Abstract
Bidens pilosa L. is an Asteraceae growing in tropical zones, and traditionally utilized worldwide in herbal medicine. The present work is based on its traditional use during child birth as a labour facilitator. In vivo tests of acute toxicity showed a weak toxic effect for both extracts but the toxicity of the ethanol extract (LD50=6.15g/kg) was upper than that of the aqueous extract (LD50=12.30g/kg). The three-days uterotrophic assay on immature mice showed body weight gain followed by a concentration-dependent decrease up to 4mg/g and a concentration dependent uterine wet weight increase. The ethanol extract exhibited the higher body weight gain representing 22.8±0.7%, (P≤0.001), at the concentration of 500µg/g/day, while the aqueous extract was significantly more efficient on the uterine wet weight gain of 0.24±0.001% (P≤0.05), at the concentration of 1000µg/g/day. In vitro isometric contraction measurement of oestrogen-primed rat uterine strips showed a significant high aqueous extract-induced contractile effect from 0.03–1.97mg/ml: on the amplitude of contraction (EC50 = 0.44±0.10mg/ml, P≤0.05), and on the rate (1.21±0.25mg/ml, P>0.05). Inspite of the higher effect of the aqueous extract on the tonus (57.23±23%), the ethanol extract showed a high effect (EC50=0.34±0.09mg/ml, P≤0.05). The weak toxicity, the estrogenic-like and the oxytocic-like activities observed could explain the empirical use of Bidens pilosa leaf aqueous extract as an uterotonic preparation to enhance labour, probably due to the presence of biologically active compound(s) which act directly on the uterine muscle.
Keywords: Bidens pilosa L. Leaf, Uterotrophic, Uterotonic, Mouse, Rat
Introduction
Bidens pilosa L. is a perennial weed herb (Asteraceae) growing in tropical and subtropical regions all around the world and widely used. All parts of the plant contain bioactive molecules which react in curing several diseases in either association or not. Previous works have been done with regard to many of its biological properties such as anti-microbial (Andersen et al., 2006; Rojas et al., 2006), anti-inflammatory (Pereira et al., 1999), anti-malarial (Krettli et al., 2001; Andrade-Neto et al., 2004; Oliveira et al., 2004) hypoglycaemic (Alarcon-Aguilar et al., 2002; Chang et al., 2004; Chang et al., 2005) and pesticidial actions (Gilbert et al., 1999; Machado et al., 2005).
In Cameroon, since the last 20 years, the framework of the laboratory of animal physiology of the Faculty of Sciences of the University of Yaounde I is to study modes of action of some medicinal plants currently used in rural zones by tradipractitionners. Different Bidens pilosa leaf extracts were tested on various smooth muscles. Its hypotensive effect on hypertensive induced rats (Dimo et al., 1998; Dimo et al., 2001; Dimo et al., 2003) and, its relaxant effect was also showed on isolated rat vascular smooth muscle (Nguelefack et al., 2005). Its antisecretory and antiulcerogenic properties on various gastric ulcers models in rat showed that the methylene chloride extract is the most potent inhibitor of gastric mucosal lesions caused by HCl/ethanol, and that the extract does not possess anti-secretory potential, and tended to increase gastric acidity when gastric outflow is obstructed by ligature; a possible cytoprotective action mediated by the cytoprotective action of endogenous prostaglandins was also demonstrated (Tan et al., 2000). Another team have tested its leaf extract on the snake venoms and found that Bidens pilosa leaf extract weakly antagonized the D. jamesoni venom and did not potentiate the SAV (Antivenon Serum) (Chippaux et al., 1997).
In this study, we focused our research on one of the empirical uses of it aqueous leaf extract by some tradipractitioners in Cameroon to facilitate labour during child birth (CSTR/OUA, 1985). According to (Balépa M et al., 1992), 36.4% of pregnant women deliver at home (a cause of mother and child mortality), and 11.7% of the cases occur in the presence of a traditional obstetrician even in urban or in rural zones (INS, 2001). The practice of obstetrics and gynaecology in traditional medicine practice is often used by traditional birth attendants (TBA) or traditional midwifes. TBAs activities have been highlighted at WHO seminars because, they play a major role even in big cities (specially in Africa), where maternity hospitals are overcrowded and women in labour have to seek help elsewhere (Sofowora, 1984; INS, 2001).
The activity of B. pilosa on the uterine muscle of pregnant women may suggest the presence of active compounds with estrogen-like and/or oxytocin-like activities. Estrogens are steroid hormones with important functions in the regulation of specific sexual processes in females; they affect the myometrium at the end of the pregnancy by decreasing cellular membrane potential which increases the excitability of the uterus (Kurowicka et al., 2005; Riley et al., 2005) and then intervene in the initiation of labour during child birth. In addition, uterotrophic substances such as oxytocin, a neurohypophysial hormone, are frequently used to enhance myometrial contractile activity during labour (Lopez Bernal, 2003), and it is known that the physiological regulation of the oxytocin system is strongly steroid-dependent (Gimpl and Fahrenholz, 2001).
Given the world-wide interest in the regulatory assessment for medicinal products, there is a need to characterize natural substances for rural population. According to the Endocrine Disruptor Screening and Testing Advisory Committee's (EDSTAC), they recommended T1 screening assays for the development of new drugs such as Non Steroidal Anti-inflammatory Drugs (NSAIDs) (Features, 1998). The in vivo three days uterotrophic bioassay using prepubertal mice has been used to determine the estrogenic activity of the aqueous and ethanol leaf extracts of B. pilosa on uterine muscle, based on the uterine tissue weight increase after several doses of the test compound (Kanno et al., 2001; Owens and Koëter., 2003). For this set of experiment, the subcutaneous administration, which is one of the two routes of administration validated by the OECD (Organisation for Economic, Co-operation and Development) was preferred to the oral route used by TBAs, because, it has been reported that during the validating protocols conducted by several laboratories, no protocol or route of administration was clearly superior in the dose-response experiments, and that the subcutaneous results on prepubescent were near those on ovariectomized rats (OECD, 2003; Owens and Koëter., 2003; Kanno et al., 2003b). In addition, isometric contraction of isolated primed oestrogenized uterine trips using the organ bath experiment was used to evaluate the plant drug/hormone-like effect and to set priorities for additional testing (Arteche et al., 1997; Greim, 2004).
