Abstract
Ageing is an unavoidable corollary to being alive; the most intuitive interpretation of ageing being that it is the consequence of progressive body degeneration. In agreement with this, current models propose that ageing occurs through a stepwise accumulation of DNA damage, which ultimately limits the regenerative capacity of tissues. On the other hand, there is increasing evidence that fetal distress can influence the development of disease in adult life, a phenomenon known as ‘intrauterine programming'. The extent to which an intrauterine exposure to DNA damage can compromise lifespan remains unclear. My group has recently generated a murine model of a human syndrome linked to defective DNA repair and observed that these animals age prematurely, but the accumulation of DNA damage is restricted mostly to the embryonic period. Here, I discuss the implications of this finding and propose that ageing can be influenced by fetal distress.
Keywords: ageing, intrauterine programming, DNA damage, ATR, Seckel syndrome
See Glossary for abbreviations used in this article.
Glossary.
- ATM
ataxia telangiectasia mutated
ATM-related and Rad3-related
phosphatidylinositol 3-kinase-like protein kinase
reactive oxygen species
- ATR
ATM-related and Rad3-related
phosphatidylinositol 3-kinase-like protein kinase
reactive oxygen species
- PIKK
phosphatidylinositol 3-kinase-like protein kinase
reactive oxygen species
- ROS
reactive oxygen species
A DNA-damage model of ageing
DNA damage has long been known to be responsible for cancer-initiating mutations, as genomic lesions can alter specifically the function of individual genes, such as tumour suppressors or proto-oncogenes. In addition, the accumulation of DNA damage was recently suggested to be involved in the onset of ageing (Garinis et al, 2008; Lombard et al, 2005). In agreement with this hypothesis, several reports have shown the existence of an activated DNA damage response in aged tissues or stem cells (SCs; Rossi et al, 2007; Sedelnikova et al, 2004). Most importantly, mutations in DNA repair genes can accelerate ageing in mice and humans, strongly supporting this model (Schumacher et al, 2008). A well-known example is that of Werner syndrome (MIM277700) patients, who age prematurely in their early years of life. The gene responsible for this disease was identified by positional cloning in 1996 (Yu et al, 1996), after many years of search. It was found to be RECQL2—also known as WRN—a DNA helicase that is now known to be involved in maintaining DNA integrity (Ozgenc & Loeb, 2005). The DNA-damage-based theory is consistent with our innate understanding of the ageing process, which is that ageing arises through a stepwise accumulation of damaged tissues. However, the model restricts the vague idea of cellular damage to the accumulation of lesions that specifically harm DNA.
But how does DNA damage lead to ageing? One possibility is through the stochastic accumulation of mutations in vital genes. However, current evidence supports an alternative view in which the main effect of DNA damage in the context of ageing is linked to the activation of cell-cycle checkpoints. In such a scenario, when the load of DNA damage rises above a certain threshold, damaged cells will either be eliminated or have their growth suppressed. If such a response is activated on SCs, it has a direct consequence in tissue regeneration, limiting the turnover of deficient tissues and ultimately leading to ageing (Rossi et al, 2008). Accordingly, DNA damage has been shown to accumulate in aged haematopoietic SCs, limiting their regenerative capacity (Rossi et al, 2007).
Protecting against DNA damage
We are constantly exposed to DNA damage, induced both by exogenous sources—which we can try to avoid—such as ultraviolet radiation, but also unavoidably by our own metabolism; for example, through the generation of ROS, which arise as by-products of mitochondrial activity. This is unfortunate in the light of the model of ageing described above. However, cells are not left alone in this continuous battle. To preserve the integrity of their genomes, living organisms have evolved a plethora of molecules, which range from ligases that join broken ends, to proteins that restrict cell growth in the presence of DNA damage. Much of the recent work on genomic integrity has focused on analysing a signalling network known as the DNA damage response (DDR; Harper & Elledge, 2007). Arguably, the main initiators of this pathway are two members of the PIKK kinase family: ATM and ATR (Shiloh, 2003). The main role of this cascade is to stimulate DNA repair while limiting the expansion of damaged cells, either by arresting their growth or by inducing apoptosis. Not surprisingly, mutations in ATM or ATR lead to severe human diseases known as ataxia telangiectasia (MIM208900) and Seckel syndrome (MIM210600), respectively, which show accelerated ageing among other phenotypes.
