Abstract
Nuclear architecture and chromatin geography are important factors in the regulation of gene expression, as these components may play a vital epigenetic role both in normal physiology as well as in the initiation and progression of malignancies. Using a modification of the chromosome conformation capture (3C) technique, we examined long-range chromatin interactions of the imprinted human IGF2 gene. We demonstrate that numerous intrachromosomal interactions occur along both parental alleles in normal tissues, where the IGF2 is paternally expressed, as well as in normal liver where gene expression is biallelic. Long-range and allele-specific interactions occur between the IGF2/H19 imprinting control region-1 (ICR1) and ICR2, a region which regulates an imprinted gene cluster nearly a megabase distant from IGF2. Loss of genomic imprinting is a common epigenetic event in cancer, and long-range interactions have not been examined in malignant cells. In cancer cell lines in which IGF2 imprinting is maintained (MOI), essentially all of the 3C interactions seen in normal cells were preserved. However, in cells in which IGF2 imprinting was lost (LOI), nearly all of the long-range chromatin interactions involving IGF2 were abrogated. A three-dimensional computer model depicts the physical interactions between the IGF2 promoter and ICR1 in MOI cells, while the model of LOI lung cancer cells is flattened with few long-range interactions. This dramatic change in the three-dimension configuration of the chromatin at the IGF2 locus in LOI cancer cells suggests that the loss of imprinting may lead to a variety of changes in gene expression in addition to changes in IGF2 transcription.
INTRODUCTION
The genes encoding insulin-like growth factor 2 (IGF2) and H19 are located ∼120 kb apart on human chromosome 11p15.5 and are reciprocally imprinted, leading to paternal expression of IGF2 and maternal expression of H19 in most tissues (1–6). Parental allelic expression is determined by an imprinting control region (ICR) located between the two genes, upstream of the H19 promoter. The current model for the mechanism underlying this reciprocal imprinting proposes a critical role for CTCF, which binds to the unmethylated maternal ICR. In the mouse, it has been shown that CTCF serves as a strategic protein that implements DNA loops (7,8) and helps silence DNA regions by binding and recruiting the polycomb repressive complex 2 (9). Using chromosome conformation capture (3C) methodology (10), Reik et al. demonstrated that the Igf2 differentially methylated region-2 (DMR2) loops out to interact with the distant methylated Igf2/H19 ICR, thereby pushing the Igf2 promoter into close contact with the H19-enhancer, which lies ∼100 kb downstream. This interaction results in Igf2 transcription from this allele. In contrast, on the maternal chromosome, DMR1 interacts with the unmethylated ICR via CTCF binding, partitioning the Igf2 promoter into a silent loop, inhibiting Igf2 and promoting H19 transcription (11). A recent modification of the maternal chromosome model posits a tighter Igf2 silencing loop of ∼25 kb formed by DMR1 and a matrix attachment region (MAR3) downstream of Igf2, while the active maternal H19 is embedded in an active chromatin hub (12). We previously extended this model to show how this inhibitory loop assumes a three-dimensional (3D) knot conformation, sequestering inter-genic and distal enhancers away from the maternal Igf2 promoter (13). While the epigenetic regulation of mouse Igf2 has been extensively studied, no studies have examined long-range interactions and chromosome looping in human IGF2.
IGF2 encodes the mitogenic, anti-apoptotic peptide IGF-II that is over-expressed in many malignant tumors. One of the seminal discoveries in epigenetics was the demonstration that there is frequently loss of IGF2 imprinting (LOI) in many tumors (14,15). Oncogenesis is also enhanced in animal models in which there is loss of Igf2 imprinting (16,17), and chimeric mice derived from embryonic stem cells in which all imprints have been ablated also develop multiple tumors (18). Mice with Igf2 loss of imprinting have also been reported to have a greater sensitivity to IGF-II signaling (19).
In theory, IGF2 LOI would lead to a doubling of IGF2 mRNA abundance, and, presumably, a 2-fold increase in IGF-II production. However, this stoichiometry does not always occur. In Wilms' tumors, we found a significant increase (>5-fold) in IGF2 mRNA expression in tumor tissues compared with the normal adjacent kidney tissue regardless of the IGF2 imprinting or the chromosome 11p15 heterozygosity status (20). LOI did not lead to an even greater abundance of IGF2 mRNA. However, LOI may occur as an early step in oncogenesis in both human tumors and in experimental murine models (17,21). The loss of differential methylation at the IGF2 imprinting control region (ICR1), located just upstream of the H19 promoter, correlates with LOI in Wilms tumors (22), bladder cancer (23) and colon cancer (24,25). Changes in CpG methylation in other regions may also be associated with LOI in colon cancer. We have only a rudimentary understanding of the epigenetic mechanisms underlying imprinting or LOI of the human gene, and we do not really know if, or understand why, LOI should lead to cancer.
The presence of elevated IGF-II levels is not in and of itself oncogenic. In a mouse tumor model of widespread LOI, over-expression of Igf2 was not sufficient to induce a high rate of spontaneous transformation even though it led to an increased growth rate (18). In transgenic mice which over-express Igf2 by 30-fold (26), tumors do not develop until senescence, yet IGF2 LOI is the most common epigenetic abnormality in Wilms tumor, a common early childhood malignancy, as well as in adult tumors like colorectal cancer. Moreover, the epigenetic status of the gene may not correlate with the circulating levels of IGF-II. In Russell–Silver syndrome, the ICR is often biallelically unmethylated, allowing CTCF binding to both alleles, thereby decreasing IGF2 transcription; yet these children do not have low levels of circulating IGF-II (27). While IGF2 often acts in a paracrine or autocrine fashion where it is not possible to assess protein abundance, the aforementioned discrepancies indicate that the epigenetic configuration that regulates imprinting may not always relate in a linear fashion to gene transcription and translation.
Instead, it is likely that IGF2 LOI predisposes to carcinogenesis not (only) by increasing levels of the mitogenic IGF-II peptide, but by additional processes that occur as a result of the disruption of the imprinting process, perhaps by altering other epigenetic inter-relationships. Gene regulation through long-range intra- and interchromosomal interactions may be a relatively common event (28). Intra-chromosomal looping has been described for several genes, including the Dlx5/Dlx6 imprinted dyad (29), immune genes (30) and the immunoglobin heavy-chain locus (31). A glucocorticoid response element proximal to the Lcn2 promoter regulates both the Lcn2 gene and the distal Ciz1 gene (32). The co-regulated human globin genes are frequently found to be in spatial proximity when they are transcriptionally active (33). Two groups have recently shown that a transient co-localization of the X-inactivation centers of the two X chromosomes is required for proper initiation of X-inactivation (34,35). There is an interchromosomal interaction between the promoter region of the IFN-γ gene on chromosome 10 and the TH2 cytokine locus on chromosome 11 that regulates gene transcription (36). We have shown that Igf2 also participates in interchromosomal regulatory interactions with the Nf1 gene in a non-stochastic allele-specific manner (7).
The network of long-range interactions that form as the cell emerges from mitosis will impose limitations and restrictions on the formation of other chromatin interactions, epigenetically limiting the cell. Thus, if the cancer cell makes or loses one chromatin interaction, new, abnormal interactions may ensue, leading to multiple changes in the physiology of that cell.
In this paper, we assess the intrachromosomal interactions of the IGF2/H19 imprinted domain in human tissues, and compare this structure with that seen in the mouse. In order to examine the IGF2 long-range intrachromosomal interactions, we have devised a novel chromosome conformation capture-original copy (3C-OC) assay to analyze global interactions across the 316 kb TH-INS-IGF2-H19-LSP1 gene region. In addition, we demonstrate that the IGF2/H19 ICR1 physically interacts with a second imprinting domain (ICR2), 887 kb upstream of ICR1. We assess these long-range interactions in cancer cells, comparing those cell lines in which IGF2 imprinting is lost (LOI) with those in which it is maintained (MOI), and we demonstrate that there is a remarkable disruption in intrachromosomal interactions when IGF2 imprinting is lost. Finally, we determine the relative interaction frequency (IF) of various domains within the IGF2/H19 by real-time quantitative PCR (Q-PCR) and illustrate a computer-generated 3D model showing the spatial chromatin organization of the 162 kb IGF2/H19 region.
RESULTS
Demonstration of intrachromosomal interactions with the IGF2/H19 imprinting control region (ICR1)
We examined intrachromosomal interactions on human chromosome 11p15 in various tissues including normal fetal tissues (liver, kidney and placenta), adult livers (35–62 year old), normal cell lines (fibroblast and fetal skin cell) and three cancer cell lines, including cells that showed maintenance or loss of imprinting (MOI or LOI) of IGF2 (Table 1). IGF2 is transcribed from the paternal allele in normal fetal liver, kidney and placenta in both mouse and in human (37,38). Maintenance of IGF2 imprinting has been demonstrated in HFB1 fetal skin cells and three cancer cell lines (HCT 116, H 146 and MCF 7) while three other cancer cell lines (HT 29, H 522 and WTCL) demonstrated LOI of IGF2 (39). IGF2 is expressed biallelically in human adult livers because of the predominant transcription from the non-imprinted promoter P1, while promoters P2, P3 and P4 retain normal IGF2 imprinting (40). Thus the molecular ‘imprint’ is present in adult liver, but most of IGF2 gene expression is driven from non-imprinted promoters in adult hepatic tissue. We used the chromosome conformation (3C) assay and a panel of oligonucleotide primers (Supplementary Material, Table S1) to examine intra-chromosomal interactions of the IGF2/H19 gene dyad in greater detail, defining how each portion of this chromosomal region interacts with nearby and distant chromatin segments.
Table 1.
