Abstract
The antibacterial activity of the aqueous, ethanol, methanol and petroleum ether Soxhlet extracts of sundried stem bark of Spathodea campanulata P. Beauv. (Bignoniaceae) was investigated by testing the extracts against B. subtilis, E. coli, P. aeruginosa and S. aureus. The minimum inhibitory concentration (MIC) of the methanol extract was determined against the four bacteria strains and C. albicans using the broth dilution method. Four topical products were prepared by incorporating the methanol extract of S. campanulata (20 % w/w) into aqueous cream, soft paraffin, emulsifying ointment and simple ointment bases and evaluated for their in vitro antimicrobial efficacy. The effect of storage time on the activity of the methanol extract of S. campanulata and S. campanulata extract incorporated in aqueous cream base was also investigated. The methanol and ethanol extracts showed good activity while the aqueous and petroleum ether extracts exhibited little activity. The methanol extract showed the best antibacterial activity. The MIC of the methanol extract of S. campanulata was: C. albicans (45 – 50 mg/ml), B. subtilis and E. coli (50 – 55 mg/ml), P. aeruginosa (60 – 65 mg/ml), S. aureus (145 – 150 mg/ml). Antimicrobial activity of S. campanulata in the topical bases was in the order: aqueous cream > emulsifying ointment > simple ointment > white soft paraffin. Antimicrobial activity of S. campanulata in aqueous cream decreased (p < 0.05) upon storage at room temperature for 6-months. The antifungal activity of the methanol extract of S. campanulata was reduced (p < 0.05) upon storage while antibacterial activity was largely unaffected.
Keywords: Spathodea campanulata, antimicrobial activity, minimum inhibitory concentration, antibacterial, antifungal, wound healing
Introduction
Spathodea campanulata P. Beauv. (Bignoniaceae) is a medium-sized plant that grows commonly in several African countries such as Ghana, Nigeria, Gabon, Cameroon and Senegal. Various parts of S. campanulata are used in Ghanaian traditional medicine for the treatment of diseases. The stem bark is used for wound healing, where it is applied as a paste to the wound (Mensah et al., 2003). Other folklore uses of the plant in Ghana include the treatment of dyspepsia and peptic ulcer (stem bark and leaf); arthritis and fracture (leaf, root bark and fruit); toothache and stomach ache (stem bark); and stomach ulcer (root bark and seed) (Agbovie et al., 2002).
The stem bark preparations of the plant are reported to have wound healing (Houghton et al., 2005; Sy et al., 2005; Mensah et al., 2006), antimalarial (Makinde et al., 1988; Amusan et al., 1996), molluscicidal (Mendes et al., 1986; Amusan et al., 1995), and hypoglycemic, anticomplement and anti-HIV activities (Niyonzima et al., 1993, 1999). The stem bark contains several chemical substances such as 3β;-acetoxyoleanolic acid, siaresinolic acid, 3β;-acetoxy-12-hydroxyoleanan-28, 13-olide and oleanolic acid (Ngouela et al., 1988). Others include triterpine, spathodic acid, spathoside, p-hydroxybenzoic acid and sitosterol-3-O-β;-D-glucopyranoside (Ngouela et al., 1990; Mbosso et al., 2008).
The wound healing property of the stem bark of S. campanulata has been attributed to its antimicrobial and antioxidant properties (Mensah et al., 2003, 2006). The extract is reported to exhibit a broad spectrum antimicrobial activity when tested against four strains of bacteria and a yeast (Mensah et al., 2003), even though the level of antimicrobial activity was reportedly weak at the concentration used (Mensah et al., 2006). The plant is known to be active against Pseudomonas solanecearum (Amusan et al., 1994). Chemical compounds isolated from the stem bark of S. campanulata such as spathoside and p-hydroxybenzoic acid, significantly inhibited the growth of both gram-positive and gram negative bacteria (Mbosso et al., 2008). The antioxidant property of the stem bark extract was demonstrated using the DPPH (2, 2-diphenyl-1-picrylhydrazyl) test, with the extract showing a strong reactive oxygen species scavenging effects (Houghton et al., 2005).
