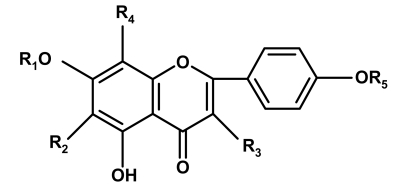
Abstract
Ethyl acetate and ethanol extracts of unflowering aerial part of Macfadyena unguis-cati L. (Fam. Bignoniaceae) were found to be rich in phenolic compounds. From ethyl acetate extract, six flavonoids were identified, 8, methoxy, acacetin, 7-O glucoside; 6, methoxy apigenin 7-O glucoside; 4‵-O methyl scutellarin, 6-O apiosyl galactoside; acacetin, 7-O glucosyl, 8-C rhamnosyl, 3-O-α arabinofuranoside; 4‵-methyl, 6- methoxy kaempferol, 7-O, 8-C diglucoside and vicenin II were isolated, while 6, methoxy, acacetin 7-O glucoside; and quercitrin were isolated from ethanol extract. These compounds were characterized and identified by their physicochemical and spectral data. The crude ethanol extract exhibited significant anti-inflammatory activity (80.47%) and moderate cytotoxic activity against lung cell line.
Keywords: Macfadyena unguis-cati L., Bignoniaceae, flavonoids, anti-inflammatory, cytotoxicity
Introduction
Macfadyena unguis-cati, (Bignoniaceae) is an ornamental climbing plant, widespread in Egypt, America and Western India. Iridoids in Bignonieae proved to be mainly C-4 carboxylated, the only exception being Macfadyena cynanchoides with decarboxylated iridoids (Poser et al., 2000). In addition leaf extracts of M. cynanchoides contain the iridoids macfadienoside (Bianco, et al., 1974) cynanchoside (Bonini, et al. 1981), and 5, 7-bisdeoxycynanchoside (Adriani et al., 1982). Root extracts of M.unguis-cati were found to contain lapachol; quinovic acid; 3-(O-fucosyl) alcohol; β-amyrin and β-sitosterol (Joshi et al., 1985). Subramanian et al., (1972) examined the flavonoids of eight Bignoniaceous plants comprising Bignonia gracilis and B. megapotamica Spreng. They found that, the two contained quercetin-3-rutinoside and the later contained also quercetin-3-galactoside. Harborne, (1967) made a search of sixteen representative species and failed to reveal any further occurrences of these rare plant pigments in Bignoniaceae. An examination of the other flavonoids in leaves and petals of bignoniads showed that most species contained flavones rather than flavonols. The nectary structure and chemical nectar composition of 15 species of Bignoniaceae (M. dentata, M.unguis-cati, Tecoma garrucha and T. stans) were analysed by Graletto (1995).
Also M. unguis-cati is used in folk medicine to treat snakebite (Hougton and Osibogun, 1993), dysentery, inflammation and rheumatism (PioCorrea, 1978). In addition, there are reports on its use in the treatment of venereal disease and as a quinine substitute for malaria (Ferrari et al., 1981). The biological screening of fractions derived from leaves and liana of M.unguis-cati revealed antitumoral and antitrypanosomal activities. In addition the anti-lipoxygenase and anti-cyclo-oxygenase activities observed in M. unguis-cati fractions showed partial correlation with the anti-inflammatory property attributed to this plant (Duarte et al., 2000).
The extracts of the whole plant did not show antiprotozoal activity against Leshmania spp. or Trypanosoma cruzi (Fournet et al., 1994)
This study aims at the isolation and identification of flavonoids in M. unguis-cati and evaluation of the potential cytotoxic and anti-inflammatory activities of the plant.
Experimental
Plant material
The fresh unflowering aerial part of Macfadyena unguis- cati (L) A. Gentry, (Syn: Doxantha unguis-cat or Bignonia unguis-cati) was collected from Manial palace, Kaser El-Aini, Cairo in August 2004. Voucher-specimens (Vouchee Number B36) were identified by Dr. Mohamed El-Gebaly and deposited at the herbarium of Orman Garden.
Preparation of successive extractives
The powdered air-dried unflowering aerial part of M.unguis-cati (500g) was successively extracted in a Soxhlet apparatus using petroleum ether, chloroform, ethyl acetate and ethanol 95%. These extracts were evaporated to dryness under vacuum at 40°C yielding dark oily residues (yield, 2.0, 0.916, 1.96, and 3.33% w/w of solvent free extracts). The crude extract was prepared by percolating 500g dry powder with 90% ethanol till exhaustion the filtered percolate was concentrated under vacuum at 40°C while the coumarin fraction was prepared as mentioned before in (Aboutabl et al., 2006).
Isolation of flavonoids
The ethyl acetate and the ethanol 95% fractions were examined by paper chromatography (Whatmann No. 1) with (a) n-butanol-acetic acid-water (3:1:1) and (b)15% acetic acid. Chromatograms were visualized under UV, UV & NH3 and UV & AlCl3. The presence of the detected spots in each extract, their Rf values and their colours were recorded. These compounds were isolated by preparative paper chromatography on Whatmann 3MM, using solvent system (a), then purified by repeated PPC using solvent system (b). Final purification was performed on Sephadex LH-20 column and eluted with methanol.