In the present study, we investigated the estrogenic/uterotrophic (in vivo) and oxytocic/uterotonic (in vitro) effects of the aqueous and ethanol leaf extracts; we then proceeded to perform further experiments on the effect of the aqueous extract of B. pilosa leaves (which showed a more potent uterotonic activity) on the contractile uterine in rats, in the presence of some modulators.
Material and Methods
Collection and Preparation of Aqueous and Ethanol Extracts
B.s pilosa plants were collected from Yaounde's suburb (Nkolbisson) from July to November, 2004. A voucher specimen (No 65112/HNC.) was deposited in the National Herbarium of Cameroon and authenticated by Professor Sonke Bonaventure, University of Yaounde I. Plants were dried and extracted in accordance with international herbal rules in traditional medicine (Drugs, 1995). Following information obtained from traditional birth attendants (TBA), two hundred grams of sun dried leaves of B.s pilosa were boiled in one litre of water for twenty minutes. The liquid obtained was freeze-dried giving 38g of a brownish powder. A stock solution of 100mg/ml in distilled water was prepared daily because of the high instability of B. pilosa's components (Brandao et al., 1997). The ethanol extract was prepared by macerating 200g of sun dried leaves for three days in one litre of ethanol (95% purity). After filtering, the collected extract was concentrated using a rotary evaporator giving about 17.25g of greenish dough. A stock solution concentrated at 100mg/ml was prepared daily by dilution with Tween 80 (0.1%).
Animals
Immature female mice (Mus musculus) aged between 19–21 days, weighing 12–20g at the beginning of the experiment were obtained from the Centre Pasteur of Yaoundé-Cameroon. Access to food and water by bottle-feed was unrestricted throughout the four days assay. The animals were housed in ambient room temperature and humidity. The assays started two days after their arrival for acclimatisation and they were sacrificed by euthanasia with ethylene ether. Adult virgin female Wistar rats weighting 200–250g were purchased from Janvier Breeding Centre (France). Twenty-four (24) hours before the experiment, they received an intraperitoneal injection of 17β Oestradiol Benzoate [20µg × Kg-1]. The rats were humanely killed by inhalation of carbon dioxide (CO2). These animals (mice and rats) were treated following the international ethical conduct of animal's treatment (Guidelines, 2003).
Pharmacological Experiments
Acute Toxicity Test: (LD50)
The acute toxicity test followed the method used by (Chearskul et al., 2004). Seventy (70) mice aged between 5–6 weeks with average weight between 28–35g were divided into twelve groups of five mice receiving an intraperitoneal injection of a single concentration (per body weight) of aqueous or alcohol extract of B. pilosa. Two control groups (for each extract) receiving the corresponding amount of vehicle distilled water or Tween 80 (0.1%) were studied. Five single concentrations: 0.78; 1.56; 2.34; 3.13; 3.90 mg/g/day were given once and the death cases were recorded for three days (72 hours).
Uterotrophic bioassay
180 mice were used and divided into groups of six animals. For the aqueous and ethanol extracts, the test groups received 0.25, 0.5, 1.0, 2.0, 4.0, 8.0mg/g/day, respectively; while the control groups received Tween 80 (0.1%) and distilled water at 2.5, 5.0, 10.0, 20, 40.0, 80.0µl/g/day respectively. Six concentrations (0.02, 0.04, 0.08, 0.16, 0.32, 0.64µg/g/day) of Premarin, a standard sulfoconjugated oestrogen solution, were given as positive control, while 2.5, 5, 10, 20, 40 and 80.0µl/g/day, respectively of Olive oil, used as vehicle to the sulfoconjugated estrogen (Yamasaki et al., 2001), were added to the corresponding solutions. Subcutaneous injections of all extracts were administered once a day for three consecutive days, at about 9h ±15min daily. The LD50 of both extracts were calculated using the Spearman-Karb>r method (W.H.O., 1981).
Log LD50 = X100±(d/n) .(Σr-n/2) where X100 is the logarithm of the concentration producing 100% of mice death; n is the number of mice used for each concentration; r is the number of dead mice for each concentration; d=2.20 for p=0.05 with an d.d.l.= Σ (n-1) including all mice groups except X0 and X100.
Determination of body Weight and Uterine Weight
Body weight of mice was recorded during the first and the fourth days of the experiments (approximately twenty four (24) hours after the last injection), and expressed as a percentage of the difference of body weight between the first and the fourth days. Mice were humanely sacrificed by anaesthesia with ethylene ether on the fourth day of the experiment. The uterine horns were removed free of ovaries and adhering fats. Care was taken not to lose the uterine luminal fluid. Wet weights were then recorded on an electronic digital balance (AND FX-300 Japan), and expressed as percentage of the uterine wet weight.
Isometric Contraction Measurement
Uterine horns were dissected and mounted in the organ bath containing a Krebs Heinseleit (KH) solution with the following composition (in mM): NaCl 118.4; KCl 4.7; KH2PO4, 1.2; MgSO47H2O, 1.2; C6H12O6, 11.1; NaHCO3 25.0 and CaCl2 2H2O, 2.5 and equilibrated as previously described by (Savineau & Mironneau, 1990; Arteche et al., 1997). After the equilibration period, the preparations were challenged by administration of a maximal effective concentration of acetylcholine (Ach), 100µM. The Ach-induced contraction was considered as a reference standard contraction and used to normalize the following contractile responses. Uterine strips were then washed 3 times with fresh KH solution and kept not stimulated until stable and regular spontaneous contractions appeared for a further 30-min period before tests. For each experiment, a ring obtained from the same horn was used as a paired temporal control. Stock solutions of 25mg/ml per extract were prepared daily. Different concentrations of aqueous and ethanol stock solutions were cumulatively injected in the bath at ten minute intervals for final concentrations of 0.03; 0.09; 0.22; 0.47; 0.97; 1.97; 3.97mg/ml.