Human tissues and cell lines used in this study
| Tissues, cell type | Source, ATCC# | R.Enzymea | IGF2b | 3C data | |
|---|---|---|---|---|---|
| Normal human tissues | |||||
| Fetal kidney | First tri-semester | CLHET | N/A | MOI | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| Fetal liver | First tri-semester | CLHET | N/A | MOI | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| Placenta | First tri-semester | CLHET | N/A | MOI | Figs 2–6 |
| Adult liver, f62 | Female, 62 year old | Stanford | N/A | ‘LOI’ | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| Adult liver, f56 | Female, 56 year old | Stanford | N/A | ‘LOI’ | Supplementary Material, Figs S1 and S2 |
| Adult liver, f35 | Female, 35 year old | Stanford | N/A | ‘LOI’ | Supplementary Material, Figs S1 and S3 |
| Normal human cell lines | |||||
| GM 00498 | Skin fibroblast | Coriell | N/A | MOI | Fig. 1 |
| HFB 1 | Fetal skin fibroblast | Our lab. | Apa 1 | MOI | Fig. 5 |
| Cancer cell lines | |||||
| HCT 116 | Colon cancer | CCL-247 | Apa 1 | MOI | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| H 146 | Lung cancer | HTB 173 | Apa 1 | MOI | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| HT29 | Colon cancer | HTB 38 | Apa 1 | LOI | Figs 1–4, Supplementary Material, Figs S3 and S4 |
| H 522 | Lung cancer | CRL-5810 | Apa 1 | LOI | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| WTCL | Kidney, Wilms' tumor | Giftc | Hha1 | LOI | Figs 2–4, Supplementary Material, Figs S3 and S4 |
| MCF 7 | Breast cancer | HTB 22 | Alu 1 | MOI | Supplementary Material, Figs S1 and S2 |
| K 562 | CM-leukemia | CCL-243 | ? | Supplementary Material, Figs S1 and S2 | |
aPolymorphic restriction enzymes used to determine the imprinting status of IGF2 in HFB 1 and cancer cell lines.
bNormal IGF2 imprinting (MOI) has been reported in normal tissues.
Adult livers show apparent LOI (‘LOI’) due to the usage of biallelically expressed promoter P1
cA gift from Dr B. Tycko.
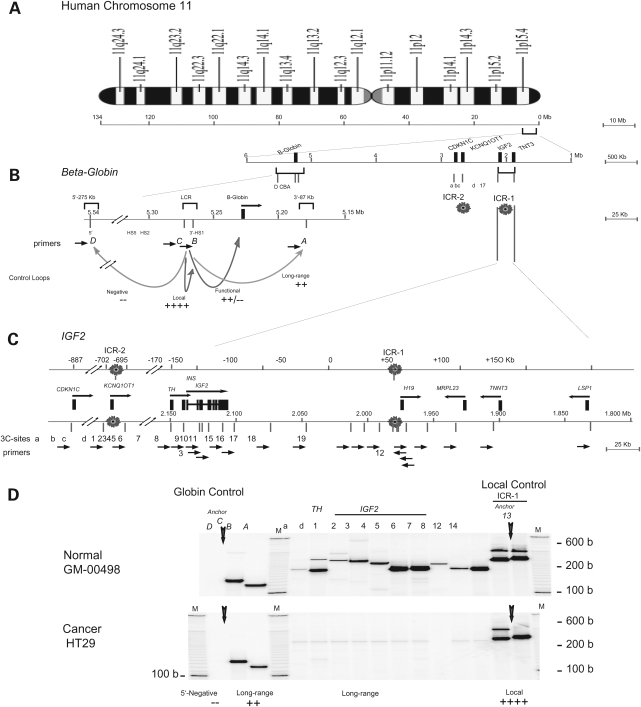
In both the normal fibroblast cell line GM-00498 (MOI) and in the colon cancer cell line HT29 (LOI), we observed strong local interactions from the imprinting control region (anchor site 13) within the IGF2/H19 locus (Fig. 1D, columns 12 and 14). Multiple interactions between anchor site 13 in ICR1 and other sites, including sites within the IGF2 gene (sites d, 1 through 8) and site ‘a’ in the ICR2 region, were evident in the normal GM-00498 cell line (Fig. 1D, upper panel). Each 3C product was verified by its unique size that comprises the sum of two DNA fragments linked by 3C ligation (Supplementary Material, Table S2). In contrast, in the HT29 colon cancer cell line, while local interactions within the ICR1 are similar to those in non-malignant cells, the long-range interactions from the ICR1 to the CDKN1C-TH-INS-IGF2 region (sites a, d, 1–8) are no longer detected (Fig. 1D, lower panel). The control beta-globin locus shows the expected, strong local LCR interaction from anchor site C (Fig. 1D, column B), a strong long-range interaction (Fig. 1D, column A) and a negative 5′ interaction (Fig. 1D, column D) in both MOI and LOI cells. These data indicate that long-range interactions in the IGF2/H19 locus are greatly modified or attenuated in colon cancer HT29 cells.
Figure 1.
IGF2 long-range chromosomal interactions in normal fibroblasts and in the HT29 cancer cell line. (A) Human chromosome 11. IGF2/H19 and beta-globin are located within 4 Mb on chromosome 11p15. Two imprinting domains, IGF2/H19 (ICR1) and KCNQ1OT1 (ICR2), are within ∼700 kb of each other. (B) Beta-globin 3C control. Within the globin locus, the locus control region (LCR) interacts with the beta-globin promoter in globin expressing cells, forming a functional active loop [C]-Beta-globin. LCR also interacts with the 3′ HS1 (DNAse I hypersensitivity 1) in normal cells, forming a ‘long-range’ [C-A] (87 kb) loop. A strong local interaction [C-B] (HS5–HS2) serves as a positive control while the absence of interaction between LCR and the 5′ region, [C-D], serves as a negative control. (C) Map of the IGF2/H19 locus. Top line: distance from ICR1 in kilobase. Two imprinting control regions, ICR1 and ICR2, are 700 kb apart. Bottom line: Map of the IGF2/H19 locus and location of 3C primers (Supplementary Material, Table S1). Forward primers (a, d, 1 through 8) and reverse ICR primers (12–14) were used to detect long-range and local chromosome interactions. (D) Chromosomal interactions in GM-00498 and HT29 cells. Control 3C interactions in globin region consists of anchor primer C (vertical arrow) and one of the forward primers A, B and D. Local C-B and long-range C-A interactions were detected in (B) and (A), respectively. Absence of C-D product denotes a negative 3C control in these cells. In the IGF2/H19 region, anchor primer 13 (vertical arrow) formed local 3C products (13-12 and 13-14) in panels 12 and 14 in both GM-0498 and HT29 cells. In GM-00498, but not in HT29 cells, anchor primer 13 in combination with each primers a, d, 1 through 8 formed specific 3C products of expected sizes (Supplementary Material, Table S2). M, 100 b and 10 b DNA markers.
Local and long-range interactions in the vicinity of the CTCF-binding site of the H19 DMR
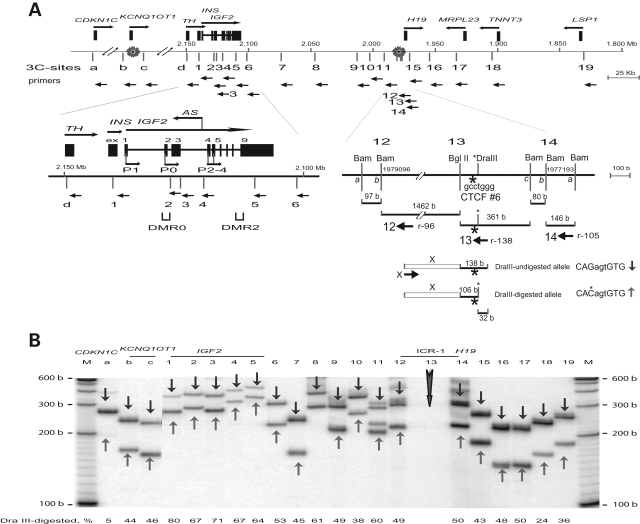
To further examine the various chromosomal interactions in normal and malignant cells, we investigated 3C interactions from multiple target sites in this gene locus in various cell lines and tissues in finer detail. In particular, we were interested in learning if differences in long-range interactions were a general feature of malignancy or were associated with imprinting status. As numerous strong local interactions were detected in both normal and cancer cell lines, we devised a multiplex PCR consisting of two or three anchor primers that allows the validation of amplification efficiency across the PCR panels.