Apparatus
* 1H-NMR Spectrophotometer Jeol EX 270, 300 and 500 NMR spectrometer .
* 13C-NMR spectrophotometer Jeol EX, 75 and 125 spectrometer.
* Ultraviolet visible recording spectrophotometer, UV-VIS. Double Beam, UVD-3500, Lambomed, Inc.
Identification of compounds
The purified compounds were subjected to UV, 1H-NMR and 13C-NMR spectral analysis. The chromatographic and UV data of these compounds were compared with published data (Mabry et al., 1970 and Markham 1982).
Investigation of bioactivities
LD50 of crude ethanol extract: was reported in a previous publication (Aboutabl et al., 2006). following Miller and Tainter (1944), procedure and found to be (4.9 g/kg b.wt.).
Anti-inflammatory activity
Acute anti-inflammatory activity of the successive extracts, as well as crude ethanol extract and coumarin fraction were evaluated according to the method described by Winter et al. (1962). 42 adult male albino rats were divided into seven groups, each group of six animals. Group 1: normal control received 1mL saline. Group 2, 3, 4, 5, 6: received orally a dose of 100mg/kg b.wt. of the successive extracts (petroleum ether, chloroform, combined ethyl acetate and ethanol, coumarin fraction , crude ethanol extract. Group 7: positive control, received orally 20 mg/kg b. wt. Indomethacin. One hour after oral administration of the different extracts or indomethacin, the animals were given a subplantar injection of 0.1 mL of 1% carrageenan solution in saline in the right hind paw and 0.1 mL saline in the left hind paw. Four hours after drugs administration, the rats were sacrificed. Both paws were excised and weighed separately. The percentage oedema was calculated and compared with that of the control group. The data obtained were analyzed by using the Student's “t” test according to Snedecor & Cochran (1971) as in table 6.
Table 6.
Acute anti-inflammatory activity of different extracts of Macfadyena unguis-cati L. and Indomethacin in rats (n=6)
| Group | Dose in mg/kg b.wt. |
% Oedema | ||
| Mean ± SE | % of change * | Relative potency | ||
| Control | 1mL saline | 59.8 ± 1.2 | ||
| Petroleum ether | 100 | 42.3 ± 0.7 | 29.26 | 45.57 |
| Chloroform | 100 | 34.7 ± 0.6 | 41.97 | 65.36 |
| Ethyl acetate +ethanol | 100 | 36.1 ± 0.9 | 39.63 | 61.72 |
| Crude ethanol extract | 100 | 28.9 ± 0.8 | 51.67 | 80.47 |
| Coumarin fraction | 100 | 39.4 ± 0.5 | 34.11 | 53.12 |
| Indomethacin | 20 | 21.4 ± 0.3 | 64.21 | 100% |
Significantly different from control group at P < 0.01% of change calculated as regard the control group. Potency is calculated relative to Indomethacin.
Cytotoxic activity of total ethanol extract of M.unguis-cati by SRB assay
Potential cytotoxicity of 90% ethanol extract of M. unguis-cati was tested using the method of Shehan et al. (1990). Tumor cells were plated in 96-multiwell plate (104 cells/well) for 24 h. before treatment with the extract to allow attachment of cells to the wall of the plate. Different concentrations of the extract (0, 1, 2.5, 5 and 10 µg/mL DMSO) were added to the cell monolayer. Triplicate wells were prepared for each individual dose. Monolayer cells were incubated with the extract for 48 hrs. at 37°C in atmosphere of 5% CO2. After 48 h., cells were fixed, washed and stained with sulforhodamine B stain (Sigma). Excess stain was washed with acetic acid and attached stain was recovered with Tris EDTA buffer (Sigma). Colour intensity was measured in an ELISA reader. The relation between surviving fraction and extract concentration was plotted to get a survival curve of each tumor cell line after incubation with the extract. The potency of the extract was compared with reference Cisplatin (Glaxo) (Table7).
Table 7.
