Abstract
Direct detection of saponins in medicinal plants using Fourier Transform Infrared (FTIR) spectroscopy is reported in this paper. Crude dry plant powders were mixed with potassium bromide (KBr) powder and compressed to a thin pellet for infrared examination. FTIR spectra of the test samples showed -OH, -C=O, C-H, and C=C absorptions characteristic of oleanane triterpenoid saponins. The C-O-C absorptions indicated glycoside linkages to the sapogenins. Phytochemical analysis confirmed the presence of saponins in the tested specimens. Entada leptostachya was used as a reference sample. Dry plant powder was extracted sequentially with hexane, dichloromethane, ethyl acetate and methanol. FTIR spectra of the reference sample powder and its organic solvent extracts showed characteristic saponin absorption peaks. These results indicated that direct detection of saponins in medicinal plants was possible by infrared analysis. Lengthy exhaustive chemical analyses necessary for detection of saponins could be avoided.
Keywords: Medicinal plants, saponins, infrared spectra, potassium bromide, glycoside
Introduction
Saponins are glycosides of triterpenes, steroids, and sometimes alkaloids, which occur primarily, but not exclusively in plants. The saponins can therefore be classified as steroidal, triterpenoidal or alkaloidal depending on the nature of the aglycone. The aglycone part of a saponin referred to as a sapogenin, while the glycone parts of the saponins are generally oligosaccharides. Oligosaccharides may be linked to the sapogenin through an ether or ester linkage at one or two glycosylation sites, giving the corresponding monodesmosidic or bidesmosidic saponins, respectively. Attachment of the glycone to three sites (tridesmosidic) in a sapogenin is rare (Natori et al., 1981).
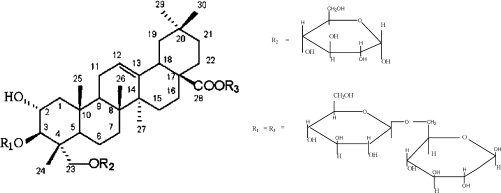
Many saponins are present in higher plants in the form of glycosides of complex alicyclic compounds and show characteristic foaming properties in aqueous solution. Many plants contain little or no saponin; in others, the triterpenoid saponins predominate. The triterpenes are subdivided into 20 groups, depending on their particular structures. The base structure found in the largest variety of medicinal plants is the oleanane-type triterpene (Figure 1). In fact isolated oleanolic acid, the basic oleanane triterpene, is a substance of therapeutic interest found in the Chinese herbal plant Panax japonicum (Liu, 1995).
Figure 1.
General chemical structure of Ginsenosides of the Oleanane class.
Investigation of 1730 species of 104 families of plants growing in the Central Asia found that tritepenoid saponins were present in 627 species, and steroidal saponins in 127 species (Table 1, Long et al., 1989). About 76% of plant families so far examined contain saponins, suggesting a wide distribution of saponins in the plant kingdom. The saponin-containing plants have been used for medical purposes. Among the Oriental drugs, many herbal drugs (Table 1) contain saponin as their principal constituents (Lacaille-Dubois M. A. et al., 1996; Osamu Tanaka, 1990). In addition, saponins are precursors of important therapeutic drugs such as cortisone, and contraceptive estrogens. Pharmacological activities associated with saponins include cytotoxicity, anti-tumor, anti-mutagenic, anti-inflammatory, anti-viral and cardiac activities (Lacaille-Dubois et al., 1996), in addition to anthelmintic activity (Watt and Brayer, 1962), among others.
Table 1.
Widely used Chinese herbs with oleanolic acid saponins as main active constituents (Long et al., 1989).
| Common Name | Constituent group | Content of saponins |
| Bupleurum | Saikosaponins | 1.2–4.7% |
| Ginseng | Ginsenosides | 2.2–5.2% |
| American ginseng | 6.2–7.4% | |
| Platycodon | Platycodins | >6% |
| Polygala | Onjisaponins | ∼2% |
Analyses of saponins in plants could be achieved by many methods (Okwu et al., 2006; Edeogu et al., 2005; Horbone, 1973; Long et al., 1989; Hsu et al., 1982; Marles et al., 1996). These methods involve extraction of plant matrix with aqueous or organic solvents prior to analyses. However, saponins in medicinal plants could be detected by the highly specific infrared absorption spectra of their sapogenins.