For this set of experiments, three parameters [the amplitude of spontaneous and transient contractions (named oscillations), the amplitude of the tonic contraction and the rate of oscillations] were selected, measured and calculated in order to characterize the effect of B. pilosa extracts on the myometrial tissue. These parameters were calculated as follows:
Amplitude of oscillations: = [(Ac-A0)/ (AAch-T)/100]
Tonus: = [(Tc-T0)/ (AAch-T0)/100]
Rate of oscillations: = [(Fc-F0)×100]
Ac = amplitude recorded during B. pilosa cumulative addition in the bath in presence or in absence of the pharmacological drug at c concentration.; A0 = amplitude recorded after the equilibrating period which follows wash of the reference Ach contraction (control strip) and after the effect of a given pharmacological substance (treated strip); AAch = amplitude of the reference contraction of Ach 100µM; T = basal tonus recorded before the reference Ach contraction.; T0 = basal tone recorded after the equilibrating period who follows wash of the reference Ach 100µM (control strip) and after the effect of the pharmacological substance (treated strip); Fc = Rate recorded during B. pilosa cumulative addition in the bath in presence or in absence of the pharmacological drug at c concentration.; F0 = Rate recorded after the equilibrating period which follows wash of the reference Ach 100µM (control strip) and after the effect of a given pharmacological substance (treated strip).
Chemicals
Premarin, a sulfoconjugated oestradiol, was purchased from Wyeth-Medica (Newbridge-Ireland); 17β-Oestradiol benzoate was purchased from Sigma Aldrich chemicals (France).
Data Analysis
Data obtained from the in vivo and in vitro tests between control and treated groups were analyzed by a multiple comparison analysis using a One-Way ANOVA: Duncan or Tukey tests and the difference between two means were assessed by the t-test or Mann-Whitney test where appropriate. For the uterotrophic analysis, the five concentrations of B. pilosa's extracts were compared with the vehicles and Premarin treated groups. Uterotonic analysis were assessed by comparing B. pilosa extracts (ethanol and aqueous) with vehicles. Body weight was reported as the mean ± S.E.M for three replicates. The significance was determined at P≤0.05. All statistics were performed using Sigma Stat version 2.0 and curves draw using Sigma plot 9.0.
Results
In Vivo Study: Uterotonic Effect
Acute Toxicity: LD50
From the intraperitoneal acute toxicity study of both extracts of the leaves of B. Pilosa in mice, the LD50 of the aqueous and ethanol extracts were 12.30g/kg and 6.15g/kg respectively.
Mice Body Weight
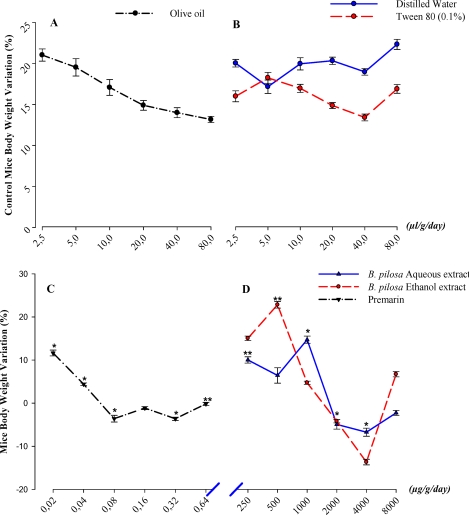
Compared with negative control, both B. pilosa ethanol and aqueous leaf extracts effects were similar and induced significant (P≤0.05) variations in mice body weight [Figure1B&D] and the effect produced by the aqueous extract was lowest in general, even at the maximal effect induced. On contrary to the aqueous extract, the ethanol extract, in spite of the general decreased effect observed, at concentrations up to 500µg/g/day, we noted a body weight increase higher than that of the negative control at corresponding concentrations.
Figure 1.
In vivo effects of Bidens pilosa leaf aqueous and ethanol extracts on immature mice Body Weight. Representative curves of vehicles (negative controls): olive oil (A), distilled water and Tween 80 (B), and representative curves of Premarin (positive control) (C) and Bidens pilosa aqueous, ethanol extracts (D) effects on the mice body weight variation during the four day uterotrophic essay. The significance of the extracts effects was expressed versus the positive control (Premarin). *P≤.05; **P≤.001.
However, compared with the standard [Premarin], mice treated with both aqueous and ethanol extracts [Figure1C&D], while presenting same variations, showed significant (P≤0.05) body weight increase with peak values at 0.5mg/g/day (22.8±0.76%, P≤0.05) for the ethanol extract and at 1mg/g/day (14.7±0.84%, P≤0.001) for the aqueous extract. Up to 4mg/g, a concentration-dependent body weight loss followed by an increase at 8mg/g was observed. However, these values were inferior to those observed at low concentrations of ethanol (+6.71±0.61%; P>0.05) and aqueous (−2.26±0.57%, P>0.05) extracts. Comparison of the effects of the two extracts showed that they are both efficient at low concentrations but the ethanol extract induced the highest body weight gain during the experiment.
Uterine Wet Weight
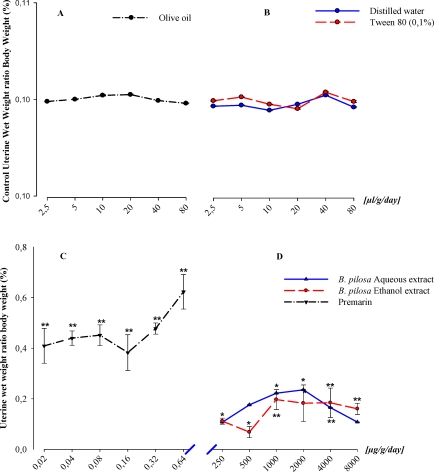
Both ethanol and aqueous extracts of B. pilosa administered to immature female mice from 0.25–8mg/g/day tended to increase uterine wet weight (P≤0.05) with peaks of 0.20±0.04% (P≤0.001) and 0.24±0.001% (P≤0.05) at 1mg/g/day and 0.2mg/g/day respectively [Figure2D]. Higher concentrations (>2mg/g/day) showed a marked decreasing effect of the aqueous extract. Though the ethanol extract exhibited a decreased effect, its values were similar to those observed at 1mg/g peak value. This suggested that increased uterine weight gain occurred at low and high concentrations of aqueous and ethanol extracts of B. pilosa leaf. The three vehicles tested showed no significant (P>0.05) variation on mice uterine wet weight [Figure 2A&B]. Comparatively to the positive control [Figure 2C] which caused a high significant (P≤0.001) uterine wet weight gain of +0.62±0.07 % at the concentration of 0.64 µg/g, and the negative control groups [Figure2B], B. pilosa extracts showed significant (P≤0.05) weak uterotrophic activity on mice uterine muscle. These results indicated an uterotrophic effect of B. pilosa leaf extracts on the mouse uterine smooth muscle.