Cytotoxic activity of total ethanol extract of M. unguis-cati
| Cell line | Conc.Mg/mL | 90% ethanol extract | Cisplatin | ||
| SF | MSE | SF | MSE | ||
| 1. lung | 0.0 | 1.000 | 0.038 | 1.000 | 0.072 |
| 1.0 | 0.746 | 0.048 | 0.796 | 0.020 | |
| 2.5 | 0.697 | 0.006 | 0.665 | 0.050 | |
| 5.0 | 0.658 | 0.015 | 0.484 | 0.026 | |
| 10.0 | 0.644 | 0.006 | 0.401 | 0.007 | |
| 2. Brain | 0.0 | 1.000 | 0.116 | 1.000 | 0.068 |
| 1.0 | 1.012 | 0.026 | 0.609 | 0.074 | |
| 2.5 | 0.836 | 0.028 | 0.564 | 0.045 | |
| 5.0 | 0.790 | 0.028 | 0.555 | 0.067 | |
| 10.0 | 0.722 | 0.045 | 0.494 | 0.051 | |
| 3. Cervix | 0.0 | 1.000 | 0.052 | 1.000 | 0.075 |
| 1.0 | 0.925 | 0.030 | 0.903 | 0.057 | |
| 2.5 | 0.799 | 0.039 | 0.424 | 0.210 | |
| 5.0 | 0.784 | 0.010 | 0.170 | 0.420 | |
| 10.0 | 0.733 | 0.006 | 0.059 | 0.024 | |
| 4. Colon | 0.0 | 1.000 | 0.116 | No significant effect |
|
| 1.0 | 0.978 | 0.14 | |||
| 2.5 | 0.902 | 0.007 | |||
| 5.0 | 0.856 | 0.007 | |||
| 10.0 | 0.822 | 0.013 | |||
| 5. Breast | 0.0 | 1.000 | 0.070 | No significant effect |
|
| 1.0 | 0.955 | 0.022 | |||
| 2.5 | 0.969 | 0.006 | |||
| 5.0 | 0.902 | 0.016 | |||
| 10.0 | 0.909 | 0.035 | |||
| 6. liver | 0.0 | 1.000 | 0.072 | 1.000 | 0.104 |
| 1.0 | 1.035 | 0.032 | 0.601 | 0.043 | |
| 2.5 | 1.035 | 0.004 | 0.593 | 0.055 | |
| 5.0 | 1.009 | 0.013 | 0.524 | 0.040 | |
| 10.0 | 0.932 | 0.032 | 0.518 | 0.054 | |
SF: Survival Fraction; MSE: Mean Standard Error.
Results and Discussion
Compound I was isolated from ethanol extract with purple colour on paper chromatography at Rf a 0.48 and Rf b 0.24 under UV, unchanged on exposure to ammonia. It was observed that compound I has free hydroxyl group at C-5 and absence of free hydroxyl at C-4‵. The difference in band I in methanol (325) and after addition of NaOMe (377) is ac 52 nm (between 45 and 65 nm) with decrease in intensity , indicated absence of free 4‵-OH. The bathochromic shift of band I from 325 to 345 ac 20 nm after addition of AlCl3 / HCl (relative to methanol) indicates 5-OH with 6-oxygenation. The terminal methoxy group appeared in 1H-NMR at 3.7432 is due to OCH3-4‵ which is differentiated from methoxy at C-6 (3.9373 ppm). The doublet signal at δ 5.0332–5.0469 ppm with J= 6.85 Hz, is attributed to H-1‶ of glucose. H- 8 and H-3 appeared as singlets at δ 6.9590 and 6.9865 ppm, respectively. AA‵XX‵ spin coupling system of two equivalent protons at δ 7.2173-71990 ppm (H-3‵/5‵) and 8.0717-8.0901 (H-2‵/6‵) with J=9.15 and 9.19 Hz respectively, indicating 1–4 distributed B-ring. The terminal methoxy group at C-4‵ was confirmed from its characteristic position in 13C-NMR at 57.0203 and differentiated from OCH3 - 6, with down field shift at 60. 5973 , due to the presence of 7-O glucosyl and 5-OH.
Also the down field shift of C-6 from 98 to 132.4971 ppm is attributed to methoxy at C-6. The O-glucosyl moiety at C-7 is confirmed from the signals of C-3‶ and C-5‶ (77.0889 and 77.7280) Δ + 0.6392.
Direct correlation observable in HMBC (Heteronuclear Multi Bond Connectivity) spectrum of compound 1 confirmed its suggested structure. Table 5 showed 2J and 3J set of correlations between hydrogens and carbons, H-3 (6.9590 ppm) is recognized by 2J coupling with carbons 2, 4 (163.9640, 182.8499); and 3J coupling with carbons 10, 1‵ (105.7230, 124.4277). H-8 (6.9865 ppm) is determined in its location by 2J with carbons 7,9 (153.2334, 159.2807). H-2‵/6‵ (8.0717–8.0901) showed 2J coupling with carbon 1‵ (124.4277) and H-2‵ has 3J with carbons 2, 4‵, 6‵ while H-6‵ has 3J with carbons 2, 2‵, 4‵. H-3‵/5‵(7.1990–7.2173) also showed 2J coupling with carbon 4‵ (160.9117) and 3J for H-3‵ at carbons 1‵, 5‵, 3J for H-5‵ at carbons 1‵, 3‵.
Table 5.
HMBC of compound I, (500MHz, DMSO):
| Proton No. | 2J | 3J |
| H-3 | 2,4 | 10,1‵ |
| H-8 | 7,9 | 6,10 |
| H-2‵ | 1‵ | 2, 4‵, 6‵ |
| H-6‵ | 1‵ | 2, 2‵, 4‵ |
| H-3‵ | 4‵ | 1‵, 5‵ |
| H-5‵ | 4‵ | 1‵, 3‵ |
| -OCH3 | 6 | - |
| -OCH3 | 4‵ | - |
The direct coupling between the two protons 2‵, 6‵ and 3‵, 5‵ is not observable on HMBC spectrum.