This procedure involves mixing a small amount of plant powder with potassium bromide salt, compressing the mixture to a pellet with subsequent recording of infrared spectrum using Fourier Transform Infrared (FTIR) spectrometer. Consequently, the detection for these valuable saponins in medicinal plants could be done in a short time. Furthermore, saponin-containing herbal drugs such as Ginseng could easily be identified and standardized using saponin as a marker phytochemical by recording FTIR spectra of the herbal powders. The latter can be accomplished without resulting to expensive and time consuming chemical methods.
In this study FTIR, spectroscopy was used to identify saponins in medicinal plants used by herbalists from Embu and Mbeere districts of Eastern province, Kenya. Phytochemical screening of saponins in the selected medicinal plants validated the FTIR technique.
Materials and methods
Collection and identification of plant specimens
The plant specimens were collected from Embu and Mbeere districts of Eastern Province, Kenya. A plant taxonomist from the East African Herbarium identified all the eleven plants. The voucher specimens were deposited in the Botany department laboratory of Jomo Kenyatta University of Agriculture and Technology. Authentic samples were air-dried under the shade and ground into a fine powder, and stored in airtight plastic containers.
Phytochemical screening
Saponin tests were carried out on the aqueous extracts using standard procedures of plant constituents' identification as described by Sofowora et al., (1978), Edeoga et al., (2005) and Horbone (1973). Three grams of each dry plant powder were weighed and extracted with 300 ml of hot distilled water in a beaker. After filtration, the aqueous extracts were cooled and stored in a refrigerator at 4°C until needed for the tests.
Five milliliters of each plant extract was placed into a test tube and diluted with 5 ml of distilled water. The mixture was shaken vigorously for two minutes. Persistent appearance of foam lasting for at least fifteen minutes or the forming of an emulsion when olive oil was added confirmed the presence of saponins.
Infrared Spectra
A little powder of plant specimen was mixed with KBr salt, using a mortar and pestle, and compressed into a thin pellet. Infrared spectra were recorded as KBr pellets on a Schimadzu FTIR Spectrometer 8000 series, between 4000 − 500 cm −1. Infrared absorptions were recorded in Tables 3 and 4.
Table 3.
FTIR spectra of medicinal plant powders (KBr disc), cm−1
| Albizia anthelmintica |
Rapanea Rhododendroides* |
Entada Leptostachya* |
Senna singueana |
Mytenus senegalensis |
Senna didmobotrya |
Terminalia brownii |
Vitex doniana |
Prunus africana |
| 3399 | 3316 | 3418 | 3394 | 3395 | 3396 | 3422 | 3429 | 3364 |
| 2923 | 2922 | 2926 | 2926 | 2925 | 2924 | 3360 | 2926 | 2924 |
| 2855 | 2853 | 1632 | 2854 | 2855 | 2855 | 2929 | 2864 | 2854 |
| 1737 | 1619 | 1457 | 1736 | 1738 | 1737 | 1736 | 1736 | 1740 |
| 1638 | 1380 | 1034 | 1622 | 1651 | 1622 | 1622 | 1648 | 1620 |
| 1559 | 1318 | 1510 | 1544 | 1515 | 1542 | 1517 | 1513 | |
| 1318 | 1038 | 1036 | 1514 | 1338 | 1505 | 1047 | 1457 | |
| 1035 | 1071 | 1458 | 1072 | 1318 | 1317 | |||
| 1256 | 1030 | 1037 | ||||||
| 1072 | 779 |
No C=O observed
Table 4.
FTIR spectra of reference sample and its organic extracts (KBr disc), cm−1
| Entada leptostachya (powder)** |
Entada leptostachya Hexane extract |
Entada leptostachya Dichloromethane extract |
Entada leptostachya Ethyl acetate extract |
Entada leptostachya Methanol Extract |
| 3418 | 3395* | 3371 | 3368 | 3383 |
| 2926 | 2924 | 2921 | 2927 | 2924 |
| 1633 | 2854 | 2850 | 2855 | 1701 |
| 1457 | 1736 | 1732 | 1719 | 1560 |
| 1561 | 1655 | 1638 | 1608 | 1542 |
| 1542 | 1542 | 1606 | 1509 | 1455 |
| 1511 | 1462 | 1462 | 1460 | 1243 |
| 1035 | 1422 | 1379 | 1043 | 1048 |
| 1378 | 1262 | |||
| 1168 | 1094 | |||
| 1097 |
Weak absorbance
No C=O peak observed
Results
Table 2 shows the phytochemical test results for saponins in the plants under investigation. Some of these plants were purported to have anthelmintic activity (Kareru et al., 2007).