Figure 2.
In vivo effects of Bidens pilosa leaves aqueous and ethanol extracts on immature mice relative uterine wet weight. Representative curves of vehicles (negative controls): olive oil (A), distilled water and Tween 80 (B), and representative curves of Premarin (positive control) (C) and Bidens pilosa aqueous, ethanol extracts(D) effects on the mice body weight variation during the four day uterotrophic essay. The significance of the extracts effects was expressed versus the positive control (Premarin). *P≤.05; **P≤.001.
The maximal percent increase in both water and ethanol extracts were 38.01 and 31.73% respectively, at concentrations of 2000µg/g/day and 1000µg/g/day respectively (concentrations producing the greatest uterotrophic response), equivalent to approximately 1/3 the highest uterine increase of the standard Premarin, at concentration of 0.64µg/g/day.
In Vitro Study: Uterotonic Effect
Mechanical Contractile Induced Effect of Bidens pilosa Aqueous and Ethanol Extracts
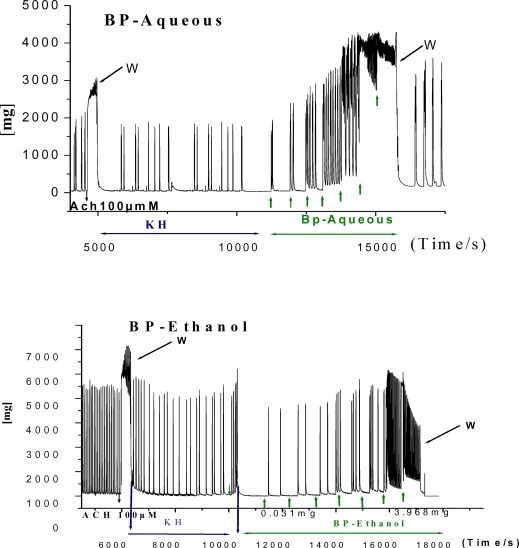
To evaluate the difference in uterotonic potency between both extracts, the same concentrations of the aqueous and ethanol extracts of B. pilosa were tested on the uterine strips. We observed that both leaf extracts generally induced concentration-dependent increases on selected parameters of the uterine strip. For high B. pilosa concentrations, the muscle exhibited a rapid contractile response characterized by a brief steady state, followed by a decrease with spike-types which could became a stable steady state with some rhythmic oscillations (aqueous extract) or could reach the level of the basal tonus (ethanol extract) [Figure3a].
Figure 3a.
Original recordings of in vitro effects of aqueous and ethanol Bidens pilosa leaves extracts on the contractile activity of rat primed estrogenized uterine strip. Seven different volumes of Bidens pilosa aqueous and ethanol extract were injected in the organ bath in presence of Normal Krebs solution cumulatively at 10 minutes intervals, respectively. Contractions are expressed in mg (ordinate). Each concentration is represented by an arrow. Ach = acetylcholine, W= wash with Normal Krebs [2.25mM].
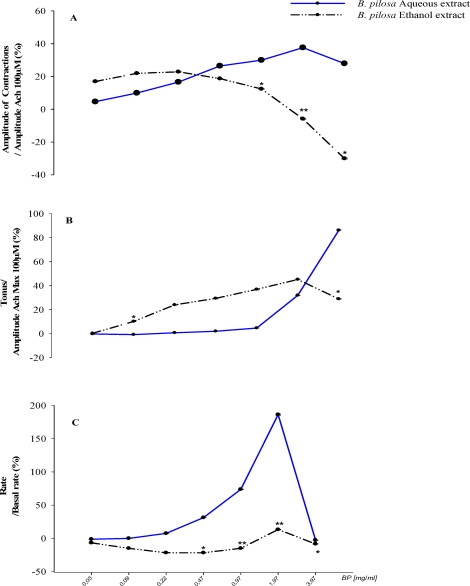
The aqueous extract showed highest increases on the three parameters studied. Its maximal effect on the amplitude (+37.61±0.15%) and the rate (+186.22±0.89%) of contractions were observed at the concentration of 1.97 mg/ml. While the maximal tonus increased (+86.21±0.89%) was observed at the concentration of 3.97mg/ml [Figure3bB]. The effects of the ethanol extract on the amplitude [Figure3bA] and the rate [Figure3bC] of oscillations were more complex and clearly biphasic according to the concentration used. Taken together and in comparison of the EC50 of each extract on the different parameters, we noted in general, a significant highest activity (P≤0.05) of the aqueous extract on the amplitude of contractions while, the ethanol extract showed a significant (P≤0.05) superior effect on the tonus of the uterine spontaneous contractile activity. The aqueous extract also showed a higher but non significant (P>0.05) effect on the rate of oscillations contrary to the ethanol extract which presented a lower but significant EC50 value [Table 1].
Figure 3b.
Curves of in vitro effects of aqueous and ethanol Bidens pilosa leaf extracts on the amplitude (A) the tonus (B); and the rate (C) of rat primed estrogenized uterine strip contractile activity. (n=6) *P≤.05; **P≤.001. The P value was determined versus the effect of aqueous extract in Krebs solution (standard).
Table 1.
