al 1981). Piperine has various pharmacological activities namely anti-asthmatic along with Adhatoda vasaca (Wealth of India, 1969), antifertility (Piyachaturwat et al., 1982), CNS depressant and anti-inflammatory (Lee et. al., 1984, Majumdar et al., 1990a, Dhuley et al., 1993), inhibition of hepatic monooxygenase and UDP-glucouronyl transferase and intestinal glucouronidation (Atal et. al 1981, Atal et. al 1985, Singh et. al 1986), inhibition of CYP3A4 and pglycoprotein (PGP) (Bhardwaj et. al., 2002). In addition Piperine has also been shown to enhance the bioavailability of drugs like vascicine, sparteine, curcumin, barbiturate and oxyphenylbutazone, zoxazolamine, propranalol and theophylline in animal experiments (Atal et al., 1981, Majumdar et al., 1990a, Shobha et al., 1998, Majumdar et al., 1990b, Majumdar et al., 1999, Bano et al., 1991). Ampicillin and Norfloxacin are extensively used in various infections (Sweetman, 2002) and they exhibit low oral-bioavailability. The oral bioavailability oif Ampicillin is 62±17% and that of Norfloxacin is 30% to 40% (Leslie et al., 1991, Sweetman, 2002). The study was aimed to find out the effect of Piperine in enhancing oral bioavailability of Ampicillin and Norfloxacin in rabbits.
Materials and Methods
Piperine sample was received from M/s Sami labs, Bangalore. The antibiotics (Ampicillin Trihydrate and Norfloxacin) were purchased from M/s MMC laboratories, Chennai and the samples were found to comply with specifications as per Indian Pharmacopoeia (Pharmacopoeia of India, 1996). The test microorganisms Micrococcus luteus (ATCC9341) and Bacillus subtilis (ATCC 6633) were obtained from National Chemical Laboratories (NCL), Pune. The culture and assay media were obtained from M/s Hi-Media Ltd., Mumbai. Rabbits of either sex were procured from central animal house of Rajah Muthiah Medical College and Hospital, Annamalai University.
The plasma concentrations of Ampicillin and Norfloxacin were determined microbiologically (Filter paper disc - diffusion assay) using calibration curve, plotted between logarithm of spiked plasma concentration (X-axis) and diameter of zone of inhibition (Y-axis) to get a straight line response. The Plasma concentrations of antibiotics were estimated from calibration curve by extrapolation of diameter of zone of inhibition (Pharmacopoeia of India, 1985, Prameela Rani 2004). The apparent elimination rate constant (Kel) for antibiotics was determined by non-linear regression analysis of the plasma concentration versus time data of the terminal phase (Gibaldi, 2005). Elimination half-life (t½) of antibiotics was calculated from relationship t½ = 0.693/ Kel, the area under curve (AUC) was calculated using the trapezoidal rule (Gibaldi, 2005). The significance between the respective treatment group were calculated by paired Student's ‘t’ test.
Experimental Design
Ampicillin Trihydrate, Norfloxacin and Piperine were prepared as suspension in carboxy methyl cellulose (CMC) (1% w/v) using pestle and mortar for oral administration to rabbits. The suspensions were fed to the rabbits using a apparatus called mouth-gag. 4 groups of rabbits (6 rabbits per group) weighing 1.5 to 2 kg were housed at constant temperature (25° ± 1°C) and light (10hr) and dark (14 hr) cycle was maintained throughout the experimental period. All animals were maintained on diet and quantity of water as prescribed by central animal house, Rajah Muthiah Medical College and Hospital, Annamalai University. Each rabbit group was administered with antibiotic as per Table 1:. Experimental Design
Table 1.