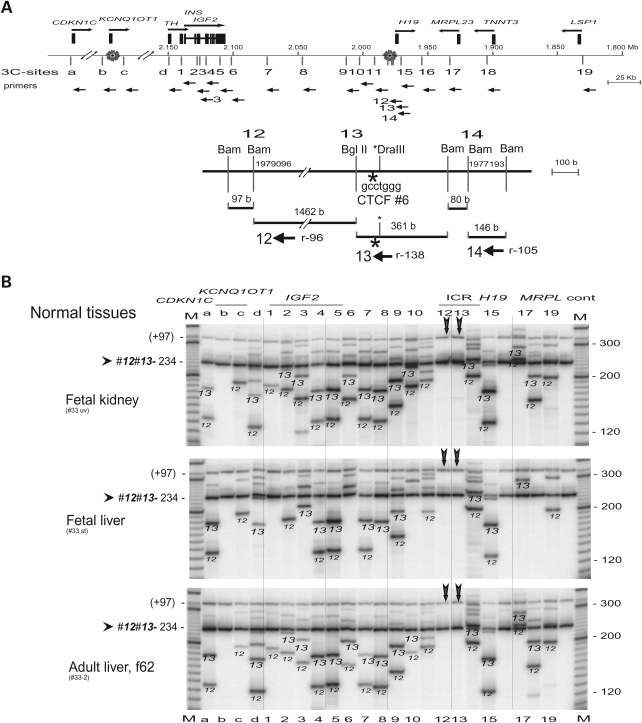
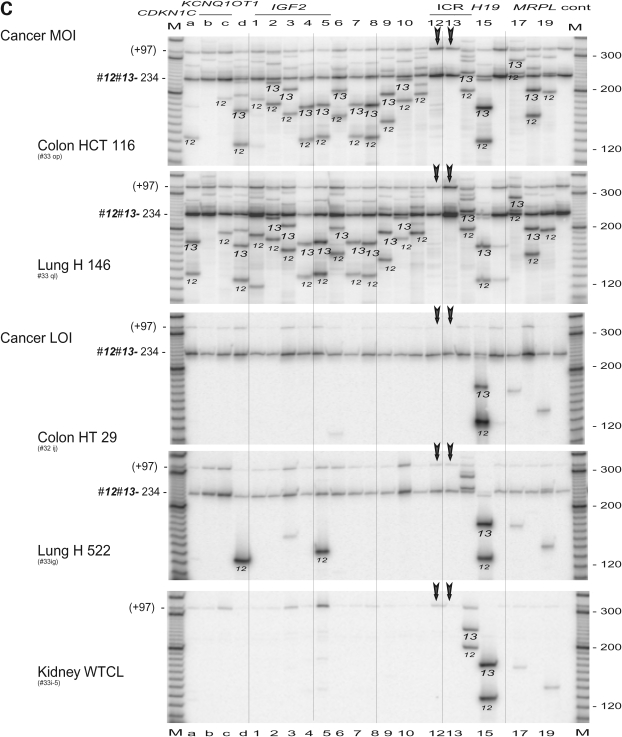
The H19 DMR within ICR1 plays a vital role in the imprinting of IGF2/H19 in human and mouse. In the mouse, binding of CTCF to the unmethylated H19 DMR has been proposed to result in silencing of the maternal Igf2 (41). The human H19 DMR harbors CTCF-binding site 6 that is located near sites 12, 13 and 14 (Fig. 2A). We performed 3C scanning across the ICR1/ICR2 region using double anchor primers 12 (r96, i.e. reverse orientation, 96 b from the restriction site) and 13 (r138). Primer 13 was located in the BglII–BamHI fragment that contains the CTCF 6 binding site, while primer 12 was 1.5 kb upstream of primer 13 (Fig. 2A). The anchor primer set 12 and 13 yielded a universal, local interaction of 234 b (96 b + 138 b) product across the CDKN1C/IGF2/H19 region in normal tissues and in MOI cancer cell lines (Fig. 2B and C). In addition to the common 234 b product, each set of anchor primers produced specific 3C products that were verified by their identification (ID) sizes (example shown in Supplementary Material, Table S2) and were marked by the anchor primer origin (12 or 13) across the panel (Fig. 2B and C). The fetal tissues, adult liver and MOI cancer cells showed a similar (or universal) pattern of interactions that includes a unique pattern of interactions close to ICR1 and long-range interactions up to ICR2 (Fig. 2B and C). The ‘signature’ banding pattern across the gels reflects the unique size of 3C products at each interaction location. In contrast, LOI cancer cell lines showed fewer bands of interaction, including fewer products of both of the local interactions while a local interaction with the H19 enhancer site 15 was predominant in all cell types (Fig. 2B and C). This indicates disruption of chromosomal structure near the CTCF binding site.
Figure 2.
Scanning analysis of chromosomal interactions from CTCF-binding region of the H19 DMR. (A) Map of the two imprinted domains. A complete set of reverse primers (orientation by horizontal arrows) was employed in this experiment. Bottom, detailed restriction map of CTCF-binding site 6. Primers are indicated by orientation (r, reverse) and the distance to the symmetric center of the target restriction sites. (B) 3C interactions in normal fetal tissues and adult liver. The anchor primers 12 and 13 (vertical arrows) were used in combination with each target primer shown on top of the panel. Each column shows 3C products from anchor primers (12 or 13, as marked) along with the common 3C product (12–13, 96 b + 138 b = 234 b) that is marked by an arrow head. A minor band (+97) is likely derived from a stuffer 97 b BamHI—BamHI fragment upstream of site 12. Columns 12, 13 and control (cont) depict only common 3C products. M, marker. (C) 3C interactions in cancer cell lines with maintenance (MOI) or lack (LOI) of imprinting. See legend in (B). Note sporadic detection of 3C products 12-d and 12-5 in H522 cells, and retention of 3C products in the H19 enhancer region (column 15) in all tissues and cell lines including the LOI cancer cells.
Long-range chromosomal interactions between the two imprinted regions ICR1 and ICR2
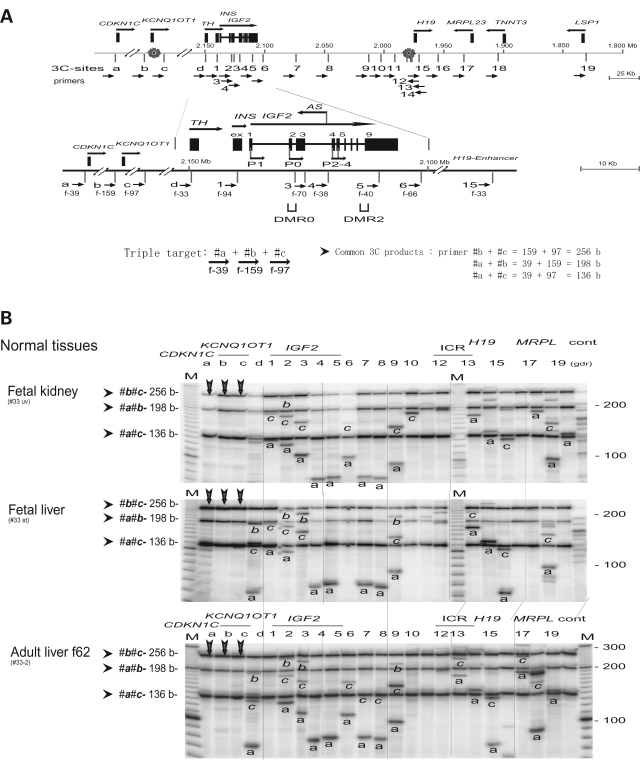
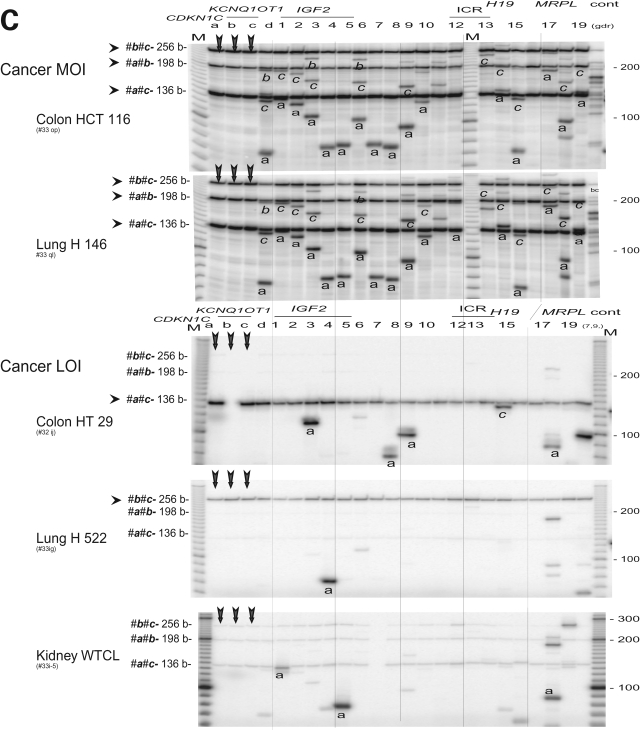
Figure 2B and C demonstrate specific 3C products from ICR1 (sites 12 and 13) and the imprinting control region-2 (ICR2, sites a and c) in cells in which IGF2 is imprinted. These products were further verified by restriction mapping, which indicated a potential interaction between the two imprinting control domains that are >800 kb apart. Three anchor primers (a, b and c) in the vicinity of CDKN1C and KCNQ1OT1 (ICR2) were used to scan the interaction between ICR2 and the IGF2/H19 gene cluster (Fig. 3A). The use of three anchor primers allows the detection of six 3C products in each lane. Three products represent ‘local’ interaction within the ICR2 while the other three are specific by its anchor origin (Fig. 3B and C).
Figure 3.
Interactions between the two imprinting control regions. (A) Map of the IGF2/H19 locus as shown in Fig. 1C. Bottom, details of the TH-INS-IGF2 locus. IGF2 is transcribed from five promoters (P1, P0 and P2–P4) across nine exons (ex 1–9). The IGF2 DMR is located in exon 9. Three anchor primers a, b and c result in three PCR products (a-b, a-c and b-c); their sizes are determined by the sum of two specified DNA segments. (B) 3C interaction in fetal tissues and adult liver. The anchor primers a, b and c (vertical arrows) show common 3C products and specific products from each anchor primers (marked as a, b and c). Columns a, b and c only contain the triple anchor primers. Cont, control of multiple 3C (unspecified). M, marker. (C) 3C interaction in cancer MOI and LOI cells. Legends are in (B). Note a failed PCR (panel HT29, column ‘b’) that represents <2% of all reactions in our PCR assays.
Within the ICR2, ‘local’ interactions include the interactions between the two imprinted genes, CDKN1C and KCNQ1OT1, (a-b, 185 kb apart; and a-c 192 kb apart) and the interaction within the KCNQ1OT1 gene (b-c, 7 kb apart). These interactions were evident in all MOI cells and in all normal tissues, including adult livers (Fig. 3B, C and Supplementary Material, Fig. S1).
The interaction between the two imprinting control regions, ICR1and ICR2, is demonstrated by patterns of specific 3C products that were identified by their sizes and were marked by their anchor origin (a, b or c) across the panel. Strong 3C interactions between CDKN1C (anchor primer a) and IGF2 (primers d, 1 through 9) were detected. Anchor primers b and c showed a similar (albeit weaker) pattern of 3C interaction. Similar results were obtained in fetal kidney, fetal liver, adult livers, placenta, breast MCF7 cells and leukocyte K562 cells (Fig. 3B, C and Supplementary Material, Fig. S1). In contrast, three LOI cancer cell lines (HT29, H522 and WTCL) show only traces of 3C products (a-c, b-c and a-b) within ICR 2 and sporadic 3C products between CDKN1C and IGF2 (Fig. 3C, anchor primer a). Overall the results indicate global loss of 3C interaction within ICR 2 and loss of interaction between the two imprinted regions in all three LOI cancer cell lines.