Table 2.
Saponin Phytochemical test results in selected medicinal plants
| Plant Name |
Saponins |
| Terminalia Brownii | + |
| Entada leptostachya | + |
| Fagaropsis angolensis | + |
| Erythrina abyssinica | + |
| Senna didymobotrya | + |
| Maytenus putterkoides | + |
| Senna singueana | + |
| Harrisonia abyssinica | + |
| Vitex doniana | + |
| Prunus Africana | + |
| Albizia anthelmintica | + |
Key: + = positive
Discussion
Phytochemical analyses of selected medicinal plants gave positive results for saponins. These results were confirmed by infrared absorptions recorded. Saponins showed characteristic infrared absorbance (Table 3) of the hydroxyl group (-OH) ranging from 3429 cm−1 (Vitex doniana) to 3316 cm−1 (Rapanea rhododendroides); C-H ranging from 2922 cm−1 (Rapanea rhododendroides) to 2929 cm−1 (Terminalia brownii); C = C absorbance ranging from 1619 cm−1 (Rapanea rhododendroides) to 1651 cm−1 (Maytenus senegalensis); C=O ranging from 1740 cm−1 (Prunus africana) to 1736 cm−1 (Senna singueana). Oligosaccharide linkage absorptions to sapogenins, that is C-O-C, were evident between 1034 cm−1 (Entada leptostachya) to 1072 cm−1 (Maytenus senegalensis and Senna didymobotrya).
The above infrared functional group absorptions characteristic of saponins were cited in literature (Toshiyuki et al., 2001; Bernadete P. da Silva et al., 2002; Meltem et al., 2002; Kirmizigul et al., 2002; Sengmin et al., 2001; Evangelista et al., 2002; Natori et al., 1981; and Toshiyuki et al., 2001), among others. The -OH, C-H, C=C, C=O and C-O-C found in Albizia anthelmintica, Senna singueana, Maytenus senegalensis, Senna didymobotrya, Terminalia brownii, and Prunus africana are suggestive of oleanane triterpenoid saponins (Kirmizigul et al., 2002; Evangelista et al., 2002; Toshiyuki et al., 2001). These oleanane-type triterpenoid saponins are characterized by the C=O infrared absorbance due to oleanolic acid/ester. Such triterpenoid saponins are also likely to be bidesmosides (Toshiyuki et al., 2001; Kirmizigul et al., 2002; and Natori et al., 1981) since they have two attachments of glycones (i.e. glycosidic and ester groups) to the sapogenin. In this study, saponins detected in Albizia anthelmintica, Senna singueana, Maytenus senegalensis, Senna didymomotrya, Terminalia brownii, and Prunus africana, were likely to be bidesmosidic, oleanane-type triterpenoids, while those detected in Entada leptostachya and Rapanea rhododendroides might be monodesmosidic saponins since ester linkages were not detected by FTIR. However, these observations need clarification using nuclear magnetic and mass spectroscopy.
The FTIR spectra of Entada leptostachya-organic solvent extracts exhibited characteristic triterpenoid saponin absorptions. Similarly, the crude powder of Entada leptostacya exhibited almost similar absorbances to those of its organic solvents (Table 4). This indicated that saponins were directly detectable in the crude powder of the former medicinal plant. The importance of this observation was that saponins could be detected in crude medicinal plant powders by FTIR spectroscopy, thus saving time and the need for extraction prior to analysis.
Conclusions
Direct detection of saponins in medicinal plants can be performed directly on crude sample powders using FTIR spectroscopy. The method is simple, fast, and economical.
Acknowledgements
This research was funded by the African Institute for Capacity Development (AICAD), and Jomo Kenyatta University of Agriculture and Technology, and is highly appreciated. Thanks to Tom Odera for recording FTIR spectra.