This study was undertaken to determine the antibacterial activity of different extracts of S. campanulata using the standard antimicrobial zone of inhibition assay. The in vitro antimicrobial efficacy of topical products prepared by incorporation of the methanol extract of S. campanulata into topical bases of varying aqueous/anhydrous character was studied. The influence of storage time on the antimicrobial activity of the crude methanol extract and the extract incorporated in aqueous cream base was investigated.
Materials and Methods
Plant collection and preparation
The stem bark of S. campanulata (Voucher number FP/PH/SC201303/KOK) was obtained from the plant situated on the campus of Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana in December, 2005. Authentication of the sample was done by a staff member of the Department of Pharmacognosy, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi. The collected stem bark was cleaned of unwanted foreign materials, cut up into small pieces and dried in sunlight for a week. The dried material was coarsely milled with an electric mill, packed into a brown paper bag and stored at room temperature in the laboratory until used.
Test microorganisms
The microbial organisms used for the study were Gram - positive bacteria: Bacillus subtilis ATCC 6633, Staphylococcus aureus NCTC 10788; Gram - negative bacteria: Escherichia coli ATCC 25922, Pseudomonas aeruginosa NCTC 10662; and the yeast Candida albicans ATCC 10231. The test organisms were obtained from the stocks of the Pharmaceutical Microbiology laboratory of the Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi, Ghana. The bacterial strains were grown and maintained on nutrient agar at 37 °C, while Candida albicans, was grown and maintained on Sabouraud's dextrose agar at 30 °C.
Microbiological media, chemicals and standard drugs
Nutrient broth, nutrient agar, Sabouraud's dextrose agar, white soft paraffin, yellow soft paraffin, wool fat, hard paraffin, cetostearyl alcohol, phenoxyethanol, chlorocresol, emulsifying wax and liquid paraffin were obtained from the Chemicals store of the Department of Pharmaceutics, Faculty of Pharmacy and Pharmaceutical Sciences, KNUST, Kumasi, Ghana. Cicatrin® antibiotic cream (GlaxoSmithKline, UK), each gram containing: neomycin sulphate 3300 units, bacitracin zinc 250 units, L-cysteine 2 mg, glycine 10 mg, dl-threonine 1 mg; and chloramphenicol (1 % w/v) were used as standard antibacterial agents. Miconazole nitrate cream (Luex, India) containing miconazole nitrate 2 % w/v was used as a standard antifungal cream.
Plant extraction
The coarsely milled stem bark of S. campanulata was extracted separately using water, ethanol (96 % w/v), methanol and petroleum ether as solvents. About 100 g of the powdered sample was continuously extracted with a particular solvent by use of a Soxhlet extraction apparatus for 24 h. The extracts were filtered and concentrated to dryness under reduced pressure and controlled temperature (50 – 55 °C) to obtain solvent-free semisolid extracts. The solvent-free semisolid extracts obtained were used for the antimicrobial studies.
Antibacterial sensitivity testing
The antibacterial activity of extracts of the stem bark of S. campanulata (10 % w/v) reconstituted with methanol was determined using the cup plate method. A molten nutrient agar stabilized at 45 °C was seeded with 0.1 ml of a 24 h broth culture of the test organism (B. subtilis, E. coli, P. aeruginosa and S. aureus) containing approximately 10 5 cfu / ml in a sterile petri dish and allowed to set. Wells of 10 mm diameter were created with a sterile cork borer and filled to about three-quarters full with 10 % w/v solutions of the aqueous, ethanol, methanol, and petroleum ether extracts of the stem bark of S. campanulata. The plates were pre-incubated for 1 h at room temperature to allow for diffusion of the solutions and then incubated at 37 °C for 24 h. The zones of inhibition were measured (mean ± SD, n=3). Chloramphenicol (1 % w/v) and methanol were used as positive and negative controls respectively.