Although our 3C data indicate the presence of a physical interaction between the two imprinting control regions ICR1 and ICR2, we do not yet know the role and allele specificity, if any, of these interactions in the regulation of the two imprinting domains in human. In the mouse, we (unpublished data) and others (42) did not detect any physical interaction between the two imprinting control regions, and it has been shown that deletion of ICR2 does not affect the imprinting of the mouse Igf2/H19 genes (43).
It is important to note that the chromosomal interactions in adult liver, where the majority of the IGF2 is expressed from both parental alleles, is identical to the interactions seen in the tissues with mono-allelic expression (MOI). This maintenance of 3D architecture despite biallelic expression in adult liver suggests that an imprint is still present in this tissue. This imprint presumably maintains the short- and long-range intrachromosomal interactions. It is possible that these interactions themselves may actually constitute or be the physical manifestation of the imprint. We propose that it is only when the imprint is lost, as in some cancer cells, that biallelic expression is associated with loss of normal chromatin interactions.
Global loss of long-range interaction from the IGF2 promoters in LOI cancer cells
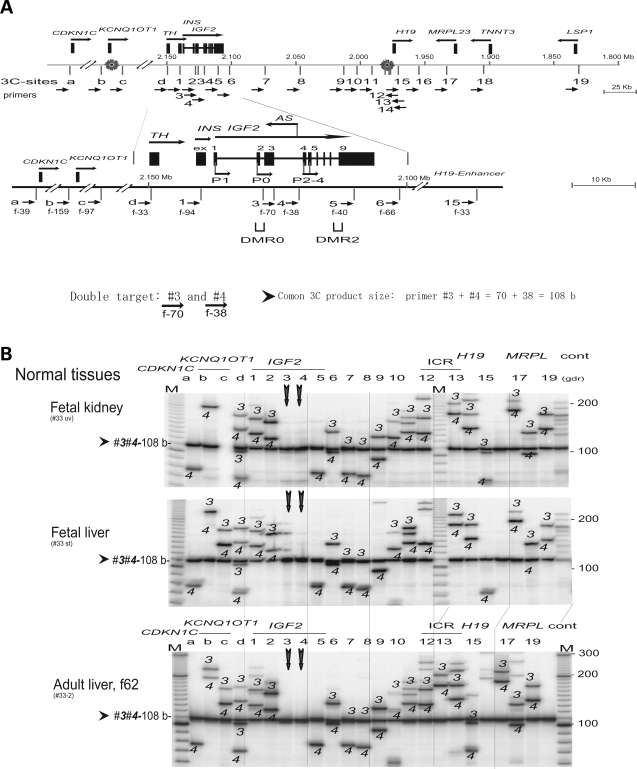
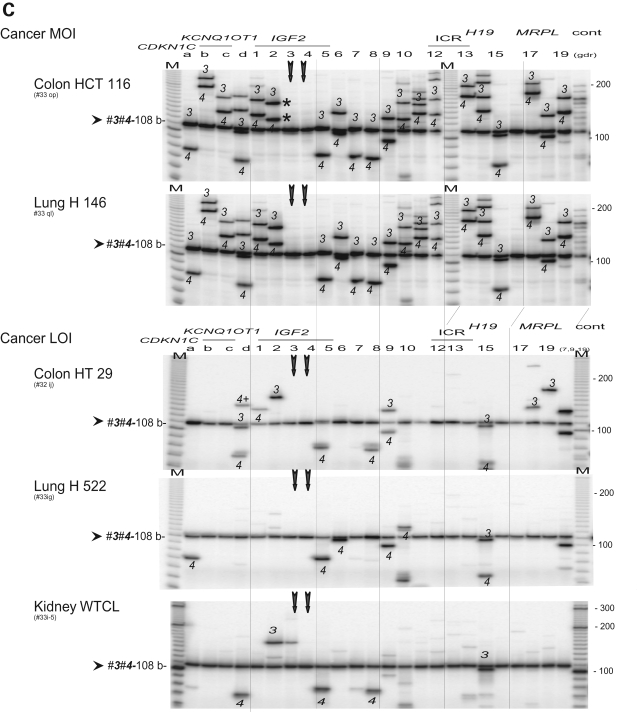
We studied details of chromosomal interactions by using BamHI and BglII restriction sites that are located near the IGF2 promoters (sites 1–4), IGF2 DMR (site 5), a putative matrix attachment site (MAR; site 6), the H19 ICR 1(site 12–14) and a putative H19 enhancer (site 15) (Fig. 4A). Scanning assays using a set of anchor primers 3 and 4 in the IGF2 promoter region (P0, P2–4) showed universal interaction across the IGF2/H19 and CDKN1C/KCNQ1OT1 regions in normal tissues and in MOI cancer cell lines (Fig. 4B,C and Supplementary Material, Fig. S2). In contrast, LOI cancer cell lines (HT29, H522 and WTCL) showed only a few 3C products, mostly local interactions within the TH/INS/IGF2 region, suggesting both the loss of long-range interactions and the disruption of local interactions in LOI cancer cells (Fig. 4C). Similar patterns of universal interaction in MOI cells and tissues, and global loss of long-range interaction in LOI cells were also observed in 3C scanning assays using paired anchor primers d and 1 that are located within a ∼10 kb DNA region adjacent to promoter P1 of IGF2 (Supplementary Material, Figs S1 and S3), as well as in the 3C assays using triple anchor primer 5 (IGF2 DMR), primer 6 (MAR) and primer 15 (H19 enhancer) (Supplementary Material, Figs S2 and S4).
Figure 4.
Scanning analysis of chromosomal interactions from the IGF2 promoter region. (A) Map of the IGF2/H19 locus as shown in Fig. 3A. A set of anchor primers 3 (forward 70 b) and 4 (forward 38 b) in the P0 and P2 promoter region results in one common 3C product (108 b). (B) 3C interaction in fetal tissues and adult liver. The anchor primers 3 and 4 (vertical arrows) produced common 3C product (3-4, 108 b) along with 3C products from primer 3 or 4 (as marked). Note one failed PCR (fetal kidney, column c). (C) 3C interaction in cancer MOI and LOI groups. Legends are in (B).
Allele-specific interaction from CTCF-binding site of the H19 DMR
It has been proposed that in the mouse, CTCF binding to the unmethylated H19 DMR followed by recruitment of other factors, including the PRC2 complex, organizes a silencing chromosomal loop between the H19 DMR and Igf2 DMR1 on the maternal allele (9). The chromosomal H19-Igf2 loop that may encompass Igf2/H19 enhancers is allele-specific (13). To learn whether a similar model also applies to the human IGF2/H19 genes, we identified a polymorphic DraIII site near CTCF-binding 6 in the ICR1 region (Fig. 5A) in the skin cell line HFB1. We performed 3C scanning across the IGF2/H19 region using a single target primer 13(-r138) and subsequently digested the 3C products with the DraIII restriction enzyme. To quantify the relative contribution of each parental allele, we employed a ‘hot-stop’ protocol followed by PhosphoImager scanning. Both alleles were involved in the 3C interactions at all sites that were studied. In the IGF2 promoter region, a slight predominance of the digested allele (80–67%) was observed (Fig. 5B, columns 1, 2, 3 and 4). In contrast, the undigested allele (95%) was predominant in the interaction between ICR1 and CDKN1C (Fig. 5B, columns a). Two other normal skin fibroblast cell lines, GM-00498 and GM-00523 were also informative for the DraIII polymorphism. Preliminary data (not shown) also indicate allele-specific interactions between ICR1 and CDKN1C, sites 13 and a in these cells as well. We are unable to identify the parental origin of this allele-specific interaction. However, we know that in the mouse, the maternal allele is involved with the ICR1-IGF2 loop; assuming that this is also true in human cells, it suggests that the CDKN1C-ICR1 interaction predominantly involves the paternal allele.
Figure 5.
Allele-specific interactions from H19 DMR. (A) Map of 3C primers in the ICR1 and ICR2 domains. Legends are in Fig. 2A. Bottom: detailed map of the H19 DMR and location of the polymorphic DraIII site. Primer 13 (r138) spans the DraIII site and the CTCF-binding 6. Amplicon primers 13 and primer x results in a 3C product (138 b + x b). Digestion with DraIII differentiates DraIII digested/undigested alleles (+/−32 b). (B) Allele-specific chromosomal interactions across CDKN1C/IGF2/H19 in normal human skin cells. Human fetal skin-derived fibroblast cell line HFB1 harbors the DraIII polymorphic site. In each column 3C products that were amplified by target primer 13 (vertical arrow) and a specified primer (marked by column number) were radioisotope labeled at the last amplification cycle (hot-stop PCR) and subsequently digested with DraIII. Undigested (downward, vertical arrow) and digested (upward, vertical arrow) products were sized on a polyacrylamide-urea gel and identified by predicted size. PhosphoImager scanning indicates a biased ratio digested/undigested allele of 2/1 in the IGF2 promoter region (1– 4). Note all 3C primers were reverse primers.