The direct correlation between -OCH3 (3.7432 ppm) and carbon 4‵ (160.9117) confirmed the substitution of OH-4‵ by -OCH3 (terminal methoxy 57.0203 ppm), also the recognized correlation between -OCH3-6(3.9373) with carbon 6 (down field at δ 132.4971 ppm).
The correlations recognized in HMBC together with 1H-NMR and 13C-NMR spectra therefore led to the complete assignments of all of the carbon resonances (Table 5), thus confirming the structure of compound 1 to be 6, methoxy, acacetin 7-O glucoside.
Compound II
It was isolated from ethyl acetate ext. as a buff precipitate, showing purple colour under UV unchanged on exposure to ammonia vapour, at Rf 0.60 and 0.66 in solvent systems a & b. From UV spectral data compound II showed apigenin skeleton with free hydroxyl group at position 5 and substitution of 4‵-hydroxy group. The bathochromic shift of band I from 334 to 386 with decrease in intensity indicates absence of free 4‵-OH. Absence of shift in band II in methanol (283 nm) and after addition of NaOAc confirmed the presence of 7-O glucosyl. The methoxy groups produced as singlet signals at δ 3.62 ppm (terminal methoxy at 4‵), and 3.92 at position 8. The two equivalent protons at δ 7.17–7.20 ppm (H-3‵/5‵) and 8.06–8.09ppm (H-2‵/6‵) confirm the AA‵XX‵ spin coupling system.
The down field shift of C-8 from 92 to 131.64 ppm indicated substitution of its proton by methoxy group. Compound II was found to be 8-methoxy acacetin -7-O glucoside.
Compound III
This compound showed purple colour under UV, unchanged on exposure to ammonia vapour, it has 5-OH and substitution on 4‵-OH. UV data of this compound revealed apigenin like skeleton. No shift in band II after addition of NaOAc (less, than 4 nm) is attributed to 7-O-glucosyl. Bathochromic shift of band I from 320 to 350 nm after addition of AlCl3/HCl (more than 20 nm) is due to 5-OH with no 6-oxygenation. Bathochromic shift of band I from 320 to 386 after addition of NaOMe with decrease in intensity is ascribed to substitution of 4‵-OH.
1H-NMR of compound III showed terminal methoxy group at δ 3.6698 ppm for OCH3–4‵. The aneumeric proton of C-sugar and O-sugar appeared at δ 4.7626 and 5.0041 ppm, respectively with J value of the last signal = 6.8 which is characteristic of glucose moiety.
13C-NMR showed signals of three sugar moieties, O-glucosyl at C-7 (1‶–6‶), C-rhamnosyl at C-8 (1‷–6‷) and O-α arabinofuranoside at C-3 (1‵‷–5‵‷). The down field shift of C-8 at 107.9073 indicates presence of C-sugar on this carbon , and the up field of C-4 to 177.6897 indicated presence of O-arabinose at C-3 which is characterized by C-1‵‷ at δ 107.0488 ppm. Compound III identified as acacetin-7-O-glucosyl-8-C rhamnosyl 3-O-α arabinofuranoside.
Compound IV
This compound showed apigenin-like skeleton, due to the deep purple colour unchanged on exposure to ammonia vapour. It indicated the presence of 5-OH and substitution of 4‵-OH. Bathochromic shift of band II from 274 to 284 nm after addition of NaOAc (more than 4nm) indicated a free 7-OH. The bathochromic shift of band I from 330 to 386 after NaOMe with decrease in intensity is attributed to substitution of 4‵-OH. Shift of band I by ac 20 nm after addition of AlCl3/HCl is ascribable to 5-OH with 6-oxygenation.
Apiosyl moiety showed signal at δ 4.7718 ppm, while O-galactoside at 4.9843 ppm.
13C-NMR showed presence of C-8 at 92.1786 and downfield of C-6 from 98 to 132.3540 was ascribed to O-sugar. The terminal methoxy is produced at its charactisistic positions in 1H and 13C-NMR 3.6515 and 56.9249 ppm, respectively. The apiosyl moiety is confirmed from its H-1‷ at 109.5097 ppm and its remaining protons 2‷-5‷.
This compound is characterized by two sugar moieties, one is 6-O linked, (galactose), the other is apiosyl. (their signals were illustrated in the experimental section as 1‶–6‶ and 1‷–5‷).
Compound IV can be identified as 4‵-O methyl scutellarin, 6-O, apiosyl galactoside.