Determination of minimum inhibitory concentration (MIC)
The broth dilution technique was used to determine the MIC of the methanol extract of the stem bark of S. campanulata (Yield = 15.4 % w/w) against the test organisms. Sterile test tubes containing 5 ml double strength nutrient broth were added graded concentrations of the methanol extract of S. campanulata (0 – 16 % w/v). The contents of the tubes were diluted with calculated volumes of sterile water and inoculated with 0.2 ml of the test organisms previously diluted to contain approximately 10 5 cfu / ml. A tube without an extract and another without a test organism were used as controls. The tubes were incubated at 37 °C for 24 h (bacteria) and at 30 °C for 72 h (fungus) and observed for growth in the form of turbidity. The experiments were conducted in triplicate. The tube with the lowest concentration of the extract which showed no growth after incubation was taken and recorded as the MIC.
Formulation of topical products
Four (4) topical bases of varying degrees of aqueous/anhydrous character, namely: simple ointment BP, emulsifying ointment BP, aqueous cream BP and white soft paraffin BP were prepared by trituration in a ceramic mortar with a pestle (British Pharmacopoeia, 1993). Twenty grams (20 g) of the semisolid methanol extract of S. campanulata was incorporated into the various bases to obtain 100 g of topical products containing 20 % w/w of S. campanulata extract. The topical products of the extract were stored in the refrigerator until they were used.
In vitro antimicrobial efficacy of topical products
The cup-plate method was used to assess the relative antimicrobial efficacy of the four topical products prepared with the methanol extract of the stem bark of S. campanulata. A molten nutrient agar stabilized at 45 °C, seeded with 0.1 ml of a 24 h broth culture of the test organism (B. subtilis, E. coli, P. aeruginosa and S. aureus) and containing approximately 10 5 cfu / ml was used. Wells of 10 mm diameter were created and filled to three-quarters full with the topical products of the extract. Cicatrin® cream and miconazole cream were used as standards for the bacteria strains and C. albicans, respectively. The plates were pre-incubated for 1 h at room temperature to ensure adequate diffusion and finally incubated at 37 °C for 24 h. Sabouraud's dextrose agar was used to test the antifungal activity of the topical products against C. albicans and the seeded plates were incubated at 30 °C for 3 days. The experiments were run in triplicate and the zones of inhibition were determined and recorded (mean ± SD, n = 3).
Effect of storage time on antimicrobial activity
The antimicrobial activity of freshly prepared methanol extract of S. campanulata (20 % w/v) and S. campanulata extract incorporated in aqueous cream (20 % w/w) was investigated using the cup-plate method. The diameter of the wells was 10 mm. Nutrient agar plates were seeded with 0.1 ml of a bacterial strain and incubated at 37 °C for 24 h whilst Sabouraud's dextrose agar plates were seeded with 0.1 ml of C. albicans and incubated at 30 °C for 3 days. The antimicrobial activity of the two samples was again determined after storage at room temperature for six months. Tests were carried out in triplicate. The zones of inhibition (mean ± SD, n = 3) were determined after incubation.
Results
Table 1 shows the antibacterial activity of aqueous, methanol, ethanol, and petroleum ether extracts of the stem bark of S. campanulata (10 % w/v) against four bacterial strains, E. coli, B. subtilis, P. aeruginosa and S. aureus. The methanol and ethanol extracts exhibited high activity while the aqueous and petroleum ether extracts showed little activity against the test organisms. For the methanol extract, antibacterial activity was in the order: E. coli > B. subtilis > P. aeruginosa > S. aureus. For the ethanol extract, antibacterial activity followed the order: B. subtilis > E. coli > P. aeruginosa > S. aureus. On the whole, the methanol extract of the stem bark of S. campanulata showed the highest antibacterial activity against the test organisms. Chloramphenicol (1 % w/v), a standard antibiotic showed a significantly higher (p < 0.05) activity compared to the methanol, ethanol, aqueous and petroleum ether extracts of S. campanulata (10 % w/v). The MIC of the methanol extract of the stem bark of S. campanulata was as follows: C. albicans (45 – 50 mg/ml), B. subtilis and E. coli (50 – 55 mg/ml), for P. aeruginosa (60 – 65 mg/ml), and S. aureus (145 – 150 mg/ml).
Table 1.