3C interaction between IGF2 and H19 DMR by Q-PCR
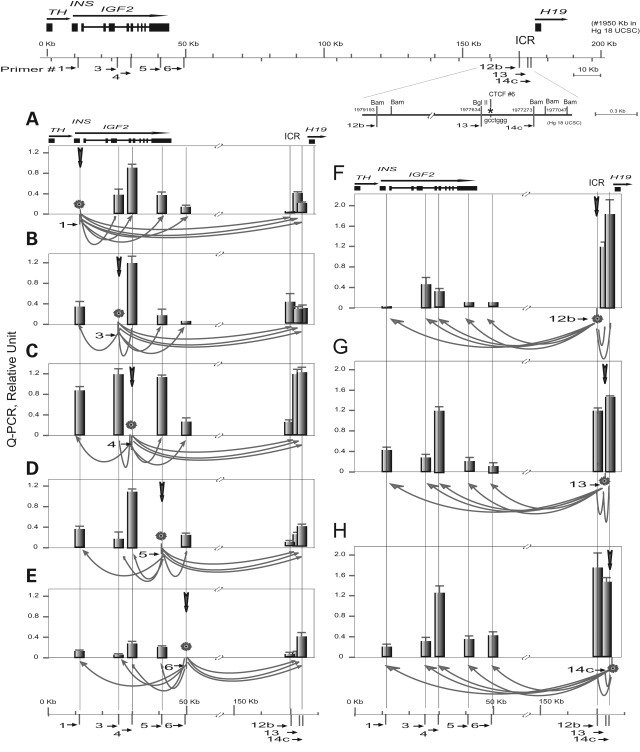
The 3C IF between IGF2 and H19 DMR was quantified by Q-PCR using control 3C DNA from BAC clones that encompass IGF2 and H19. Since a clone that covers the entire IGF2/H19 region was unavailable, we chose a combination of BAC clone RP11-542J6 (IGF2 clone) and a cosmid G248P8570B6 (H19 clone including H19 DMR). This allowed us to quantify IF between the IGF2 (sites 1 to 6) and H19 DMR (sites 12–14), but not the intergenic IGF2/H19 region.
In fetal liver, systematic Q-PCR scanning using anchor primers 1 through 14c (Fig. 6, panels A through H) yielded consistent IF values. DNA regions which are close together exhibit high IF values: three sites 12b, 13 and 14c in the H19 ICR1 show the strongest local interactions with highest IFs (Fig. 6F–H). Local interaction across the IGF2 gene shows a tendency of decreasing values with increasing distance (Fig. 6A–E). The IGF2 promoters P2–P4 (site 4) exhibits strong interaction with sites 13 and 14c of the H19 DMR, and vice versa (Fig. 6C, G and H). Cancer cell lines H116 and H146 that demonstrate IGF2 MOI also displayed panels of consistent IF values while very little IF data could be obtained for LOI cell lines HT29 and H522 because of the paucity of 3C interactions. The complete panels of IF data from fetal liver and from MOI cancer cells H116 and H146, and the relatively incomplete data set of LOI H522 lung cancer cells were used to compute 3D models of IGF2/H19 chromosomal organization.
Figure 6.
3C Interactions across 162 kb IGF2/H19 by Q-PCR. Top, Map of IGF2/H19 and detail map of the H19 DMR. Bottom (A–H), Q-PCR scanning in fetal liver using anchor primers 1 through 14c. Anchor primer (marked as a star) and target site are linked by an arrow at the bottom of each panel. Relative interaction frequency (IF) values shown in the y-axis are mean values and standard errors from 6 to 8 assays. The IFs are calibrated against a 3C control using BAC and cosmid DNA (8 assays). The x-axis shows genomic distance in kilobase.
3D model of IGF2/H19 chromosome
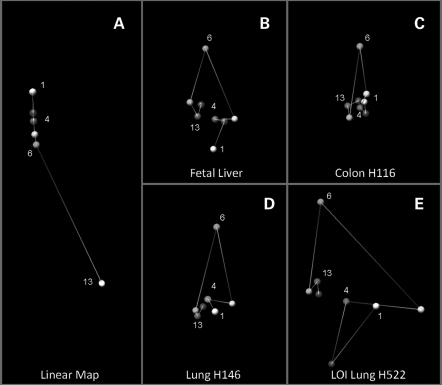
The spatial chromatin organization of the 162 kb DNA of IGF2/H19 was modeled by a software program recently developed by Dostie's group (44). This program was originally designed to identify chromosome conformation signatures by computing the spatial location of the selected points from IF data. We have modified and enhanced the program to illustrate 3D models of the IGF2/H19 region. The models depict the spatial organization of five locations in the IGF2 region (sites 1, 3, 4, 5 and 6) and three nearby locations (sites 12, 13 and 14) in the H19-ICR.
Figure 7A demonstrates a computer model of a control, linear chromatin fiber of 162 kb comprising IGF2/H19 that is devoid of long-range looping. Theoretical IF values were calculated merely by using the distance in nucleotide base pair between the eight selected sites. Each site is shown as a color-coded ball, creating a map of the IGF2/H19 chromosome segment. When we replace the input data of the linear map model by the 3C Q-PCR data sets, the software generates a 3D configuration of the IGF2/H19 chromosome segment in fetal liver and MOI cancer cells (Fig. 7B–D). Since the 3C data sets did not differentiate the two parental alleles, the model represents only the average 3D conformation for both alleles from cell populations at various cell cycle states, rather than in vivo individual structures. Nevertheless our 3D model depicts the proximity of the IGF2 promoters (site 4) and the H19 DMR (sites 13 and 14) in all three types of cells. Of note, the spatial distance between IGF2 (site 6) and H19 ICR (site 12), which are 122 kb apart, was compacted to a distance similar to the spatial distance of sites 5 and 6 which are 7.56 kb apart. The intergenic IGF2/H19 chromosomal DNA may have to loop out to a great extent to bring the H19 ICR close to the IGF2 promoter region. We speculate that the accurate 3C quantification by direct Q-PCR used in this study, rather than semi-quantification by PCR or by hybridization used by others, eliminates most of experimental noise and allows these observations to become more certain.
Figure 7.
Three-dimensional model of IGF2/H19. (A) 3D map of the 162 kb linear IGF2/H19 chromosomal segment. Theoretical IFs are calculated from distances between the sites in nucleotide base pair. (B–D) 3D configuration of the IGF2/H19 in fetal liver, MOI colon cancer cell line H116 and MOI lung cancer cell line H146. Experimental IFs are derived from 3C Q-PCR data set (such as the one shown in Fig. 6). Each color ball and number denotes the location of the selected site on the IGF2/H19 map. Color bands connecting the balls represents 3D spatial distance while virtually linear DNA length is shown in (A). Chromosome looping to bring the IGF2 promoters (4, red) in close contact to the ICR (13, purple) is evident in fetal liver, H116 and H146 cells. IGF2 LOI correlates with loss of measurable IGF2-H19 long-range interactions and a flattened structure in panel E (H522 cell line).
Q-PCR of the LOI cell lines HT29 and H522 demonstrated universal loss of long-range interactions and yielded only local interactions within the IGF2 or the H19 ICR. Most of the 3C interactions between IGF2 and the ICR were undetectable by Q-PCR. These data indicate the loss of 53 and 73% long-range interactions (8 out of 15, 11 out of 15 in HT29 and H522 cell lines, respectively). The 3D images of LOI cells created by the computer program are based on an insufficient data set (because of the paucity of interactions), and appear to be somewhat unstable, spread-out and flatter (Fig. 7E).
DISCUSSION
The 3D configuration of a gene can affect its transcription and interactions with other genes, and knowledge of this spatial arrangement is therefore necessary to understand its physiologic function. In this study, we have examined the intrachromosomal interactions of the human IGF2 gene for the first time. Multiple long- and short-range interactions occur, including potentially important physical interactions between the two imprinted regions on chromosome 11p15, the IGF2/H19 imprinting region ICR1 and the KCNQ1OT1 imprinting cluster ICR2. All of these interactions are preserved in tumor cell lines in which IGF2 imprinting is maintained (MOI), and in normal adult liver, where promoters P2–P4 are imprinted but where IGF2 is expressed biallelically from the non-imprinted promoter P1. However, in tumors in which there is LOI for IGF2, the pattern of interactions is completely disrupted as nearly all of the long-range interactions are abrogated.
Previous studies examining the 3D structure of these important imprinted genes focused on the well-characterized mouse region on chromosome 7 which is syntenic to human chromosome 11p15.5. Human IGF2 differs from the mouse gene in several important ways. While the mouse gene is normally transcribed from three maternally imprinted promoters, we showed that human IGF2 is also transcribed from an upstream non-imprinted promoter (P1) (40). An imprinted human P0 promoter and two novel transcripts which use the insulin promoter are also present (45). There is no DMR1, and the ICR contains 7 CTCF binding sites, while there are only 4 in the mouse (46). This ICR is differentially methylated (47–49), with the sixth of the seven CTCF-binding sites demonstrating paternal allele-specific differential methylation (23). Human IGF2 harbors two DMRs, a DMR0 that is located at the P0 promoter and an analog DMR2 at exon 9. Hypomethylation of three specific CpG sites in DMR0 has been shown to be associated with IGF2 LOI which was proposed as a constitutive biomarker risk for colorectal cancer (9). A recent study has shown that the DMR0 hypomethylation which was detected in 80% colorectal tumor tissues is somatically acquired in both breast and colorectal cancer (24). Human IGF2 harbors two DMRs, a DMR0 that is located at the P0 promoter and an analog DMR2 at exon 9. Hypomethylation of three specific CpG sites in DMR0 has been shown to be associated with IGF2 LOI which was proposed as a constitutive biomarker risk for colorectal cancer (9). A recent study has shown that the DMR0 hypomethylation which was detected in 80% colorectal tumor tissues is somatically acquired in both breast and colorectal cancer (24). Human DMR0, which does not bind CTCF, may act as a silencer element that can suppress IGF2 independent of the ICR-CTCF regulatory pathway. We have shown that the ICR-CTCF regulatory pathway that involves three proximal promoters (P1–P3 in the mouse and P2–P4 in human) is allele-specific (31). Two CTCF binding sites have been mapped near human promoters P3 and P4 (27), and the regulatory pathway involves intrachromosomal looping of the promoter-ICR and the polycomb repressive complex 2.
Most studies utilizing the chromosome conformation capture technique have examined the interactions of a relatively small number of DNA segments which were selected because of they were presumed to interact based on genetic experiments and a molecular biologic understanding of gene regulation. These studies have led to elegant models of DNA looping between promoter and enhancer regions that have provided a physical basis for long-range interactions between DNA segments. Multiple DNA loops that include local, short-range and long-range, transcriptionally active loops (and the absence of DNA looping as negative control) have been established for the beta-globin locus of both human and mouse (50). We have used DNA looping in the human beta-globin locus as 3C controls in the present study.