The available mouse models of genomic instability syndromes often recapitulate many of the symptoms observed in the human patients, such as the development of cancer, immunodeficiencies, neurodegeneration and progeria. In this regard, the viability of ATM-deficient mice has allowed numerous genetic studies of the DDR in the past decade (Barlow et al, 1996; Xu & Baltimore, 1996; Yu et al, 1996). By contrast, the essential nature of ATR has significantly limited research on the physiological roles of this kinase (Brown & Baltimore, 2000; de Klein et al, 2000). However, although a complete elimination of ATR is incompatible with life, a splicing mutation that produces extremely low levels of ATR was found to cause Seckel syndrome in a subset of patients (O'Driscoll et al, 2003). On the basis of this information, my group has recently generated a mouse model of ATR-Seckel (Murga et al, 2009). As could perhaps be anticipated, ATR-Seckel mice also develop a progeroid syndrome, but analysis of its causes revealed an unexpected twist.
Embryonic DNA damage and ageing in Seckel mice
Our murine model of Seckel recapitulated all of the symptoms described in patients, including the characteristic ‘bird-headed dwarfism', after which the disease was named (Seckel, 1960). Two months after birth, several—but not all—tissues of the Seckel mice showed anomalies that are associated with normal ageing, including osteoporosis, accumulation of fat in the bone marrow, thinning of the skin and kyphosis. Mutant animals succumb to this segmental progeroid syndrome after less than six months of age. Notably, evidence of premature ageing has also been described in human patients (Arnold et al, 1999; Boscherini et al, 1981; Butler et al, 1987; Fathizadeh et al, 1979). Surprisingly, we could find almost no evidence of DNA damage in adult tissues of Seckel mice. This is in marked contrast to all of the previously published progeroid models, in which DNA damage gradually accumulates after birth (Lombard et al, 2005; Rossi et al, 2007). Thus, adult ATR-Seckel organs were ageing in the absence of overt signs of DNA damage. How could these potentially conflicting observations be reconciled?
The fact that Seckel mice were born smaller than wild-type mice was crucial to solving this puzzle. We reasoned that, even if adult organs did not show contemporaneous DNA damage, they could perhaps have been exposed to it at a previous stage. Indeed, Seckel embryos present a generalized accumulation of DNA damage in all tissues. Notably, the main effect of prenatal exposure to DNA damage is a decreased head size (Murphy, 1928), and perhaps the most notorious phenotype of Seckel mice and patients is a severe microcephaly. Hence, one possible interpretation of the Seckel phenotype is that the DNA damage to which mutant cells are exposed during embryonic development has a lasting effect that is manifested later in life.
To understand why limited ATR activity is particularly detrimental for embryonic stages, one must understand the type of damage to which this kinase responds. In contrast to ATM—which is activated exclusively by DNA double-stranded breaks—ATR responds to chromosomal breaks and to the still poorly defined type of damage known as ‘replicative stress' (Cimprich & Cortez, 2008). At the molecular level, Replicative stress is associated with the generation of single-stranded DNA stretches and abnormal replicative structures at sites where replication forks stall. In other words, the amount of Replicative stress is directly proportional to replication rates. As such, it is not surprising that protection against Replicative stress is particularly important during development. After all, organisms never replicate faster or more frequently than during embryonic development.