Antibacterial activity of the stem bark extracts of Spathodea campanulata (10 % w/v)
| Extract | Zone of inhibition (mm) | |||
|
Bacillus Subtilis |
Escherichia coli |
Pseudomonas aeruginosa |
Staphylococcus aureus |
|
| Methanol extract | 7.50 ± 1.29 | 8.75 ± 1.26 | 6.75 ± 0.50 | 5.75 ± 1.89 |
| Ethanol extract | 6.00 ± 1.41 | 5.25 ± 0.96 | 4.75 ± 0.96 | 3.50 ± 0.58 |
| Aqueous extract | 2.00 ± 1.41 | 3.50 ± 1.29 | 2.25 ± 0.50 | 1.25 ± 0.96 |
| Petroleum ether extract | 5.50 ± 1.29 | 3.00 ± 0.82 | 3.00 ± 1.83 | 2.50 ± 1.29 |
|
*Chloramphenicol (1 % w/v) |
25.00 ± 0.00 | 24.33 ± 1.15 | 25.33 ± 0.58 | 22.33 ± 0.58 |
Chloramphenicol (1 % w/v) and methanol were used as positive and negative controls, respectively. Methanol did not show any zones of inhibition.
Table 2 depicts the antimicrobial activity of the methanol extract of the stem bark of S. campanulata in four topical bases. S. campanulata in aqueous cream base had a significantly higher activity (p < 0.05) than the other topical bases when tested against the test organisms. The order of antimicrobial activity of S. campanulata in the topical bases was as follows: aqueous cream > emulsifying ointment > simple ointment > white soft paraffin. Table 3 compares the antimicrobial activity of S. campanulata in aqueous cream base against two standard drugs, Cicatrin® cream and miconazole cream. For antibacterial activity, S. campanulata in aqueous cream had high activity (p < 0.05) (B. subtilis, S. aureus), similar activity (E. coli) and low activity (p < 0.05) (P. aeruginosa) compared to Cicatrin® cream. For antifungal activity, miconazole cream exhibited a high activity (p < 0.05) than S. campanulata in aqueous cream against C. albicans.
Table 2.
Antimicrobial activity of the methanol stem bark extract of Spathodea campanulata incorporated in different topical bases (20 % w/w)
| Zone of inhibition (mm) | ||||
| Microbial Organisms | Soft paraffin | Simple ointment | Emulsifying ointment |
Aqueous cream |
| Bacillus subtilis | 11.00 ± 0.00 | 11.50 ± 0.58 | 11.50 ± 0.58 | 22.00 ± 0.82 |
| Escherichia coli | 11.00 ± 0.00 | 11.50 ± 0.58 | 12.25 ± 0.50 | 20.25 ± 0.96 |
| Pseudomonas aeruginosa | 0 | 0 | 11.00 ± 0.00 | 15.50 ± 0.58 |
| Staphylococcus aureus | 15.50 ± 0.58 | 15.00 ± 0.00 | 15.00 ± 0.00 | 20.25 ± 0.50 |
| Candida albicans | 0 | 11.50 ± 0.58 | 11.00 ± 0.00 | 17.50 ± 0.58 |
Table 3.
In vitro antimicrobial efficacy of the methanol stem bark extract of Spathodea campanulata incorporated in aqueous cream base (20 % w/w) against standard antibacterial and antifungal creams
| Microbial Organisms | Zone of inhibition (mm) |
||
|
S. campanulata (20% w/w) in aqueous cream |
Cicatrin cream | Miconazole cream | |
| Bacillus subtilis | 22.00 ± 0.82 | 15.00 ± 0.00 | N/A |
| Escherichia coli | 20.25 ± 0.96 | 20.00 ± 0.00 | N/A |
| Pseudomonas aeruginosa | 15.50 ± 0.58 | 21.25 ± 0.50 | N/A |
| Staphylococcus aureus | 20.25 ± 0.50 | 18.5 ± 1.91 | N/A |
| Candida albicans | 17.50 ± 0.58 | N/A | 22.5 ± 2.38 |
N/A = not applicable
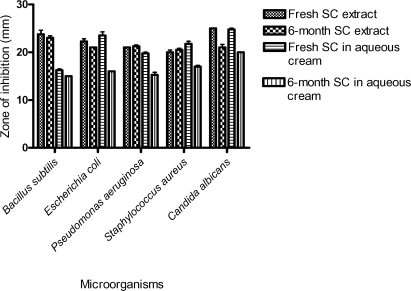
Figure 1 shows the effect of storage time on the antimicrobial activity of the methanol extract of S. campanulata and S. campanulata in aqueous cream base. Storage of the methanol extract of S. campanulata had no effect on the antibacterial activity while the antifungal activity was lowered. Storage of S. campanulata in aqueous cream base resulted in a reduction of the antibacterial and antifungal activity of the product.