In the mouse Igf2-H19 locus, a simple model of switching interactions between the ICR1 and one of the two Igf2 DMRs as the basis to achieve promoter–enhancer contact has been proposed (11). Silencing of Igf2 on the maternal allele can be viewed as a result of a tight, inactive loop around the maternal Igf2 (12,27,28), or as a result of enhancer-blocking by sequestering enhancers by CTCF complex(es) (13,51). Activation of the paternal Igf2 is suggested to be the result of direct enhancer contact. A newer model of enhancer tracking allows direct and exclusive paternal Igf2-enhancer interaction and the absence of paternal H19-enhancer contact (52). Most of these mouse models of chromosomal interaction are based on qualitative 3C results.
However, we believe that it is important to understand that DNA segments have numerous and myriad local and long-range interactions, and that studying only the known or hypothesized long-range interactions can lead to a biased interpretation of the 3D conformation and structure of these genes. Therefore in this study, we have performed multiple 3C studies, choosing numerous segments on the IGF2/H19 gene dyad and selected sites near the TH gene and a second imprinting control region (ICR2), nearly 1 Mb away, to carefully delineate both local and long-range interactions. As might be expected, in general, local interactions are far more prevalent than long-range ones. As one looks farther from the DNA segment of interest, one finds fewer (probably stochastic) interactions and more distinct and specific long-range contacts. There is a rather remarkable consistency to these interactions, indicating that the 3D configuration of IGF2/H19 does not differ substantially among the various tissues and that it remains fairly constant throughout development. Even in adult liver, where the great preponderance of IGF2 mRNA is expressed biallelically from promoter P1 and only a small amount is transcribed from a single allele from promoters P2–P4, the 3C data shows a similar pattern of interactions as is seen in tissues where IGF2/H19 transcription is uniformly monoallelic. These interactions include physical contacts from the ICR1 to the IGF2 gene, to the CDKN1C gene near the ICR2 and to regions downstream of H19.
Careful examination of the IGF2-H19 interaction by Q-PCR reveals a predominant interaction between ICR1 (sites 13 and 14) and the IGF2 promoter (site 4). Since both sites 13 and 14 are within ∼400 b from CTCF-binding site 6 (Fig. 6), it is likely that binding of CTCF at this binding site plays a role in organizing the IGF2 promter-ICR1 loop. The interaction IGF2-ICR1 is preferentially derived from one parental allele (Fig. 5). As both mouse and human IGF2 are expressed from the paternal allele, it is likely that the human IGF2-ICR1 loop is preferentially occurring on the maternal allele, similar to the mouse maternal Igf2-ICR interaction. This looping is illustrated in 3D models computed from the 3C Q-PCR data sets which shows physical intimacy between the IGF2 promoter (site 4) and the ICR1 (sites 13 and 14), even though the data sets are from both parental alleles (Fig. 7). Since a majority of genes in a diploid cell are expressed equally from each parental allele, there would be no allelic contrast between two parental chromosomal structures. Our combination of 3C-OC assay, Q-PCR and 3D computer software would provide a universal approach to modeling conformation structure of most chromosomal domains.
The 3D structure is dramatically disrupted in cells where IGF2 imprinting is lost. Instead of the frequent long-range intrachromosomal interactions seen in normal cells that demonstrate a normal karyotype and in cancer cells where imprinting is maintained, LOI cells lose nearly all of the interactions at the IGF2 promoter region and at other downstream regions. It is likely that the loss of these physical interactions alters IGF2 transcription, and, perhaps more significantly, the abrogation of long-range interactions may change the transcription of the normally-interacting genes, which may have physiologically significant affects on cell growth and division. For example, in the mouse, Igf2 normally interacts in an interchromosomal fashion via CTCF with Nf1; disruption of this physical interaction leads to loss of Igf2 imprinting and to changes in Nf1 mRNA abundance (7).
It is of particular interest that there is a physical interaction between the two ICRs, which are nearly 1 Mb apart. This interaction suggests that these two imprinting centers may be regulated by a common factor or that the centers may actually play a role in modulating each others' activity. The long-range interaction between ICR1 and CDKN1C (ICR2) is predominantly from one parental allele, opposite to the allele involved in IGF2-ICR looping. Assuming that the IGF2-ICR loop is maternally derived, as is the case with the mouse genes, then looping between the two ICRs is likely derived from the paternal allele. This is consistent with the known maternal expression of CDKN1C, as opposed to paternal IGF2 expression. While we observed allele-specific ICR1–ICR2 interactions in skin-derived cells, further studies in various tissues and cell types may help shed light on the function of this long-range chromosomal looping. In this regard, it is important to note that some patients with Beckwith–Wiedemann syndrome who harbor mutations in the CDKN1C gene, part of the ICR2 cluster of genes, also have loss of IGF2 imprinting (53), suggesting the presence of a physiologic interaction between these imprinting control regions on chromosome 11p15.
Based on these data, we would like to suggest a new paradigm to help explain how loss of IGF2 imprinting may be involved in oncogenesis. Alterations in the IGF2 epigenome, including LOI, will disrupt long-range intrachromosomal and, potentially, interchromosomal interactions, leading to the dysregulation of a host of genes. This disruption of (potentially) multiple pathways leads to an oncogenic diathesis, and, ultimately malignant transformation. An in-depth examination of long-range intrachromosomal and interchromosomal interactions will provide new insights into the normal physiologic regulation of gene expression and may suggest new insights into carcinogenesis.
MATERIALS AND METHODS
Human tissues, cell lines and DNA sources
Normal fetal tissues of 12 weeks of gestation were obtained from the Central Laboratory for Human Embryology Tissue, University of Washington, Seattle. Normal adult livers were fresh frozen tissues from Stanford Medical School, Stanford, CA, USA. Cancer cell lines HCT116, HT29, H522 and H146 were purchased ATCC (Rockville, MD, USA). Normal fibroblast cell line GM-00498 was obtained from the Coriell Cell Repositories, Camden, NJ, USA. Human fibroblast cell line HFB1 was cultured from fetal skin in our lab (39). Wilms’ tumor cell line WTCL was kindly provided by Dr Benjamin Tycko. Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum and 100 U/ml of penicillin and 100 µg/ml of streptomycin, and grown at 37°C with 5% CO2.
Bacterial artificial chromosome (BAC) RP11-542J6 and cosmid G248P8570B6 clones were obtained from Children's Hospital Oakland Research Institute (CHORI), Oakland, CA, USA. BAC and cosmid DNAs were prepared by standard methods and quantified using Power SYBR Green PCR kit from Applied Biosystems, Foster City, CA, USA.
Chromosome conformation capture original copy (3C-OC) assay
The chromosome conformation capture original copy (3C-OC) assay consists of three steps: (i) preparation of 3C DNA, (ii) construction of a multiplex PCR library and (iii) detection and quantification of specific 3C products by PCR or Q-PCR. In contrast to the 3C-Carbon Copy (5C) methodology (54), 3C-OC does not contain a ligation step. The 3C-OC is based on the specificity of the multiplex PCR primers to construct the multiplex library, and the specificity of semi-nested primers to single out specific 3C products.
Step 1: 3C DNA preparation
Tissues were dissected, rinsed with PBS, minced and sieved through a 70-µm nylon cell strainer (BD Biosciences, MA) into PBS. Cell suspensions were fixed immediately with formaldehyde in PBS at room temperature for 10 min. Cells that attach to the culture plates were rinsed with PBS and directly fixed with 2% formaldehyde in PBS (5 ml for 10 cm plates) at room temperature for 10 min. 3C assays were carried out as described (55) with some modifications. Briefly, cells were fixed in formaldehyde, quenched with 0.125 m glycine and then lysed with 0.2% NP-40 on ice for 2 h with stirring. Cells that were fixed and quenched on culture plates were collected on ice in NP-40 lysis buffer using a flat blade. Nuclei were collected by centrifugation, resuspended in NEB BamHI buffer (New England Biolab, NJ, USA) containing 0.3% SDS, treated at 37C for 1 h and quenched with 1.8% Triton X at 37°C for 1 h. Treated nuclei (∼107) were digested with BamHI and BglII (1000 units each) in 500 µl of NEB BamHI buffer at 37 C for ∼18 h. After enzyme deactivation (1% SDS, 65°C for 20 min) and quenching (2.6% Triton X, 37°C for 1 h), aliquots of digested nuclei (∼3 µg DNA) were ligated with T4 DNA ligase (2000 U T4 ligase in 1.2 ml ligation buffer) at 16°C for 4 h. Cross-linked DNAs were treated with Proteinase K (100 µg, 65°C overnight) and RNase A (1 µg, 37°C, 30 min), and then concentrated in a speedVac (Savant, NY, USA) to reduce to ∼1/5 volume before phenol–chloroform extraction. Control samples (before/after digestion and before/after ligation) were also treated with Proteinase K and RNase A before phenol–chloroform extraction. DNAs were precipitated with ammonium acetate (2 m) and 2-propanol (1.5 vol.). The pellet was washed with cold 75% ethanol three times and then dissolved in low TE buffer (1 mm Tris, 0.1 mm EDTA, pH 8.0).
Step 2: construction of a multiplex PCR library
The multiplex library consists of multiple, primary PCR primers. Sets of unidirectional, forward or reverse primers were designed to be close to the selected restriction sites near the DNA region of interest. We have tested as many as 55 primary primers in one experiment, but it is likely that as many as ∼100 primers can be used in the multiplex assay. Primers from other loci can be added as 3C controls (such as β-globin in Fig. 1). The selection of appropriate primers is critical to avoid repetitive sequences or SNP sequences that could produce erroneous results. We used available software (http://frodo.wi.mit.edu/primer3) following these criteria: (i) avoidance of human mispriming, (ii) primer sizes of 28 ± 3 bases, (iii) Tm = 74°C ± 2°C, (iv) avoidance of DNA repeat regions and (v) avoidance of all SNPs. Since each 3C product is formed by two DNA fragments of specified size (that is the distance of the primer to the restriction site), the final 3C product has a defined size which can be identified on a short DNA sequencing gel as a specified ID size tag. Multiplex libraries were constructed from 3C cross-linked DNAs (0.5–1.0 µg) in a 40 µl PCR reaction containing 0.1 µM appropriate primers, 50 µm dNTPs and 3 units KlenTaqI polymerase. PCR conditions were 97°C for 120 s followed by 30 cycles of 97°C for 20 s and 67°C for 120 s and finally 72°C for 5 min.