Experimental Design
| Rabbit groups | Drug | Adjuvant |
| I | Ampicillin Trihydrate (Equivalent to 150mg of Ampicillin/kg body weight) |
No adjuvant (control) |
| II | Ampicillin Trihydrate (Equivalent to150mg of Ampicillin/kg body weight) |
Piperine (20mg/kg body weight) (Bano et. al 1991,Hiwale et. al 2002,) |
| III | Norfloxacin (150 mg/kg body weight) |
No adjuvant (control) |
| IV | Norfloxacin (150 mg/kg body weight) |
Piperine (20mg/kg body weight) (Bano et. al 1991,Hiwale et. al 2002,) |
After administration of antibiotics with and without Piperine, each rabbit was fed with 12ml water. Exactly 0.5ml of blood samples were collected from the ear veins of rabbits using sterile syringes at time intervals equal to 3 half-lives of antibiotics [ t½ of Ampicillin= 1–2hrs, t½av-1.5hrs. t½ of Norfloxacin = 4hrs] (Sweetman, 2002) and then transferred to sterile eppendorf tubes (2ml capacity) containing 10µl sterile anticoagulant solution (10% w/v sodium citrate). Then the blood samples were centrifuged at 2000 rpm for 20 mins to separate the plasma. The above procedure was approved by Institutional Animal Ethics Committee [IAEC, Registration No. 160/1999/CPCSEA], of Rajah Muthiah College and Hospital, Annamalai University.
Results and Discussion
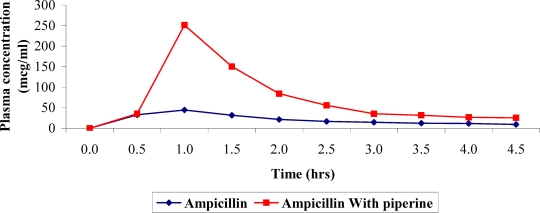
The plasma concentration versus time profile of Ampicillin administered with and without Piperine is given in Table-2 and depicted as a plot in Figure 1. The data fitted to a one-compartment model with first order kinetics and other pharmacokinetic parameters derived from these data are given in Table 3. From Table 3 differences in pharmacokinetic parameters in rabbits administered with Ampicillin and Piperine are inferred. There is greater increase (about 338%) in AUC of rabbits administered with Ampicillin and Piperine. This may be due to changes in permeability of gastrointestinal epithelial cells (Johri et al., 1992) and inhibition of enzymes involved in conversion of ampicillin to penicilloic acid brought about by Piperine (Atal et al., 1985, Singh et al., 1986). (Ampicillin is partly metabolized and excreted as such (20–40%) in Urine) (Sweetman, 2002).
Table 2.
Effect of Piperine on plasma concentration of Ampicillin in rabbits. (Values expressed as mcg/ml are Mean ± S.D.)*
| Time (hrs) | Ampicillin | Ampicillin + Piperine |
| 0.0 | 0 | 0 |
| 0.5 | 30.584 ± 0.38 | 35.481 ± 0.26 |
| 1.0 | 44.668 ± 0.32 | 251.188 ± 0.27 |
| 1.5 | 31.672 ± 0.49 | 188.364 ± 0.31 |
| 2.0 | 26.607 ± 0.37 | 84.139 ± 0.31 |
| 2.5 | 18.836 ± 0.38 | 66.834 ± 0.26 |
| 3.0 | 17.782 ± 0.32 | 35.481 ± 0.27 |
| 3.5 | 12.589 ± 0.31 | 33.496 ± 0.26 |
| 4.0 | 12.589 ± 0.32 | 26.607 ± 0.27 |
| 4.5 | 9.441 ± 0.26 | 26.607 ± 0.27 |
n = 6, p<0.05
Figure 1.
Effect of Piperine on bioavaialbility of Ampicillin
Table 3.