Figure 1.
Effect of storage time on antimicrobial activity of methanol stem bark extract of S. campanulata (SC) and SC in aqueous cream
Discussion
Aqueous decoctions of the stem bark of S. campanulata are used in Ghanaian traditional medicine for wound healing where it is applied as a paste to the wound. The plant material was sundried, as it is the method used in traditional application, being also the commonest mode of drying plant materials in Africa (Fennell et al., 2004). The method and conditions used for the extraction of the plant material, though not used in traditional application, were controlled in order to minimize possible artifact formation. The antimicrobial activity of the stem bark extracts of the plant was investigated as the presence of such compounds may prevent wound and other skin infections, especially by bacteria (Houghton et al., 2005). The ability of the stem bark extracts to prevent infections is one of the basis of its use in wound healing, as infected wounds takes a longer time to heal. The bacteria strains chosen for the study, namely: B. subtilis, S. aureus, E. coli, and P. aeruginosa, are commonly found in infected wounds (Jones et al., 2000) while C. albicans is a known human opportunistic pathogen (Mensah et al., 2004). Activity of S. campanulata extract against these organisms and others implicated in wound infections would confirm the wound healing property of the plant and validate its use in folklore medicine.
The aqueous, methanol, ethanol and petroleum ether extracts of S. campanulata demonstrated varying levels of antibacterial activity against the test organisms. The methanol extract of S. campanulata showed the best antibacterial activity. The higher activity of the methanol and ethanol extracts may be due to higher solubility of the active compounds in these solvents. Methanol and ethanol were better able to extract the active antibacterial compounds in the plant which exhibited higher activity with higher zones of inhibition. The aqueous and petroleum ether extracts had little activity against the test organisms. This may possibly be due to lack of sufficient quantities of active compounds in the extracts at the dose levels used (Taylor et al., 2001). The aqueous and petroleum ether extracts may possibly be active against bacterial strains which were not tested in the current study (Shale et al., 1999).
Chloramphenicol, a standard antibiotic, had a better antibacterial activity than methanol extract of S. campanulata (10 % w/v). The antibacterial activity of various plant species has been compared to that of standard antibiotics (Parekh and Chanda, 2008). The antimicrobial activity of the extracts could be attributed to the phytochemical constituents of the stem bark of S. campanulata which includes flavonoids, glycosides, tannins, triterpenes and sterols (Ngouela et al., 1990; Tsuchyia et al., 1996; Kwapong, 2007). Recently, various chemical compounds have been isolated from the stem bark of S. campanulata including; p-hydroxybenzoic acid, spathoside and oleanolic acid which have shown significant antibacterial activity against a broad spectrum of bacteria (Mbosso et al., 2008).
The MIC of the methanol extract of S. campanulata against the test organisms varied from 45 – 150 mg/ml, with E. coli and B. subtilis being the most susceptible bacteria while S. aureus was the least susceptible. C. albicans was the most sensitive of the test organisms. Mensah et al. (2006) have reported that the methanol extracts of the stem bark of S. campanulata though active against E. coli and B. subtilis, had no activity against S. aureus, P. aeruginosa and three yeast strains, including C. albicans, at the maximum test concentration of 1000 µg/ml. In the current study, a much higher test concentration was employed which was active against all the test organisms. This confirms the report of Farnsworth (1993) who indicated that the antimicrobial activity of crude plant extracts may sometimes be demonstrated only with the utilization of large doses of the extracts.