EC50 of in vitro effects of aqueous and ethanol extracts of Bidens pilosa leaves on the amplitude, the tonus and the rate of rat primed oestrogenized uterine strip contractile activity. *P≤.05; ***P≤.0001.
| Bidens pilosa extract : | EC50 (mg/ml) | ||
| Amplitude of oscillations | Tonus | Rate of oscillations | |
| Aqueous | 0.44 | 4.08 | 1.21 |
| ±0.10* | ±0.30*** | ±0.25 | |
| Ethanol | 1.7 | 0.34 | 1.98 |
| ±0,24 | ±0.09* | ±0.69* | |
Discussion
Based on the conclusions of previous toxicological results on mice and rats which determined the LD50 <5mg/kg as highly toxic and LD50 >5g/kg as weakly toxic, (Diezi, 1989), and the values of the aqueous and ethanol B. pilosa leaf extracts obtained, we can thus characterize both extracts as weakly toxic. But when compared with each other, the ethanol extract present a higher lethal effect than the aqueous extract. This result implies that, as regards of the ethanol extract, a normal adult weighing about 60kg will need to absorb 60×6.15g = 369g to obtain a lethal effect. Yet this quantity is largely higher than any prescribable maximum effective dose of any drug which is usually 1–2g (adult dose) two or three times daily (Kamanyi, 1992). We can thus conclude the concentrations under investigations may not be toxic.
Our results on the three-day uterotrophic bioassay showed a body weight gain in mice at low concentrations but a weight loss at high concentrations. We also observed a general uterine wet weight increase for both ethanol and aqueous extracts. Different laboratories during assessments on uterotrophic protocols (Kanno et al., 2001) have demonstrated that, the three-day bioassay on immature rodent showed no consistent correlation between the body weight gain and the uterine weight gain; and that the body weight adjustment had relatively little impact on the evaluation of uterine weight in these studies.This assertion was verified in our results related to the maximal effect observed on the animal body weight gain and on the uterine wet weight increase occurred at different but low concentrations for both aqueous and ethanol extracts. In reference to previous reports, the uterine weight increase is a fundamental and early marker of the female to sufficient exposure to estrogen agonists. The response begins with the essential interaction of the estrogen with a high-affinity receptor in uterine tissues that initiates a series of responses culminating in the uterine weight increase, which express a combination of water imbibition in the tissue and the uterine lumina, to the cellular hyperplasia (Kanno et al., 2001; Kanno et al., 2003b), and to microvascular permeability which may be mediated by growth factors (Chearskul S. et al., 2004). The uterine wet weight increase observed in presence of B. pilosa aqueous and ethanol extracts, is due to similar mechanisms, and expresses an uterotrophic effect, which may thus result from the effect of some oestrogenic compounds present in the plant extracts at the level of the uterine muscle. Previous studies on the effect of the aqueous and alcoholic extracts of some tropical plants such as: Trifolium pratense (red clover) on the ovariectomized rat (Burdette et al., 2002) traditionally used as B. pilosa, to enhance labour during childbirth; Angelica sinensis (Circosta et al., 2006), Morinda citrifolia (Chearskul S. et al., 2004), mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis (P.B. Telefo et al., 2002) used to alleviate menstrual disorders and to cure infertility, have demonstrated that the uterotrophic activity observed could be caused by oestrogenic-like compounds presents in plant extracts. On the other hand, B. pilosa at low concentrations displays estrogenic activity that is not observed at higher concentrations which seems to depress the uterotrophic response to below the control levels. Conclusions of previous studies have mentioned that the uterotrophic and the estrogen agonistic effect presented by various natural estrogens [genistein (GN), daidzein (DN), and coumestrol (CN), etc], occur at low doses but not at higher doses (Hideki Wanibucchi et al., 2003; Owens et al., 2003; Yamasaki et al., 2003). Our results also confirm the calabrese's concept of hormone-like biphasic dose response model characterized by a low-dose stimulation which usually present a modest response (30–60% vs. control) and a high-dose inhibition (Calabrese EJ. and Baldwin, 2003; Chearskul S. et al., 2004); this gives proof that B. pilosa leaf active compounds act after a uterine receptor binding.
The in vitro test performed on primed-oestrogenized isolated uterine strips confirms the hormone-like (estrogenic-like) effects of both aqueous and ethanol extracts, and clearly showed their different potencies and characteristics on the uterine muscle. Each extract presents a different action on the parameters chosen to characterize B. pilosa activity. Their significant effects on the amplitude and the tonus are quite opposite as concentrations increased, with a highest effect at low concentrations for the ethanol extract and a highest effect of the aqueous extract at high concentrations. This could be related to the amount of B. pilosa active chemicals which have positive effects on those parameters. On the other hand, the lack of the ethanol effect on the rate could imply a lack of biological activity of the extract on the enhancement of the rate of contractions.
These differences could be due to the existence of active substance(s) in B. pilosa's leaves, which have a variable solubility coefficient (difference in polarity) of the molecules extracted with the solvent used for extraction (water and ethanol). Natural estrogenic-like compounds are generally more extractable in ethanol than in water (Circosta et al., 2006). Earlier phytochemical screenings of different extracts of Bidens pilosa's leaves revealed the presence of biological components such as polyacetylenes and flavonoids in abundance (Alvarez et al., 1996; Chang et al., 2004). Some natural flavonoids have been tested on human and rat myometrium and presented a weak estrogenic activity (Gilbert et al., 1999; Kim et al., 2002; Kim et al., 2005), and a relaxant effect (Revuelta et al., 1999). This dual effect is in accordance with our results could be due to the nature of solvent used for extraction or the instability of polyacetylene bioactivity (Krettli et al., 2001).
Earlier researchers have reported that, estrogens possess some oxytocic effect and hence may be involved in increasing uterine contractility (G. Tafesse et al., 2005). The oxytocin hormone was reported to biologically act via binding to a uterine membrane receptor, (OTR), with seven (7) transmembrane domains, and belongs to the class I G protein-coupled receptor (GPCR) family, and thereby stimulates a cascade of events leading to the contraction of the uterine contractile proteins (actin and myosin) (Keena McKillen et al., 1999; Gimpl and Fahrenholz, 2001; Muller et al., 2006). Similarly, the increase of the uterine induced contractile activity observed in our results could be due to the presence of oxytocic-like compounds acting via the same pathways.