References
- 1.da Silva Bernadete P., de Sousa Ac, Silve G M, Mendes T P, Parente J P. Bioactive steroidal saponin from Agave attenuate. Z Naturforch. 2002;57c:423–428. doi: 10.1515/znc-2002-5-603. (Ger). [DOI] [PubMed] [Google Scholar]
- 2.Edeoga H O, Okwu D E. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685–688. [Google Scholar]
- 3.Evangelista L, Teixeira de Sousa Filho P, Bastida J, Guillermo Schmeda-Hirschmann G. Saponins from Cariniana rubra (Lecythidaceae) Bol Soc Chil Quim. 2002;47(4) Concepcion dic. [Google Scholar]
- 4.Horbone J B. Phytochemical methods: A Guide to modern techniques of plant analysis. London: Chapman and Hall; 1973. p. 279. [Google Scholar]
- 5.Hsu H Y, Chen Y P, Hong M. The Chemical Constituents of Oriental Herbs. Long Beach, CA: Oriental Healing Arts institute; 1982. [Google Scholar]
- 6.Kareru PG, Kenji G M, Gachanja A N, Keriko J M, Mungai G. Traditional Medicines and Healing Methods among Embu and Mbeere Peoples of Kenya. Afr J Trad CAM. 2007;4(1):75–86. doi: 10.4314/ajtcam.v4i1.31193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirmizigul S, Anil H. New Triterpenic saponins from Celphalaria transsylvanica. Turk J Chem. 2002;26:947–954. [Google Scholar]
- 8.Lacaille-Dubois M A, Wagner H A. A review of the biological and pharmacological activities of saponins. Phytomedicine. 1996;2(4):363–386. doi: 10.1016/S0944-7113(96)80081-X. [DOI] [PubMed] [Google Scholar]
- 9.Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacology. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 10.Long Q Q. Total saponin contents of the root of wild and cultivated Platycodon grandiflorum. J Chinese Medicinal Materials. 1989;12(3):37–38. [Google Scholar]
- 11.Natori S, Ikekawa N, Suzuki M, editors. Extraction and Isolation of Biologically Active Compounds. Tokyo 112, Japan: Kodansha Ltd; 1981. Advances in Natural Products Chemistry; pp. 275–287. [Google Scholar]
- 12.Marles R J, Farnsworth NR. Antidiabetic plants and their active constituents: an update. Protocol J Botanical Medicine. 1996;1(3):85–137. doi: 10.1016/S0944-7113(11)80059-0. (reprinted from Phytomedicine (1996): 2(1) [DOI] [PubMed] [Google Scholar]
- 13.Ozipek M, Saracoglu I, Ogihara Y, Callis I. Nautigenin-Type steroidal saponins from Veronica fuhsii and V. multifida. Z Naturforsch. 2002;57c:603–608. doi: 10.1515/znc-2002-7-809. (Ger). [DOI] [PubMed] [Google Scholar]
- 14.Okwu D E, Josiah C. Nigerian medicinal plants. Afr J Biotechnol. 2006;5(4):357–361. [Google Scholar]
- 15.Tanaka Osamu. Ginseng saponins from Panax species. Pure and Appl Chem. 1990;62(7):1281–1284. [Google Scholar]
- 16.Sengmin S, Shilong M, Aina L, Zhongliang C, Chi-Tang H. Steroidal saponins from the seeds of Allium tuberrosum. J Agric Food chem. 2001;49:1475–1478. doi: 10.1021/jf001062b. [DOI] [PubMed] [Google Scholar]
- 17.Sofowora E A, Odebiyi O O. Phytochemical screening of Nigerian medicinal plants. Nig J Pharm. 1978 May–Jun;:25–32. [PubMed] [Google Scholar]
- 18.Murakami T, Kohno K, Matsuda H, Yashikawa M. Structures of Oleanane-Type Triterpene Oligo-glycosides from Green Peas (Pisum stivum L.) Chem Pharm Bull. 2001;49(1):73–77. doi: 10.1248/cpb.49.73. [DOI] [PubMed] [Google Scholar]
- 19.Watt, Brayer . The Medicinal and Poisonous plants of Southern and Eastern Africa. 2nd edition. E and Livingstone Ltd; 1962. pp. 554–555. [Google Scholar]