Pharmacokinetic parameters of Ampicillin alone and in combination with Piperine. (Values expressed as mcg/ml are Mean ± S.D.)*
| Treatment | Tmax | Cmax | t½ | AUC |
| (hr) | (mcg/ml) | (hr) | (mcg/ml/hr) | |
| Ampicillin (150mg/kg) po | 1±0.31 | 44.6±0.27 | 1.3±0.46 | 103.7±0.52 |
| Ampicillin (150mg/kg) + Piperine (20mg/kg)po | 1.1±0.3 | 251.2±0.28 | 1.9±0.57 | 350.486±0.47 |
p<0.05
Effect of Piperine on bioavailability of Norfloxacin
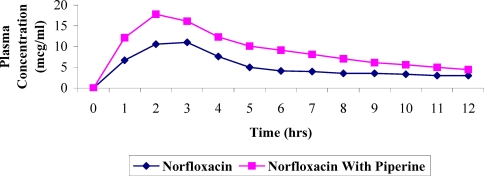
The plasma concentration versus time profile of Norfloxacin administered with and without Piperine is given in Table-4 and graphically presented in Figure 2. The data fitted one compartment model with first order kinetics and other pharmacokinetic parameters are given Table 5. There is increase (about 174.6%) in AUC and also in other pharmacokinetic parameters of rabbits administered with Norfloxacin and Piperine. The enhancement of bioavailability of Norfloxacin by Piperine is comparatively less than that of Ampicillin. The enhancement of bioavailability may be due to inhibition of enzymes responsible for metabolism of Norfloxacin in liver (Atal et al., 1985). Norfloxacin is metabolized in liver (Sweetman, 2002).
Table 4.
Effect of Piperine on plasma concentration of Norfloxacin in rabbits (Values expressed as mcg/ml are Mean ± S.D.)*
| Time (hrs) | Norfloxacin | Norfloxacin + Piperine |
| 0.0 | 0 | 0 |
| 1.0 | 10.593 ± 0.26 | 14.125 ± 0.27 |
| 2.0 | 7.079 ± 0.26 | 17.783 ± 0.32 |
| 3.0 | 5.623 ± 0.32 | 14.963 ± 0.27 |
| 4.0 | 5.011 ± 0.38 | 11.885 ± 0.32 |
| 5.0 | 4.732 ± 0.26 | 10.592 ± 0.37 |
| 6.0 | 3.981 ± 0.26 | 8.414 ± 0.25 |
| 7.0 | 3.548 ± 0.21 | 8.414 ± 0.26 |
| 8.0 | 3.349 ± 0.26 | 7.079 ± 0.26 |
| 9.0 | 3.162 ± 0.27 | 7.079 ± 0.27 |
| 10.0 | 2.512 ± 0.27 | 5.623 ± 0.27 |
| 11.0 | 2.238 ± 0.26 | 5.011 ± 0.27 |
| 12.0 | 1.778 ± 0.26 | 4.467 ± 0.27 |
n = 6, p<0.
Figure 2.
Effect of Piperine on bioavailability of Norfloxacin
Table 5.
Pharmacokinetic parameters of Norfloxacin alone and in combination with Piperine. (Values expressed as mcg/ml are Mean ± S.D.)*
| Treatment | Tmax | Cmax | t½ | AUC |
| (hr) | (mcg/ml) | (hr) | (mcg/ml/hr) | |
| Norfloxacin (150mg/kg) po | 3.1±0.32 | 11±0.26 | 1.75±0.38 | 63.976±0.51 |
| Norfloxacin (150 mg/kg) + Piperine (20 mg/kg)po | 3.2±0.31 | 16.1±0.27 | 2.97±0.38 | 111.695±0.54 |
p<0.05
Conclusion
It is concluded that co-administration of Piperine with Ampicillin and Norfloxacin improved their oral bioavailability which is also reflected in various pharmacokinetic parameters studied. Since Piperine is a natural product used by many in day today food preparation with less toxicological consequences, Piperine might prove to be useful as an adjuvant dosage preparation (oral) of these antibiotics and would cut down the frequency of administration and the related side effects. This may also bring down the cost of treatment of many infections, which require the use of the above antibiotics. However, in depth bioavailability studies are necessary with Piperine for the above antibiotics, on human volunteers to establish such a claim in clinical medicine.