The comparison of the oxytocic-like activity of both aqueous and ethanol extracts, on all parameters, showed a highest activity of the aqueous extract on the primed estrogenized uterine muscle. When considering the phytochemical composition of the different plants extracts of B. pilosa extracts, all are composed of common chemicals such as phenylheptatryine, quercetin, ferulic acid, caffeic acid, flavone glycosides, and minerals. These compounds have been reported to acts severally and/or jointly on the biological activity of plant extracts (Brandao et al., 1997; Lic. Humberto et al., 2001). During the evaluation of the antioxidant activity of some natural compounds by (Usami et al., 2004), they found that the water-soluble phase showed stronger activity than the lipo-soluble phase, which was mainly due to the stronger effect of ferulic acid, caffeic acid and quercetin. Therefore, the biological active substances of B. pilosa leaf extracts could be essentially due to those compounds which present a higher uterotrophic activity in the aqueous extract.
In conclusion, B. pilosa's leaf extracts induced a biological agonist reaction on the uterine muscle, related to the presence of active compounds, which possess estrogenic-like and oxytocin-like activity. The aqueous extract showed a higher uterotrophic/estrogenic effect while the aqueous extract showed a higher uterotonic/oxytocic-like effect. Those characteristics are probably responsible for its empirical use as a labour enhanceing substance.The present work represents the first investigation of the uterotrophic and uterotonic effects of Bidens pilosa on the myometrial smooth muscle. However, further electrophysiological experiments are going on to clearly define the proposed signalling pathway(s) and phytochemical analysis is required to isolate active components responsible for the observed uterine muscle contractile effect.
Acknowlegments
The authors thank Mrs Huguette Crevel from the Laboratoire de Physiologie Cellulaire Respiratoire; INSERM U885 (Bordeaux, France) for her technical assistance, Dr Pascal Wafo from the department of chemistry (E.N.S. Yaounde) for plant extractions, and Mr Ebone Thomas for plant collection. F. Longo was a recipient of the French Cooperation Organism (EGIDE).
References
- 1.Alarcon-Aguilar FJ, Roman-Ramos R, Flores-Saenz JL, Aguirre-Garcia F. Investigation on the hypoglycaemic effects of extracts of four Mexican medicinal plants in normal and alloxan-diabetic mice. Phytother Res. 2002;16(4):383–386. doi: 10.1002/ptr.914. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez L, Marquina S, Villarreal ML, Alonso D, Aranda E, Delgado G. Bioactive polyacetylenes from Bidens pilosa. Planta Med. 1996;62(4):355–357. doi: 10.1055/s-2006-957902. [DOI] [PubMed] [Google Scholar]
- 3.Andersen HR, Bonefeld-Jorgensen EC, Nielsen F, Jarfeldt K, Jayatissa MN, Vinggaard AM. Estrogenic effects in vitro and in vivo of the fungicide fenarimol. Toxicol Lett. 2006;163(2):142–152. doi: 10.1016/j.toxlet.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Andrade-Neto VF, Brandao MG, Oliveira FQ, Casali VW, Njaine B, Zalis MG, Oliveira LA, Krettli AU. Antimalarial activity of Bidens pilosa L. (Asteraceae) ethanol extracts from wild plants collected in various localities or plants cultivated in humus soil. Phytother Res. 2004;18(8):634–639. doi: 10.1002/ptr.1510. [DOI] [PubMed] [Google Scholar]
- 5.Arteche E, Strippoli G, Loirand G, Pacaud P, Candenas L, Molto JC, Souto L, Fernandez J, Norte M, Martin JD, Savineau JP. An analysis of the mechanisms involved in the okadaic acid-induced contraction of the estrogen-primed rat uterus. J Pharmacol Exp Ther. 1997;282(1):201–207. [PubMed] [Google Scholar]
- 6.Balépa M, Fotso M & BB. Enquête démographique et de santé au Cameroun. 1992. pp. 67–80. (Fre). Chap. 5. [Google Scholar]
- 7.Brandao MG, Krettli AU, Soares LS, Nery CG, Marinuzzi HC. Antimalarial activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. J Ethnopharmacol. 1997;57(2):131–138. doi: 10.1016/s0378-8741(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 8.Burdette JE, Liu J, Lantvit D, Lim E, Booth N, Bhat KP, Hedayat S, Van Breemen RB, Constantinou AI, Pezzuto JM, Farnsworth NR, Bolton JL. Trifolium pratense (red clover) exhibits estrogenic effects in vivo in ovariectomized Sprague-Dawley rats. J Nutr. 2002;132(1):27–30. doi: 10.1093/jn/132.1.27. [DOI] [PubMed] [Google Scholar]
- 9.Calabrese EJ, Baldwin L. The hormonetic dose-response model is more common than the threshold model in toxicology. Toxicol Sc. 2003;71:246–250. doi: 10.1093/toxsci/71.2.246. [DOI] [PubMed] [Google Scholar]
- 10.Chang CL, Kuo HK, Chang SL, Chiang YM, Lee TH, Wu WM, Shyur LF, Yang WC. The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. J Biomed Sc. 2005;12(1):79–89. doi: 10.1007/s11373-004-8172-x. [DOI] [PubMed] [Google Scholar]
- 11.Chang SL, Chang CL, Chiang YM, Hsieh RH, Tzeng CR, Wu TK, Sytwu HK, Shyur LF, Yang WC. Polyacetylenic compounds and butanol fraction from Bidens pilosa can modulate the differentiation of helper T cells and prevent autoimmune diabetes in non-obese diabetic mice. Planta Med. 2004;70(11):1045–1051. doi: 10.1055/s-2004-832645. [DOI] [PubMed] [Google Scholar]