Compound V
It gave purple colour under UV indicating 5-OH group, unchanged on expousure to ammonia vapour due to substitution of 4‵-OH by methoxy group. UV absorption showed bathochromic shift of band I from 326 to 386 nm after addition of NaOMe with decrease in intensity confirmed 4‵-OH substitution. Bathochromic shift of band I from 326 to 345 ac 19 nm (less than 20), was attributed to 5-hydroxy with 6-oxygenation. Small shift in band II after addition of NaOAc (from 276 to 278) was ascribed to 7-O glucosyl. It is also confirmed from the doublet of anumeric proton of O-glucose moiety in 1H-NMR at 4.9996–50134 ppm with J=6.9 Hz; while C-glucosyl moiety at position 8-appeared at 4.7749 ppm. The two methoxy groups at δ 3.6667 and 3.8517 ppm were attributed to OCH3-4‵ (terminal) and OCH3-6. 13C-NMR confirmed the substitution of proton 3- and the kaempferol skeleton from the up field of C-4 from 182 to 177.5943 ppm. The appearance of C-8 at 107.1100 (downfield ) is ascribed to substitution of its proton by C-sugar.
O-glucosyl moiety appeared at signals of glucose with C-1‶ at 100.4292 and Δ 0.6486 between C-5‶ and C-3‶, while C-glucosyl moiety at position 8 produced from 73.7124 for C-1‷ and confirmed by signal of C-5‷ at 81.8104, compound V was identified as 4‵, methyl, 6 methoxy kaempferol, 7-O, 8-C diglucoside
Compound VI
This compound was isolated from ethyl acetate extract. of unflowering aerial part of M.unguis-cati at Rf 0.56 and 0.50 (in solvent systems a and b respectively ), with purple colour under UV which changed to greenish on exposure to ammonia. vapour, indicating free hydroxyl groups at positions 5 and 4‵. The bathochromic shift of band II from 271 to 276 (after addition of NaOMe) indicated presence of OH- on ring A. Bathochromic shift of band I from 326 in methanol to 395 on addition of NaOMe with increase in intensity indicated free 4‵-OH. Bathochromic shift of band I from 326 to 352 after addition of AlCl3/HCl (less than 20 nm) indicates 5-OH with 6-oxygenation. The multiple signal at δ 5.1784 ppm was for H-1‶ of O-glucosyl moiety at position 7. Appearance of singlet signal at d 3.7692 ppm indicated presence of - OCH3 at position 6. Two doublet protons of H-3‵/5‵ and H-2‵/6‵ appeared at δ 6.9360–6.9529 and 7.9433–7.9602 ppm with J = 8.45 Hz.
Compound VI is 6-methoxy apigenin-7-O glucoside
Compound VII
It was produced from ethanol extract. at Rf 0.74 and 0.63 (in solvent systems a and b), deep purple under UV, changed to greenish on exposure to ammonia vapour.
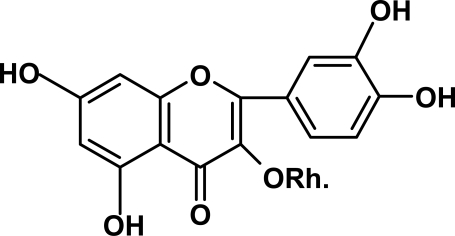
This compound was identified as quercitrin as characterized by O-rhamnosyl moiety at position 3. the UV spectroscopy showed small maxima at 326 after addition of NaOMe indicates free 7-OH. The difference in band I after addition of NaOAc which is more than 5 nm indicates free 7-OH. 1H-NMR showed the characteristic doublet signal of rhamnose at 1.1188–1.1066 with J=6.1 for CH3-6‶. H-6‵ appeared as m (d.d.) at 7.5231 and H-2‵ at 8.0947 while H-5‵ produced as d. at 7.2173–7.2005 with J= 8.4
Compound VIII
It appeared purple colour under UV indicating free 5-OH, changed to yellowish on exposure to ammonia vapour due to free 4‵-OH. It was confirmed from UV spectroscopic data which showed great bathochromic shift of band I after addition of NaOMe (332 to 400) with increase in intensity ascribable to a free 4‵-OH. Also the large bathochromic shift of band I after addition of AlCl3/HCl (332 to 388) is attributed to 5-OH with no 6-oxygenation. Shift of band II from 268 to 280 (more than 4 nm ) after addition of NaOAc was attributed to free 7-OH. It is also confirmed from the appearance of new band at 328 with NaOMe. Compound VIII has apigenin like skeleton with free hydroxyls at 5, 7 and 4‵. 1H-NMR showed two glucose moieties attached through C-link (two doublets at δ 4.123 and 4.147 ppm. It was identified as vicenin II.
Quercitrin and vicenin II were isolated before from M.unguis-cati by Duarte et al. (2000). They also described flavonoid containing arabinose moiety called corymboside, with 6-C-arabinose and 8-C-galactose.
In this work, arabinose is produced in compound III at position 3 with O-link. The presence of scutellarin in Bignoniaceae plants was confirmed before by Subramanian and Nair (1972), who isolated it from Millingtonia hortensis and Oroxylum indicum. Also Subramanian et al. (1972) isolated quercetin glycosides and scutellarin from Bignonia and other spp. of Bignoniaceae.