References
- 1.Atal C K, Dubey R K, Singh J. Biochemical basis of enhanced drug bioavailability by piperine-evidence is apotent inhibitor of drug metabolism. Pharmacol Exp Ther. 1985;232:258. [PubMed] [Google Scholar]
- 2.Atal C K, Zutshi U, Rao P G. Scientific evidence on the role of Ayurvedic herbals on Bioavailability of drug. J Ethanopharmacol. 1981;4:117. doi: 10.1016/0378-8741(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 3.Bano G, Raina R K, Zutshi U. Effect of piperine on bioavailability and pharmacokinetics of propanolol and theophylline in healthy volunteers. Eur J Clin Pharmacol. 1991;41:615–617. doi: 10.1007/BF00314996. [DOI] [PubMed] [Google Scholar]
- 4.Bhardwaj R K, Glaeser H, Becquemont L, Klotz U, Gupta S K, Fromm M F. Piperine, a major constituent of black pepper inhibits human p-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–650. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 5.Dhuley J N, Raman P H, Mujumdar A M, Naik S R. Inhibition of lipid peroxidation by piperine during experimental inflammation in rats. Indian J Exp Biol. 1993;31:443. [PubMed] [Google Scholar]
- 6.Gibaldi M. Biopharmaceutics and Clinical Pharmacokinetics. 4th Ed. Hyderabad: 2005. p. 16. Reprint. 377–386. [Google Scholar]
- 7.Hiwale A R, Dhuley J N, Naik S R. Effect of co-administration of piperine on pharmacokinetics of β-lactam antibiotics in rats. Indian J Exp Biol. 2002;40:277–281. [PubMed] [Google Scholar]
- 8.Johri R K, Thusi N, Khajuria A. Piperine mediated changes in the permeability of rat intestinal epithelial cells. The status of gamma-glutamyl transpeptidase activity, uptake of amino acids and lipid per oxidation. Biochem Pharmacol. 1992;43(7):1401–1407. doi: 10.1016/0006-2952(92)90195-o. [DOI] [PubMed] [Google Scholar]
- 9.Lee E B, Shin K H, Woo W S. Central nervous system depressant and anti-inflammatory activity of piperine. Arch Pharmac Res. 1984;7:127. [Google Scholar]
- 10.Leslie Z B, Williams R L. Goodman and Gillman's The Pharmacological Basis of therapeutics. 8th edn. II. New York: 1991. p. 1658. 1697. [Google Scholar]
- 11.Majumdar A M, Dhuley J N, Deshmukh V K, Raman P H, Naik S R. Antiinflammatory activity of piperine. Japan J Med Sc Biol. 1990a;43:95. doi: 10.7883/yoken1952.43.95. [DOI] [PubMed] [Google Scholar]
- 12.Majumdar A M, Dhuley J N, Deshmukh V K, Raman P H, Tharat S L, Naik S R. Effect of piperine on pentobarbitore induced hypnosis in rats. Indian J Exp Biol. 1990b;28:486. [PubMed] [Google Scholar]
- 13.Majumdar A M, Dhuley J N, Deshmukh V K, Naik S R. Effect of Piperine Bioavailability of Oxyphenylbutazone in rats. Indian Drugs. 1999;36:123. [Google Scholar]
- 14.Pharmacopoeia of India. 3rd Ed. Vol. 2. New Delhi: 1985. p. A-92. [Google Scholar]
- 15.Pharmacopoeia of India. 4th Ed. Vol. 1. New Delhi: 1996. pp. 57–58. 522 – 3. [Google Scholar]
- 16.Piyachaturwat P, Glinsukon T, Peugvicha P. Post-coital antifertility effect of piperine. Contraception. 1982;26:625. doi: 10.1016/0010-7824(82)90137-8. [DOI] [PubMed] [Google Scholar]
- 17.Prameela Rani A. Biotechnological studies on Cyclodextrin Complexation for enhancing the bioavailability and for obtaining controlled release of Insoluble Drugs. Hyderabad: Jawaharlal Nehru Technological University; 2004. pp. 108–110. PhD thesis. [Google Scholar]
- 18.Shobha G, Joseph T, Majeed M, Rajendran R, Srinivas P S S R. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Plant Med. 1998;64:353. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 19.Singh J, Dubey R K, Atal C K. Piperine mediated inhibition of glucouronidation activity in isolated epithelial cells of the guinea-pig small intestine: Evidence that piperine lowers the endogenous UDP-glucuronic acid content. J Pharmacol Expt Ther. 1986;236:488. [PubMed] [Google Scholar]
- 20.Sweetman S C. Martindale, The Complete drug reference. 33rd Ed. London: 2002. pp. 150–151. p 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wealth of India. VIII. New Delhi: 1969. p. 96. [Google Scholar]