- 12.Chearskul S, Kooptiwut S, Chatchawalvanit S, Onreabroi S, Churintrapun M, Saralamp P, Soonthornchareonnon N. Morinda citrifolia has very weak estrogenic activity in vivo. Thai J Physiol Sc. 2004;17(1):22–29. [Google Scholar]
- 13.Chippaux JP, Rakotonirina VS, Rakotonirina A, Dzikouk G. Drug or plant substances which antagonize venoms or potentiate antivenins. Bull Soc Pathol Exot. 1997;90(4):282–285. [PubMed] [Google Scholar]
- 14.Circosta C, Pasquale RD, Palumbo DR, Samperi S, Occhiuto F. Estrogenic activity of standardized extract of Angelica sinensis. Phytother res. 2006;20:665–669. doi: 10.1002/ptr.1928. [DOI] [PubMed] [Google Scholar]
- 15.CSTR/OUA, author. Pharmacopée Africaine. 1íre Ed. Lagos: 1985. pp. 4–8. (Fre). [Google Scholar]
- 16.Diezi J. Toxicologie: Principes de base et répercussions cliniques dans Pharmacologie: des concepts fondamentaux aux applications thérapeutiques. Frisson-Roche. 1989:33–43. [Google Scholar]
- 17.Dimo T, Azay J, Tan PV, Pellecuer J, Cros G, Bopelet M, Serrano JJ. Effects of the aqueous and methylene chloride extracts of Bidens pilosa leaf on fructose-hypertensive rats. J Ethnopharmacol. 2001;76(3):215–221. doi: 10.1016/s0378-8741(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 18.Dimo T, Nguelefack TB, Tan PV, Yewah MP, Dongo E, Rakotonirina SV, Kamanyi A, Bopelet M. Possible mechanisms of action of the neutral extract from Bidens pilosa L. leaves on the cardiovascular system of anaesthetized rats. Phytother res. 2003;17(10):1135–1139. doi: 10.1002/ptr.1132. [DOI] [PubMed] [Google Scholar]
- 19.Dimo T, Rakotonirina S, Kamgang R, Tan PV, Kamanyi A, Bopelet M. Effects of leaf aqueous extract of Bidens pilosa (Asteraceae) on KCl-and norepinephrine-induced contractions of rat aorta. J Ethnopharmacol. 1998;60(2):179–182. doi: 10.1016/s0378-8741(97)00142-6. [DOI] [PubMed] [Google Scholar]
- 20.Drugs Guidelines, author. Medicinal Herbs in Traditional Herbal medicine. OTTAWA: 1995. Drugs Directorate Guidelines, traditional Herbal Medicine, and the related Drug Directorate Policy; pp. 2–16. [Google Scholar]
- 21.Features ACN, author. Table 1. EDSTAC's recommended T1 screening assays and possible alternatives. Amer Chem Soc. 1998:528 A–532 A. [Google Scholar]
- 22.Tafesse G, Mekonnen Y, Makonnen E. In Vivo and In Vitro Anti-fertility and Anti implantation of Leonotis ocymifolia in Rats. Afr J Trad CAM. 2005;2(2):103–112. [Google Scholar]
- 23.Gilbert B, Teixeira DF, Carvalho ES, De Paula AE, Pereira JF, Ferreira JL, Almeida MB, Machado, Rda S, Cascon V. Activities of the Pharmaceutical Technology Institute of the Oswaldo Cruz Foundation with medicinal, insecticidal and insect repellent plants. An Acad Bras Cienc. 1999;71(2):265–271. [PubMed] [Google Scholar]
- 24.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 25.Greim HA. The endocrine and reproductive system: adverse effects of hormonally active substances? Pediatrics. 2004;113(4):1070–1075. [PubMed] [Google Scholar]
- 26.Guidelines, author. Guidelines for the Used of Animals in Behavioural Projects in Schools. Amer Physiol Ass. 2003:1–2. [Google Scholar]
- 27.Hideki Wanibucchi, Jin seck kang, Elsayed I salim, Keiichirou M, Fukushima S. Toxicity vs. beneficial effects of phytoestrogens. Pure Applied Chemistry. 2003;75(11–12):2047–2053. [Google Scholar]
- 28.INS, author. Pauvreté et santé au Cameroun en 2001. 2001. 2í Enquête camerounaise auprís des ménages; pp. 1–6. (Fre). [Google Scholar]
- 29.Kamanyi A. Uterotonic, ileal and vascular smooth muscle effects of some extracts from the leaves of Musanga cecropioides (cecropiaceae) R.Brown; 1992. pp. 106–107. Doctorat d'Etat Thesis. [Google Scholar]
- 30.Kanno J, Onyon L, Haseman J, Fenner-Crisp P, Ashby J, Owens W. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ Health Perspect. 2001;109(8):785–794. doi: 10.1289/ehp.01109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanno J, Onyon L, Peddada S, Ashby J, Jacob E, Owens W. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dose-response studies. Environ Health Perspect. 2003b;111(12):1530–1549. doi: 10.1289/ehp.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keena McKillen S, Thornton, Taylor CW. Oxytocin increases the [Ca2+]i sensitivity of human myometrium during the falling phase of phasic contractions. Am J Physiol Endocrinol Metab. 1999;276(2):E345–E351. doi: 10.1152/ajpendo.1999.276.2.e345. [DOI] [PubMed] [Google Scholar]
- 33.Kim HS, Kang TS, Kang IH, Kim TS, Moon HJ, Kim IY, Ki H, Park KL, Lee BM, Yoo SD, Han SY. Validation study of OECD rodent uterotrophic assay for the assessment of estrogenic activity in Sprague-Dawley immature female rats. J Toxicol Environ Health Part A. 2005;68(23–24):2249–2262. doi: 10.1080/15287390500182354. [DOI] [PubMed] [Google Scholar]
- 34.Kim HS, Shin JH, Moon HJ, Kang IH, Kim TS, Kim IY, Seok JH, Pyo MY, Han SY. Comparative estrogenic effects of p-nonylphenol by 3-day uterotrophic assay and female pubertal onset assay. Reproduct Toxicol. 2002;16(3):259–268. doi: 10.1016/s0890-6238(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 35.Krettli AU, Andrade-Neto VF, Brandao MG, Ferrari WM. The search for new antimalarial drugs from plants used to treat fever and malaria or plants randomly selected: a review. Mem Inst Oswaldo Cruz. 2001;96(8):1033–1042. doi: 10.1590/s0074-02762001000800002. [DOI] [PubMed] [Google Scholar]