Evaluation of acute anti-inflammatory activity of different extracts of M.unguis-cati
The highest percentage of reduction of the induced oedema was produced with the crude ethanol extract (51.67%), followed by chloroform extract (41.97%). When compared with Indomethacin (64.21), relative potency of crude ethanol extract and chloroform extract, are (80.47% and 65.36%) respectively. These results agreed with PioCorea, (1978) who reported that M.unguis-cati was used in folk medicine to treat inflammation and rheumatism.
Evaluation of cytotoxic activity of total ethanol extract of M. unguis-cati
The crude ethanol extract of M.unguis-cati did not show great activity as antitumoral against different cell lines used in performing the test. The most effective result obtained from lung cell line with surviving fraction 0.644 (is still less than 50% activity).
Fournet et al., (1994) proved that the extract of the whole plant did not show antiprotozoal activity against Leshmania sp or Trypanosoma cruzi.
While Biological screening of Duarte et at. (2000) revealed antitumoral and antitrypanosomal activities.
Table 1.
Chromatographic properties of isolated flavonoids
| Compound No. |
Isolated from | Solvent system a |
Solvent system b |
UV | UV/amm. |
| I | Ethanol ext. | 0.48 | 0.24 | Purple | Purple |
| II | Ethyl acetate ext. | 0.60 | 0.66 | Purple | Purple |
| III | Ethyl acetate ext. | 0.39 | 0.38 | Purple | Purple |
| IV | Ethyl acetate ext. | 0.18 | 0.42 | Purple | Purple |
| V | Ethyl acetate ext. | 0.55 | 0.63 | Purple | Purple |
| VI | Ethyl acetate ext. | 0.56 | 0.50 | Deep purple | Greenish |
| VII | Ethanol ext. | 0.74 | 0.63 | Purple | Greenish |
| VIII | Ethyl acetate ext. | 0.16 | 0.44 | Purple | Yellowish green |
Table 2.
UV spectral data of isolated flavonoids
| Compound No. |
Methanol | NaOMe | AlCl3 | AlCl3/HCl | NaOAc | NaOAc/H3BO3 |
| I | 252(sh),276, 325. |
243(sh), 290,377. |
259(sh), 290, 299 (sh), 349. |
259(sh), 289, 300(sh), 345. |
279, 300 (sh),330, 390. |
253 (sh), 277, 328. |
| II | 255(sh), 283, 323. |
306, 333 (sh), 386. |
286, 302 (sh), 340. |
293(sh), 299, 348. |
261(sh), 283, 324. |
283, 327. |
| III | 279, 298 (sh), 320. |
292, 326 (sh), 386. |
257 (sh), 282, 298(sh), 350. |
257(sh), 282, 298(sh), 350. |
276, 301(sh), 329, 396. |
276, 329. |
| IV | 260 (sh), 274, 330. |
293, 386. | 260 (sh), 290 299,355. |
260, (sh) 290, 299, 350. |
284, 299 (sh), 330. |
280, 300 (sh), 330. |
| V | 253 (sh), 276, 296, 326. |
256 (sh), 292, 327 (sh), 386. |
256 (sh), 290, 296, 350. |
256 (sh), 290, 296, 345. |
278, 296 (sh), 324, 392. |
278, 324. |
| VI | 271, 326. | 276, 296 (sh), 395. |
276, 296, 355. | 330 (sh), 276, 296, 345. |
271, 330, 400. | 271, 330. |
| VII | 250 (sh), 272, 326. |
290, 326 (sh), 380. |
260(sh), 285, 298 (sh), 352. |
260(sh), 285, 298 (sh), 330. |
251 (sh), 279, 326, 396. |
251 (sh), 279, 326. |
| VIII | 268,332. | 284, 328, 400. | 276, 284, 326, 388. |
276, 284, 326, 388. |
280, 374. | 276, 332. |
Table 3.