The antimicrobial efficacy of the topical preparations of S. campanulata was best demonstrated when aqueous cream, a relatively hydrophilic, oil-in-water emulsion base was employed in its preparation. The antimicrobial compounds present in the extracts were released more readily in the more water-miscible aqueous cream base than in the anhydrous (simple ointment) and lipophilic (white soft paraffin) bases. Thus, the release of the antimicrobial compounds from the topical products reduced with an increase in the hydrophobic/lipophilic character of the topical bases employed. The antibacterial activity of S. campanulata in aqueous cream base (20 % w/w) was higher than Cicatrin® cream, a standard antibiotic cream used in wound healing. The antifungal activity of S. campanulata in aqueous cream base was lower than that of miconazole cream, a standard antifungal cream.
Storage of the methanol extract of S. campanulata at room temperature for 6-months had no effect on the antibacterial activity of the extract, as the zones of inhibition of the test bacterial strains were largely unaffected. Antifungal activity of the extract was however lowered upon storage. Storage of S. campanulata in aqueous cream base for 6-months caused a significant reduction in its antibacterial and antifungal activity due possibly to product instability. There is, therefore, the need for further studies into the stability of the extract and its topical preparations to ensure the formulation of stable and efficacious products for wound healing.
Conclusions
The study has demonstrated the antimicrobial activity of the stem bark extracts of S. campanulata against four strains of bacteria and a yeast, C. albicans. The methanol extract of S. campanulata showed the best antibacterial activity. Aqueous cream, a water-miscible topical base, was a better vehicle for the release of the antimicrobial compounds present in S. campanulata stem bark extract. The broad antimicrobial activity of S. campanulata is supportive of its folklore use in the treatment of wounds and other topical infections.
References
- 1.Agbovie T, Amponsah K, Crentsil O R, Dennis F, Odamtten G T, Ofosuhene-Djan W. Conservation and sustainable use of medicinal plants in Ghana - Ethnobotanical Survey. 2002. http://www.unep-wcmc.org/species/plants/ghana.
- 2.Amusan O O G, Adesogan E K, Makinde J M. Antimalarial active principles of Spathodea campanulata stem bark. Phytother Res. 1996;10(8):692–693. [Google Scholar]
- 3.Amusan O O G, Bhembe F N, Mkhatshwa F T, Thwala E Z. Antibacterial activity of Annona senegalensisi, Andrache ovalis and Spathodea campanulata against Pseudomonas solsnecearum. UNISWA J Agric. 1994;3:62–66. [Google Scholar]
- 4.Amusan O O G, Msonthi J D, Makhubu L P. Molluscicidal activity of Spathodea campanulata, Andracne ovalis, Phytolacca dodecandra and Hypoxis rooperi. Fitoter. 1995;66:113–116. [Google Scholar]
- 5.British Pharmacopoiea, author. British Pharmacopoiea Commission. II. London: HMSO Publication Centre; 1993. [Google Scholar]
- 6.Farnsworth N R. Biological approaches to the screening and evaluation of natural products. In: Rasoanaivo P, Ratsimamanga-Urverg S, editors. Biological evaluation of plants with reference to the Malagasy flora. Madagascar: 1993. pp. 35–43. [Google Scholar]
- 7.Fennel CW, Light ME, Sparg SG, Stafford GI, van Staden J. Assessing African medicinal plants for efficacy and safety: agricultural and storage practices. J Ethnopharmacol. 2004;95:113–121. doi: 10.1016/j.jep.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Houghton P J, Hylands P J, Mensah AY. In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol. 2005;100:100–107. doi: 10.1016/j.jep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Jones N P, Arnason J T, Abou-zaid M, Akpagana K, Sanchez-Vindas P, Smith M L. Antifungal activity of extracts from medicinal plants used by first nation's peoples of Eastern Canada. J Ethnopharmacol. 2000;73:191–198. doi: 10.1016/s0378-8741(00)00306-8. [DOI] [PubMed] [Google Scholar]