- 36.Kurowicka B, Franczak A, Oponowicz A, Kotwica G. In vitro contractile activity of porcine myometrium during luteolysis and early pregnancy: effect of oxytocin and progesterone. Reproduct Biol. 2005;5(2):151–169. [PubMed] [Google Scholar]
- 37.Lic Humberto , Lastra Valdés A, Lic Heidy Ponce de LR. Bidens pilosa Linné. Rev. cub plantas medicinales. 2001;1:28–33. (Fre). [Google Scholar]
- 38.Lopez Bernal A. Mechanisms of labour--biochemical aspects. Br J Obst Gynaecol. 2003;110(20):39–45. doi: 10.1046/j.1471-0528.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 39.Machado AF, Jakelaitis A, Ferreira LR, Agnes EL, Santos LD. Population dynamics of weeds in no-tillage and conventional crop systems. J Environ Sc Health Part B. 2005;40(1):119–128. doi: 10.1081/pfc-200034259. [DOI] [PubMed] [Google Scholar]
- 40.Muller A, Siemer J, Renner S, Hoffmann I, Beckmann MW, Dittrich R. Modulation of uterine contractility and peristalsis by oxytocin in estrogen-primed non-pregnant swine uteri. Eur J Med Res. 2006;11(4):157–162. [PubMed] [Google Scholar]
- 41.Nguelefack TB, Dimo T, Mbuyo EP, Tan PV, Rakotonirina SV, Kamanyi A. Relaxant effects of the neutral extract of the leaves of Bidens pilosa Linn on isolated rat vascular smooth muscle. Phytother Res. 2005;19(3):207–210. doi: 10.1002/ptr.1646. [DOI] [PubMed] [Google Scholar]
- 42.OECD, author. Detailed Background Review of the Uterotrophic Bioassay: Summary of the Available Literature in Support of the Project of the OECD Task Force on Endocrine Disrupters Testing and Assessment (EDTA) to Standardise and Validate the Uterotrophic Bioassay. Paris: OECD; 2003. (OECD Environment, Health and Safety Publication Series on Testing and Assessment. ENV/JM/MONO. N° 38(1)). [Google Scholar]
- 43.Oliveira FQ, Andrade-Neto V, Krettli AU, Brandao MG. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J Ethnopharmacol. 2004;93(1):39–42. doi: 10.1016/j.jep.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 44.Owens W, Ashby J, Odum J, Onyon L. The OECD program to validate the rat uterotrophic bioassay. Phase 2: dietary phytoestrogen analyses. Environ Health Perspect. 2003;111(12):1559–1567. doi: 10.1289/ehp.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owens W, Koëter HBWM. The OECD program to validate the rat uterotrophic bioassay: an overview. Environ Health Perspect. 2003;111(12):1527–1529. doi: 10.1289/ehp.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Telefo PB, Moundipa PF, Tchouanguep FM. Oestrogenicity and effect on hepatic metabolism of the aqueous extract of the leaf mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macranthus and Justicia insularis. Fitoter. 2002;73:472–478. doi: 10.1016/s0367-326x(02)00177-6. [DOI] [PubMed] [Google Scholar]
- 47.Pereira RL, Ibrahim T, Lucchetti L, Da Silva AJ, Gonçalves de Moraes VL. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacol. 1999;43(1):31–37. doi: 10.1016/s0162-3109(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 48.Revuelta MP, Hidalgo A, Cantabrana B. Involvement of cAMP and beta-adrenoceptors in the relaxing effect elicited by flavonoids on rat uterine smooth muscle. J Auton Pharmacol. 1999;19(6):353–358. doi: 10.1111/j.1365-2680.1999.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 49.Riley M, Baker PN, Tribe RM, Taggart MJ. Expression of scaffolding, signalling and contractile-filament proteins in human myometria: effects of pregnancy and labour. J Cell Mol Med. 2005;9(1):122–134. doi: 10.1111/j.1582-4934.2005.tb00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rojas JJ, Ochoa VJ, Ocampo SA, Munoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BMC CAM. 2006;6:2. doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Savineau JP, Mironneau J. An analysis of the action of phorbol 12, 13-dibutyrate on mechanical activity in rat uterine smooth muscle. J Pharmacol Exp Ther. 1990;255(2):133–139. [PubMed] [Google Scholar]
- 52.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. Ibadan: John Wiley & Sons; 1984. p. 44. [Google Scholar]
- 53.Tan PV, Dimo T, Dongo E. Effects of methanol, cyclohexane and methylene chloride extracts of Bidens pilosa on various gastric ulcer models in rats. J Ethnopharmacol. 2000;73(3):415–421. doi: 10.1016/s0378-8741(00)00290-7. [DOI] [PubMed] [Google Scholar]
- 54.Usami E, Kusano G, Katayose T, Wachi H, Seyama Y. Assessment of antioxidant activity of natural compound by water- and lipid-soluble antioxidant factor. Yakugaku Zasshi. 2004;124(11):847–850. doi: 10.1248/yakushi.124.847. [DOI] [PubMed] [Google Scholar]
- 55.W.H.O., author Progress in the characterization of venom and standardization of antivenoms. 1981;58:1–44. W.h.o. offset Publication. [PubMed] [Google Scholar]
- 56.Yamasaki K, Sawaki M, Noda S, Takatuki M. Effects of olive, corn, sesame or peanut oil on the body weights and reproductive organ weights of immature male and female rats. Exper An. 2001;50(2):173–177. doi: 10.1538/expanim.50.173. [DOI] [PubMed] [Google Scholar]
- 57.Yamasaki K, Takeyoshi M, Sawaki M, Imatanaka N, Shinoda K, Takatsuki M. Immature rat uterotrophic assay of 18 chemicals and Hershberger assay of 30 chemicals. Toxicology. 2003;183(1–3):93–115. doi: 10.1016/s0300-483x(02)00445-6. [DOI] [PubMed] [Google Scholar]