1H-NMR of isolated flavonoids in DMSO:
| Proton No. |
I 500 MHz |
II 270 MHz |
III 500 MHz |
IV 500 MHz |
V 500 MHz |
VI 500 MHz |
VII 500 MHz |
VIII 300 MHz |
| 3 | 6.9590 | 6.98 | - | 6.7512 | - | 6.6890 | - | 6.848 |
| 6 | - | 6.96 | 6.6839 | - | - | - | 6.9865 d.J=2.1 |
- |
| 8 | 6..9865 | - | - | 6.8444 | - | 6.8505 | 6..9865 d.J=2.1 |
- |
| 2‵ 6‵ |
8.0717-8.0901 d.J=9.19 |
8.05-8.09 d.J=8.9 |
8.0075-7.9907 d.J=8.4 |
7.9632-7.9479 d.J=7.65 |
8.0091-7.9923 d.J=8.4 |
7.9433-7.9602 d.J=8.45 |
8.0947-8.0794 d.J=7.65 (2‵) 7.5231(6‵) |
8.055-8.025 d.J=9.0 |
| 3‵ 5‵ |
7.2173-7.1990 d.J=9.15 |
7.17-7.20 d.J=8.9 |
7.1913-7.1745 d.J=8.4 |
7.1424-7.1256 d.J=8.4 |
7.1898-7.1715 d.J=9.15 |
6.9360-6.9529 d.J=8.45 |
7.2173-7.2005 d.J=8.4(5‵) | 6.894-6.864 d.J=9.0 |
| H-1‶ | 5.0332-5.0469 d.J=6.85 7, O-glucosyl |
5.01-5.04 d.J=6.92 7,O-glucosyl |
5.0177-5.0041 d.J=6.8 7,O-glucosyl |
4.9843m 6,O-galactosyl |
4.9996-5.0134 d.J=6.9 7, O-glucosyl |
5.1784m 7, O-glucosyl |
5.0332 3,O-rhamnosyl |
4.123 d. 8, C-glucosyl |
| H-2‶ to H-6‶ |
4.5899, 5.1203, 5.3832 (aliphatic glucose protons) |
3.32-3.43 (aliphatic glucose protons) |
3.32-3.43, 5.2212 5.4932 (aliphatic glucose protons) |
5.4780, m (aliphatic sugar protons) |
5.4550, 5.5437 m (aliphatic sugar protons) |
3.1715, 3.5093 5.2938, 5.3908 (aliphati c sugar protons) |
- | - |
| H-1‷ | - | - | 4.7626 m-8, C-rhamnosyl |
4.7718 apiosyl |
4.7749m 8, C-glucosyl |
- | - | 4.147 d. 6, C-glucosyl |
| H-2‷ to H-5‷ |
- | - | 4.49529 4.6404m |
- | 4.6725m | - | - | - |
| CH3-rhamnos yl |
- | - | 1.2350m | - | - | - | 1.1188-1.1066 DJ= 6.1 |
- |
| H-1‵‷ | - | - | 5.2212 3, O-arabinofuranoside |
- | - | - | - | - |
| H-2‵‷ to H-5‵‷ |
- | - | 5.4932m | - | - | - | - | - |
| OCH3-4‵ | 3.7432 | 3.62 | 3.6698 | 3.6515 | 3.6667 | - | - | - |
| OCH3-6 | 3.9373 | - | - | - | 3.8517 | 3.7692 | - | - |
| OCH3-8 | - | 3.92 | - | - | - | - | - | - |
Table 4.
13C-NMR of isolated flavonoids in DMSO
| Carbon No. |
I 125 MHz |
II 75 MHz |
III 125 MHz |
IV 125 MHz |
V 125 MHz |
| 2 | 163.9640 | 163.50 | 168.5043 | 164.4409 | 167.5313 |
| 3 | 104.1969 | 103.66 | 130.3796 | 103.9107 | 132.1346 |
| 4 | 182.8499 | 182.31 | 177.6897 | 182.8404 | 177.5943 |
| 5 | 152.5657 | 151.50 | 152.0021 | 152.0506 | 154.5000 |
| 6 | 132.4971 | 99.81 | 98.7505 | 132.3540 | 132.2300 |
| 7 | 153.2334 | 152.94 | 152.2113 | 153.3288 | 157.8000 |
| 8 | 92.2453 | 131.64 | 107.9073 | 92.1786 | 107.1100 |
| 9 | 159.2870 | 158.73 | 161.1884 | 159.3093 | 160.2441 |
| 10 | 105.7230 | 104.00 | 104.2541 | 105.5036 | 105.7000 |
| 1‵ | 124.4277 | 123.82 | 122.0012 | 124.5135 | 124.7901 |
| 2‵/6‵ | 128.7485 | 128.22 | 130.3796 | 128.7962 | 129.1873 |
| 3‵/5‵ | 117.1594 | 116.58 | 115.5856 | 117.1976 | 117.0545 |
| 4‵ | 160.9117 | 160.38 | 161.1884 | 160.5111 | 167.5313 |
| 1‶ | 100.3911 | 100.10 | 100.0382 | 100.0286 | 100.4292 |
| 2‶ | 73.7219 | 73.15 | 73.4739 | 72.1672 | 73.7124 |
| 3‶ | 77.0889 | 76.52 | 76.5739 | 73.4167 | 77.0794 |
| 4‶ | 70.2404 | 69.61 | 69.8000 | 68.0275 | 69.8000 |
| 5‶ | 77.7280 | 77.20 | 77.4132 | 75.8490 | 77.7280 |
| 6‶ | 610.2076 | 63.01 | 61.6464 | 60.7021 | 61.1504 |
| 1‷ | 77.4132 | 109.5097 | 73.7124 | ||
| 2‷ | 76.8123 | 76.4594 | 71.5000 | ||
| 3‷ | 77.1557 | 79.1874 | 78.7000 | ||
| 4‷ | 72.155 | 73.6265 | 70.2000 | ||
| 5‷ | 72.6155 | 63.4014 | 81.8104 | ||
| 6‷ | 19.9067 | - | 61.1504 | ||
| CH3 of rhamnosyl | |||||
| 1‵‷ | 107.0488 | ||||
| 2‵‷ | 82.9832 | ||||
| 3‵‷ | 77.1557 | ||||
| 4‵‷ | 98.2640 | ||||
| 5‵‷ | 61.3000 | ||||
| OCH3-6 | 60.5976 | - | - | - | 63.5731 |
| OCH3-8 | - | 60.07 | - | - | - |
| OCH3-4‵ | 57.0203 | 56.50 | 56.7151 | 56.9249 | 57.8000 |
References