Step 3: assay of specific 3C products
To assay for specific 3C products, we used semi-nested primers that had sequences identical to the multiplex library primers plus an additional 2–5 bases extended at the 3′ end (Supplementary Material, Table S1). The 3C assay contained the 3C-OC library (1000-fold diluted in the final reaction) as template, anchor primer (single or multiple) and a single target primer. PCR conditions were 97°C for 120 s followed by 20 cycles of 97°C for 20 s and 67°C for 120 s and finally 72°C for 5 min. The PCR products were radio-labeled (6 µl PCR reaction containing 0.2 µCi 32P dCTP), analyzed on a 5% polyacrylamide–urea gel and visualized by a PhosphorImager (Molecular Dynamics, Sunnyvale, CA, USA). Quantitative PCR (see in what follows) was performed using single pair primers.
Parental allelic differentiation
To analyze the relative abundance of the two parental alleles we utilized a ‘hot stop’ protocol where labeled nucleotide (32P-dCTP) or 32P end-labeled primers were added in a PCR mixture at the last 20th cycle. The extension-labeling condition was 97°C for 3 min followed by 67°C for 5 min and 72°C for 10 min. Labeled PCR products were digested with DraIII (New England Biolabs, Beverly, MA, USA) in a total volume of 10 µl at 37°C for 12 h under liquid wax. The digested products were separated on a polyacrylamide-urea gel and quantified by the PhosphorImager.
Controls for the 3C assay
We employed two types of control, an external 3C control and an in-tube internal control. The external 3C control consisted of 3C interactions of the beta-globin locus, 3 Mb upstream of IGF2-H19 on chromosome 11p15 (Fig. 1A and B). The 3C configurations of the beta- globin locus have been studied extensively in human and in mouse (54). Local and long-range interactions between the locus control region (LCR) and DNAse I-hypersensitive sites (HS5, HS2 and HS1), and the absence of interaction between the LCR and the distant 5′ region were reported. Globin primers and IGF2/H19 primer sets (Supplementary Material, Table S1) were analyzed simultaneously. For in-tube internal controls, we utilized multiplex PCR in the second PCR step. Each PCR tube contained two (or three) anchor primers and a variant that was the specified primer at each location. Pair(s) of anchor primers across all PCR tubes served as internal control(s) for PCR efficiency across scanning panels.
Real-time quantitative (Q)-PCR
Q-PCR assays were run in two quadruplicates on 384-well plates using an ABI Prism 7900HT, SYBR Green and the ABI protocol. A single set of primers containing one anchor and one target primer (Supplementary Material, Table S1) was run in a standard 3 µl PCR under clear mineral oil. PCR conditions were 95°C for 10 min, 35 cycles of 95°C for 15 s and 90 s at 65°C. Specificity of the PCR was demonstrated by the presence of single bands on polyacrylamide gels. We used a melting temperature program to further verify the purity of the PCR products. Relative levels were determined by a ‘delta Ct and delta-delta Ct’ with reference to control 3C DNA from BAC/cosmid clones. Equal molar amounts of DNA from the BAC (IGF2) and cosmid (H19-ICR) were used to generate 3C DNA controls, which were pre-determined by separate Q-PCR assay using two pairs of PCR primers in the IGF2/H19 region. Relative IF and the standard deviation from six to eight assays were calculated and graphed. We used panels of IF data to compute 3D model of chromatin folding.
3D model of IGF2/H19 chromosome
The 3D model prediction, ‘5C3D’ program, is available from the Dostie Laboratory website (http://dostielab.biochem.mcgill.ca/). Theoretical IFs between two points ‘i’ and ‘j’ in Fig. 7A are calculated as follows:
where ‘d’ is the distance (i-j) in Kb and ‘c’ is a coefficient factor to adjust IF values within a desirable range (0.01 < IF < 100). To draw a ∼200 kb DNA we select ‘c’ = 25.
In the 3D model of IGF2/H19, the input data are the IF values derived from the 3C Q-PCR (experimental sample values versus BAC and cosmid control). The IF values from the various samples (fetal liver, H116 and H146 cells) were normalized by assigning a coefficient factor ‘c’ =2.5 (IF = c × 1/d). It follows that the IF value of the closest interaction between sites 13 and 14 (441 b) is assigned as 2.5/0.441 = 5.66893 that was used to calibrate IF data sets from the various samples.
SUPPLEMENTARY MATERIAL
FUNDING
This study was supported by NIH grant HD047013, Department of Defense grant NF050184 and the Medical Research Service of the Department of Veterans Affairs.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mathieu Rousseau and Josee Dostie for communicating the 3D-5C software, Tao Li and Xin Wen Qiu for help with tissue culture, the Central Laboratory for Human Embryology Tissue, University of Washington, Seattle, for fetal tissues.
Conflict of Interest statement. None declared.
REFERENCES
- 1.DeChiara T.M., Robertson E.J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991;64:849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- 2.Bartolomei M.S., Zemel S., Tilghman S.M. Parental imprinting of the mouse H19 gene. Nature. 1991;351:153–155. doi: 10.1038/351153a0. [DOI] [PubMed] [Google Scholar]
- 3.Zemel S., Bartolomei M.S., Tilghman S.M. Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nat. Genet. 1992;2:61–65. doi: 10.1038/ng0992-61. [DOI] [PubMed] [Google Scholar]
- 4.Giannoukakis N., Deal C., Paquette J., Goodyer C.G., Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat. Genet. 1993;4:98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- 5.Ohlsson R., Nystrom A., Pfeifer O.S., Tohonen V., Hedborg F., Schofield P., Flam F., Ekstrom T.J. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith–Wiedemann syndrome. Nat. Genet. 1993;4:94–97. doi: 10.1038/ng0593-94. [DOI] [PubMed] [Google Scholar]
- 6.Leighton P.A., Ingram R.S., Eggenschwiler J., Efstratiadis A., Tilghman S.M. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature. 1995;375:34–39. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- 7.Ling J.Q., Li T., Hu J.F., Vu T.H., Chen H.L., Qiu X.W., Cherry A.M., Hoffman A.R. CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science. 2006;312:269–272. doi: 10.1126/science.1123191. [DOI] [PubMed] [Google Scholar]
- 8.Phillips J.E., Corces V.G. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li T., Hu J.F., Qiu X., Ling J., Chen H., Wang S., Hou A., Vu T.H., Hoffman A.R. CTCF regulates allelic expression of Igf2 by orchestrating a promoter-polycomb repressive complex 2 intrachromosomal loop. Mol. Cell. Biol. 2008;28:6473–6482. doi: 10.1128/MCB.00204-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dekker J., Rippe K., Dekker M., Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 11.Murrell A., Heeson S., Reik W. Interaction between differentially methylated regions partitions the imprinted genes Igf2 and H19 into parent-specific chromatin loops. Nat. Genet. 2004;36:889–893. doi: 10.1038/ng1402. [DOI] [PubMed] [Google Scholar]
- 12.Kurukuti S., Tiwari V.K., Tavoosidana G., Pugacheva E., Murrell A., Zhao Z., Lobanenkov V., Reik W., Ohlsson R. CTCF binding at the H19 imprinting control region mediates maternally inherited higher-order chromatin conformation to restrict enhancer access to Igf2. Proc. Natl Acad. Sci. USA. 2006;103:10684–10689. doi: 10.1073/pnas.0600326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu X., Vu T.H., Lu Q., Ling J.Q., Li T., Hou A., Wang S.K., Chen H.L., Hu J.F., Hoffman A.R. A complex deoxyribonucleic acid looping configuration associated with the silencing of the maternal Igf2 allele. Mol. Endocrinol. 2008;22:1476–1488. doi: 10.1210/me.2007-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa O., Eccles M.R., Szeto J., McNoe L.A., Yun K., Maw M.A., Smith P.J., Reeve A.E. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumour. Nature. 1993;362:749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- 15.Rainier S., Johnson L.A., Dobry C.J., Ping A.J., Grundy P.E., Feinberg A.P. Relaxation of imprinted genes in human cancer. Nature. 1993;362:747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- 16.Christofori G., Naik P., Hanahan D. A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature. 1994;369:414–418. doi: 10.1038/369414a0. [DOI] [PubMed] [Google Scholar]
- 17.Sakatani T., Kaneda A., Iacobuzio-Donahue C.A., Carter M.G., de Boom Witzel S., Okano H., Ko M.S., Ohlsson R., Longo D.L., Feinberg A.P. Loss of imprinting of Igf2 alters intestinal maturation and tumorigenesis in mice. Science. 2005;307:1976–1978. doi: 10.1126/science.1108080. [DOI] [PubMed] [Google Scholar]
- 18.Holm T.M., Jackson-Grusby L., Brambrink T., Yamada Y., Rideout W.M., III, Jaenisch R. Global loss of imprinting leads to widespread tumorigenesis in adult mice. Cancer Cell. 2005;8:275–285. doi: 10.1016/j.ccr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Kaneda A., Wang C.J., Cheong R., Timp W., Onyango P., Wen B., Iacobuzio-Donahue C.A., Ohlsson R., Andraos R., Pearson M.A., et al. Enhanced sensitivity to IGF-II signaling links loss of imprinting of IGF2 to increased cell proliferation and tumor risk. Proc. Natl Acad. Sci. USA. 2007;104:20926–20931. doi: 10.1073/pnas.0710359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W.H., Duan J.X., Vu T.H., Hoffman A.R. Increased expression of the insulin-like growth factor-II gene in Wilms' tumor is not dependent on loss of genomic imprinting or loss of heterozygosity. J. Biol. Chem. 1996;271:27863–27870. doi: 10.1074/jbc.271.44.27863. [DOI] [PubMed] [Google Scholar]