- 10.Kwapong A A. Formulation of a topical product from the stem bark of Spathodea campanulata for wound healing. Kumasi, Ghana: Kwame Nkrumah University of Science and Technology; 2007. p. 110. MPharm thesis. [Google Scholar]
- 11.Makinde J M, Amusan O O G, Adesogan E K. The antimalarial activity of Spathodea campanulata stem bark extract on Plasmodium berghei in mice. Planta Med. 1988;54(2):122–125. doi: 10.1055/s-2006-962367. [DOI] [PubMed] [Google Scholar]
- 12.Mbosso E J, Ngouela S, Nguedia J C, Penlap V, Rohmer M, Tsamo E. Spathoside, a cerebroside and other antibacterial constituents of the stem bark of Spathodea campanulata. Nat Prod Res. 2008;22(4):296–304. doi: 10.1080/14786410701766281. [DOI] [PubMed] [Google Scholar]
- 13.Mendes N M, de Souza C P, Araujo N, Pereira J P, Katz N. Molluscicide activity of some natural products on Biomphalaria glabrata. Mem Inst Oswaldo Cruz. 1986;81(1):87–91. doi: 10.1590/s0074-02761986000100012. [DOI] [PubMed] [Google Scholar]
- 14.Mensah A Y, Houghton P J, Dickson R A, Fleischer T C, Heinrich M, Bremner P. In Vitro evaluation of effects of two Ghanaian plants relevant to wound healing. Phytother Res. 2006;20(11):941–944. doi: 10.1002/ptr.1978. [DOI] [PubMed] [Google Scholar]
- 15.Mensah A Y, Houghton P J, Fleischer T C, Adu C, Agyare C, Ameade A E. Antimicrobial and antioxidant properties of two Ghanaian plants used traditionally for wound healing. J Pharm Pharmacol. 2003;55(Supplement):S-4. [Google Scholar]
- 16.Mensah A Y, Houghton P J, Woode E. Fungi, Friends or Foes? Ghana Pharm J. 2004;28:22–36. [Google Scholar]
- 17.Ngouela S, Tsamo E, Sondengam B L. Extractives from Bignoniaceae: Constituents of the Stem Bark of Spathodea campanulata. Planta Med. 1988;53(5):476. doi: 10.1055/s-2006-962516. [DOI] [PubMed] [Google Scholar]
- 18.Ngouela S, Nyasse B, Tsamo E, Sondengam B L. Spathodic acid: a triterpene from the stem bark of Spathodea campanulata. Phytochem. 1990;29(12):3959–3961. [Google Scholar]
- 19.Niyonzima G, Scharpe S, Van Beeck L. Hypoglycemic activity of Spathodea campanulata stem bark decoction in mice. Phytother Res. 1993;7:64–67. [Google Scholar]
- 20.Niyonzima G, Laekeman G, Witvrouw M, Van Poel B, Pieters L, Paper D, De Clercq E, Franz G, Vlietinck A J. Hypoglycemic, anticomplement and anti-HIV activities of Spathodea campanulata stem bark. Phytomed. 1999;6(1):45–49. doi: 10.1016/S0944-7113(99)80034-8. [DOI] [PubMed] [Google Scholar]
- 21.Parekh J, Chanda S V. Antibacterial activity of aqueous and alcoholic extracts of 34 Indian medicinal plants against some Staphylococcus species. Turk J Biol. 2008;32:63–71. [Google Scholar]
- 22.Shale T L, Strik W A, van Staden J. Screening of plants used by Southern African traditional healers in the treatment of dysmenorrhoea for prostaglandin-synthesis inhibitors and uterine relaxing activity. J Ethnopharmacol. 1999;64:9–14. doi: 10.1016/s0378-8741(98)00097-x. [DOI] [PubMed] [Google Scholar]
- 23.Sy GY, Nongonierma RB, Ngewou PW, Mengata DE, Dieye AM, Cisse A, Faya B. Healing activity of methanolic extract of the barks of Spathodea campanulata Beauv (Bignoniaceae) in rat experimental burn model. Dakar Med. 2005;50(2):77–81. [PubMed] [Google Scholar]
- 24.Taylor J L S, Rabe T, McGaw L J, Jager A K, van Staden J. Towards the scientific validation of traditional medicinal plants. Plant Growth Regul. 2001;34:23–37. [Google Scholar]
- 25.Tsuchyia H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanka T, Linuma M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50:27–34. doi: 10.1016/0378-8741(96)85514-0. [DOI] [PubMed] [Google Scholar]