- 1.Aboutabl E A, Hashem F A, Sleem A A, Maamoon A A. Phytochemical and bioactivity investigations of Macfadyena unguis-cati L. (Bignoniaceae) . The first international conference of the Arab Society for Medical Research. November 7–9 , 2006, National Research Centre, Cairo, Egypt. Int J Biol Biotech. 2006;3:695–702. [Google Scholar]
- 2.Adriani C, Lavarone C, Trogolo C. 5, 7-bisdeoxy-Cynanchoside, an iridoid glucoside from Macfadyena cynanchosides. Phytochemistry. 1982;21:231–233. [Google Scholar]
- 3.Bianco AD, Guiso M, Lavarone C, Trogolo C. iridoids XV. Macfadenoside structure and configuration. Gazzeta Chimica Italiana. 1974;104:731–738. [Google Scholar]
- 4.Bonini C, Davini E, Lavarone C, Trogolo C. Cynanchoside a highly oxygenated iridoid glucoside from Macfadyena cynanchoides. Phytochemistry. 1981;20:1587–1590. [Google Scholar]
- 5.Duarte DS, Dolabela MF, Salas CF, Raslan DS, Oliveiras AB, Nenninger A, Wiedemann B, Wagner H, Lombardi J, Lopes MTP. Chemical characterization and Biological activity of Macfadyena unguis-cati (Bignoniaceae) J Pharm Pharmacol. 2000;52:347–352. doi: 10.1211/0022357001773904. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari F, Cornelio k1, Delle Monache F, Marini Bettolo GB. Quinovic acid glycosides from roots of Macfadyena unguis-cati. Planta Med. 1981;43:24–27. doi: 10.1055/s-2007-971467. [DOI] [PubMed] [Google Scholar]
- 7.Fournet A, Barrios AA, Munzo V. Leishmanicidal and Trypanocidal activities of Bolivian medicinal plants. J Ethanopharmacol. 1994;41:91–97. doi: 10.1016/0378-8741(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 8.Graletto L. Nectary structure and Nectar characteristics in some Bignoniaceae. Plant Systematics and Evolution. 1995;1–2:99–121. [Google Scholar]
- 9.Harborne JB. Comparative biochemistry of the flavonoids — VI. Flavonoid patterns in the Bignoniaceae and the Gesneriaceae. Phytochemistry. 1967;6:1643–1651. [Google Scholar]
- 10.Houghton PJ, Osibogun 1M. Flowering plants used against snakebite. J Ethanopharmacol. 1993;39:1–29. doi: 10.1016/0378-8741(93)90047-9. [DOI] [PubMed] [Google Scholar]
- 11.Joshi KC, Singh P, Sharma MC. Quinones and other constituents of Markhamia platycalyx and Bignonia unguis-cati. J Natural products. 1985;48:145. [Google Scholar]
- 12.Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New york: Springer; 1970. [Google Scholar]
- 13.Markham KR. Techniques of flavonoids identification. London: Academic press; 1982. [Google Scholar]
- 14.Miller LC, Tainter MI. Estimation of LD50 and its error by Means of logarithmic probit graph paper. Proc Soc Expt Biol and Med. 1944;57:261–264. [Google Scholar]
- 15.PioCorrea M. Dicionario das plantas Lcis do Brasil e das Exoticas cultivadas. Zmprensa Nacional, Ministerio da Agricultura, IBDF, Rio de Janeiro, Brasil. 1978;6:1926–1954. [Google Scholar]
- 16.Poser G, Schripsema J, Henriques A, Jensen S. The distribution of iridoids in the Bignoniaceae. Biochemical Systematics and Ecology. 2000;28:351–366. doi: 10.1016/s0305-1978(99)00076-9. [DOI] [PubMed] [Google Scholar]
- 17.Shehan P, Storeng R, Scudiero D, Monks A, Mc Mahon J, Vistica D. New coloremetric cytotoxicity assay for anti-cancer drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 18.Snedecor WG, Cochran GW. Statistical methods. 10the ed. USA: Lowa state, university press; 1971. [Google Scholar]
- 19.Subramanian S, Nair A. flavonoids of the leaves of Oroxylum indicum and Pajanelia longifolia. Phytochemistry. 1972;11:439–440. [Google Scholar]
- 20.Subramanian S, Nagarajains, Sulochana N. Flavonoids of eight bignoniaceous plants. Phytochemistry. 1972;11:1499. [Google Scholar]
- 21.Winter GA, Risley EA, Nuss GW. Carrageenan-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med III. 1962:1544–1547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]