- 21.Cui H., Cruz-Correa M., Giardiello F.M., Hutcheon D.F., Kafonek D.R., Brandenburg S., Wu Y., He X., Powe N.R., Feinberg A.P. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science. 2003;299:1753–1755. doi: 10.1126/science.1080902. [DOI] [PubMed] [Google Scholar]
- 22.Cui H., Niemitz E.L., Ravenel J.D., Onyango P., Brandenburg S.A., Lobanenkov V.V., Feinberg A.P. Loss of imprinting of insulin-like growth factor-II in Wilms' tumor commonly involves altered methylation but not mutations of CTCF or its binding site. Cancer Res. 2001;61:4947–4950. [PubMed] [Google Scholar]
- 23.Takai D., Gonzales F.A., Tsai Y.C., Thayer M.J., Jones P.A. Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum. Mol. Genet. 2001;10:2619–2626. doi: 10.1093/hmg/10.23.2619. [DOI] [PubMed] [Google Scholar]
- 24.Cui H., Horon I.L., Ohlsson R., Hamilton S.R., Feinberg A.P. Loss of imprinting in normal tissue of colorectal cancer patients with microsatellite instability. Nat. Med. 1998;4:1276–1280. doi: 10.1038/3260. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa H., Chadwick R.B., Peltomaki P., Plass C., Nakamura Y., de La Chapelle A. Loss of imprinting of the insulin-like growth factor II gene occurs by biallelic methylation in a core region of H19-associated CTCF-binding sites in colorectal cancer. Proc. Natl Acad. Sci. USA. 2001;98:591–596. doi: 10.1073/pnas.011528698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogler C.E., Yang D., Rossetti L., Donohoe J., Alt E., Chang C.J., Rosenfeld R., Neely K., Hintz R. Altered body composition and increased frequency of diverse malignancies in insulin-like growth factor-II transgenic mice. J. Biol. Chem. 1994;269:13779–13784. [PubMed] [Google Scholar]
- 27.Binder G., Seidel A.K., Weber K., Haase M., Wollmann H.A., Ranke M.B., Eggermann T. IGF-II serum levels are normal in children with Silver-Russell syndrome who frequently carry epimutations at the IGF2 locus. J. Clin. Endocrinol. Metab. 2006;91:4709–4712. doi: 10.1210/jc.2006-1127. [DOI] [PubMed] [Google Scholar]
- 28.Spilianakis C.G., Flavell R.A. Molecular biology. Managing associations between different chromosomes. Science. 2006;312:207–208. doi: 10.1126/science.1126689. [DOI] [PubMed] [Google Scholar]
- 29.Horike S., Cai S., Miyano M., Cheng J.F., Kohwi-Shigematsu T. Loss of silent-chromatin looping and impaired imprinting of DLX5 in Rett syndrome. Nat. Genet. 2005;37:31–40. doi: 10.1038/ng1491. [DOI] [PubMed] [Google Scholar]
- 30.Spilianakis C.G., Flavell R.A. Epigenetic regulation of Ifng expression. Nat. Immunol. 2007;8:681–683. doi: 10.1038/ni0707-681. [DOI] [PubMed] [Google Scholar]
- 31.Sayegh C.E., Jhunjhunwala S., Riblet R., Murre C. Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 2005;19:322–327. doi: 10.1101/gad.1254305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakim O., John S., Ling J.Q., Biddie S.C., Hoffman A.R., Hager G.L. Glucocorticoid receptor activation of the Ciz1-Lcn2 locus by long range interactions. J. Biol. Chem. 2009;284:6048–6052. doi: 10.1074/jbc.C800212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown J.M., Leach J., Reittie J.E., Atzberger A., Lee-Prudhoe J., Wood W.G., Higgs D.R., Iborra F.J., Buckle V.J. Coregulated human globin genes are frequently in spatial proximity when active. J. Cell. Biol. 2006;172:177–187. doi: 10.1083/jcb.200507073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacher C.P., Guggiari M., Brors B., Augui S., Clerc P., Avner P., Eils R., Heard E. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell Biol. 2006;8:293–299. doi: 10.1038/ncb1365. [DOI] [PubMed] [Google Scholar]
- 35.Xu N., Tsai C.L., Lee J.T. Transient homologous chromosome pairing marks the onset of X inactivation. Science. 2006;311:1149–1152. doi: 10.1126/science.1122984. [DOI] [PubMed] [Google Scholar]
- 36.Spilianakis C.G., Lalioti M.D., Town T., Lee G.R., Flavell R.A. Interchromosomal associations between alternatively expressed loci. Nature. 2005;435:637–645. doi: 10.1038/nature03574. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y., Koessler T., Ibrahim A.E., Rai S., Vowler S.L., Abu-Amero S., Silva A.L., Maia A.T., Huddleston J.E., Uribe-Lewis S., et al. Somatically acquired hypomethylation of IGF2 in breast and colorectal cancer. Hum. Mol. Genet. 2008;17:2633–2643. doi: 10.1093/hmg/ddn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo L., Choufani S., Ferreira J., Smith A., Chitayat D., Shuman C., Uxa R., Keating S., Kingdom J., Weksberg R. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev. Biol. 2008;320:79–91. doi: 10.1016/j.ydbio.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 39.Chen H.L., Li T., Qiu X.W., Wu J., Ling J.Q., Sun Z.H., Wang W., Chen W., Hou A., Vu T.H., et al. Correction of aberrant imprinting of IGF2 in human tumors by nuclear transfer-induced epigenetic reprogramming. EMBO J. 2006;25:5329–5338. doi: 10.1038/sj.emboj.7601399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vu T.H., Hoffman A.R. Promoter-specific imprinting of the human insulin-like growth factor-II gene. Nature. 1994;371:714–717. doi: 10.1038/371714a0. [DOI] [PubMed] [Google Scholar]
- 41.Bell A.C., Felsenfeld G. Methylation of a CTCF-dependent boundary controls imprinted expression of the Igf2 gene. Nature. 2000;405:482–485. doi: 10.1038/35013100. [DOI] [PubMed] [Google Scholar]
- 42.Redrup L., Branco M.R., Perdeaux E.R., Krueger C., Lewis A., Santos F., Nagano T., Cobb B.S., Fraser P., Reik W. The long noncoding RNA Kcnq1ot1 organises a lineage-specific nuclear domain for epigenetic gene silencing. Development. 2009;136:525–530. doi: 10.1242/dev.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleary M.A., van Raamsdonk C.D., Levorse J., Zheng B., Bradley A., Tilghman S.M. Disruption of an imprinted gene cluster by a targeted chromosomal translocation in mice. Nat. Genet. 2001;29:78–82. doi: 10.1038/ng715. [DOI] [PubMed] [Google Scholar]
- 44.Fraser J., Rousseau M., Shenker S., Ferraiuolo M.A., Hayashizaki Y., Blanchette M., Dostie J. Chromatin conformation signatures of cellular differentiation. Genome Biol. 2009;10:R37. doi: 10.1186/gb-2009-10-4-r37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monk D., Sanches R., Arnaud P., Apostolidou S., Hills F.A., Abu-Amero S., Murrell A., Friess H., Reik W., Stanier P., et al. Imprinting of IGF2 P0 transcript and novel alternatively spliced INS-IGF2 isoforms show differences between mouse and human. Hum. Mol. Genet. 2006;15:1259–1269. doi: 10.1093/hmg/ddl041. [DOI] [PubMed] [Google Scholar]
- 46.Hark A.T., Schoenherr C.J., Katz D.J., Ingram R.S., Levorse J.M., Tilghman S.M. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- 47.Vu T.H., Li T., Nguyen D., Nguyen B.T., Yao X.M., Hu J.F., Hoffman A.R. Symmetric and asymmetric DNA methylation in the human IGF2-H19 imprinted region. Genomics. 2000;64:132–143. doi: 10.1006/geno.1999.6094. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y., Shields T., Crenshaw T., Hao Y., Moulton T., Tycko B. Imprinting of human H19: allele-specific CpG methylation, loss of the active allele in Wilms tumor, and potential for somatic allele switching. Am. J. Hum. Genet. 1993;53:113–124. [PMC free article] [PubMed] [Google Scholar]
- 49.Jinno Y., Sengoku K., Nakao M., Tamate K., Miyamoto T., Matsuzaka T., Sutcliffe J.S., Anan T., Takuma N., Nishiwaki K., et al. Mouse/human sequence divergence in a region with a paternal-specific methylation imprint at the human H19 locus. Hum. Mol. Genet. 1996;5:1155–1161. doi: 10.1093/hmg/5.8.1155. [DOI] [PubMed] [Google Scholar]
- 50.Tolhuis B., Palstra R.J., Splinter E., Grosveld F., de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 51.Yoon Y.S., Jeong S., Rong Q., Park K.Y., Chung J.H., Pfeifer K. Analysis of the H19ICR insulator. Mol. Cell. Biol. 2007;27:3499–34510. doi: 10.1128/MCB.02170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engel N., Raval A.K., Thorvaldsen J.L., Bartolomei S.M. Three-dimensional conformation at the H19/Igf2 locus supports a model of enhancer tracking. Hum. Mol. Genet. 2008;17:3021–3029. doi: 10.1093/hmg/ddn200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M., Squire J., Shuman C., Fei Y.L., Atkin J., Pauli R., Smith A., Nishikawa J., Chitayat D., Weksberg R. Imprinting status of 11p15 genes in Beckwith–Wiedemann syndrome patients with CDKN1C mutations. Genomics. 2001;74:370–376. doi: 10.1006/geno.2001.6549. [DOI] [PubMed] [Google Scholar]
- 54.Dostie J., Richmond T.A., Arnaout R.A., Selzer R.R., Lee W.L., Honan T.A., Rubio E.D., Krumm A., Lamb J., Nusbaum C., et al. Chromosome Conformation Capture Carbon Copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006;16:1299–1309. doi: 10.1101/gr.5571506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dostie J., Dekker J. Mapping networks of physical interactions between genomic elements using 5C technology. Nat. Protoc. 2007;2:988–1002. doi: 10.1038/nprot.2007.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.