Acceleration of ageing through the loss of p53
The fact that the DNA damage generated in Seckel embryos was linked to replication helped us to understand another unexpected outcome of our study that has important cancer-related implications. The tumour suppressor p53 is a crucial effector in eliciting cellular responses to DNA damage. It is therefore not surprising that the absence of p53 has been shown to alleviate ageing phenotypes in several progeroid models (Rodier et al, 2007). In agreement with the presence of DNA damage, p53 accumulation was also widespread on Seckel embryos. However—in notable contrast to other available models—p53 deficiency in Seckel mice exacerbated the ageing phenotype to the point that few double-mutant animals were even born. This was the first example of accelerated ageing due to the loss of p53. Similar synthetic lethal effects of ATR and p53 have also been reported with a conditional allele of ATR (Ruzankina et al, 2009), the explanation for which lies in the replicative nature of the damage: in the absence of p53, increased replication rates lead to even higher amounts of Replicative stress, which are able to activate apoptosis independently of p53.
Beyond its importance in the context of Seckel syndrome, the synthetic lethal effects of the loss of p53 and ATR are of obvious relevance in cancer. Furthermore, if the model postulated here is correct, this implies that the loss of ATR should not only be synthetically lethal with the loss of tumour suppressors, but also with the activation of oncogenes that boost replication. In other words, an ATR inhibitor might be particularly toxic for cells bearing carcinogenic mutations. This hypothesis also implies that diminishing the intrauterine replication rate might be sufficient to alleviate the adult phenotypes associated to Seckel syndrome. My group is actively exploring these two possibilities.
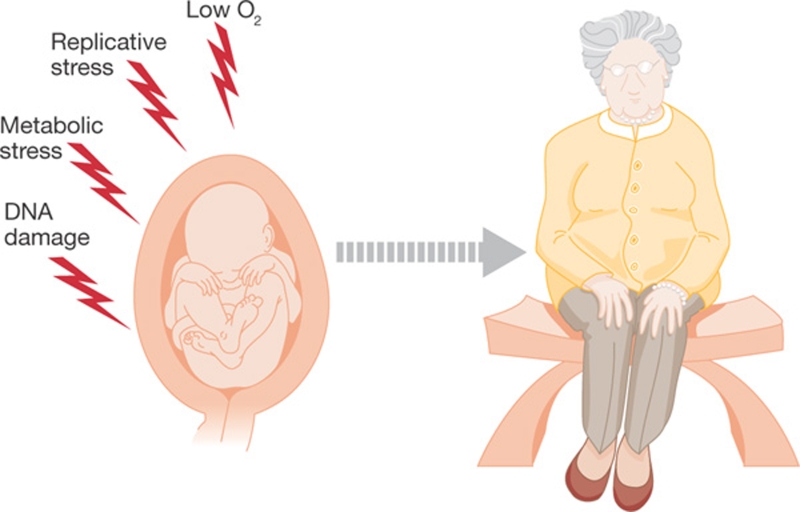
Perhaps the main conceptual implication of these results is that mammals can succumb to a progeroid disease even if its cause—in this case DNA damage—occurs during embryonic development. This led us to propose that prenatal exposure to DNA damage can determine the onset of ageing later in life (Fig 1).
Figure 1.
Intrauterine programming of ageing. The stresses to which embryos are subjected during development can determine the onset of ageing many years later.
Intrauterine programming
Human epidemiological studies were the first to reveal that impaired intrauterine growth could be associated with a later onset of disease (Barker, 1994). This phenomenon is now known as ‘intrauterine programming' and has been associated particularly with metabolic disorders such as diabetes, but also linked to early menopause and symptoms associated frequently with old age, such as cardiovascular problems and osteoporosis (Fowden et al, 2006). Importantly, these correlations between fetal distress and adult disease were found to exist independently of the level of obesity or exercise in the adult (Barker, 1994; Gluckman & Hanson, 2005). Given the inherent difficulties of assessing embryonic stress in humans, these studies are essentially based in the evaluation of statistical correlations between a given disease and birth weights. Much of the research has centred on the effects of nutritional deficits during gestation, known as ‘nutritional programming'. In this context, the shortage of key metabolites or hormones is thought to be responsible for the poor development of specific cell types, which will only be manifested in adulthood. Recent epidemiological studies have shown that Muslim women who observe the one-month fast of Ramadan early in their pregnancy are more likely to have children with low birth weights and mental—mainly learning—disabilities (Almond & Mazumder, 2008). Nutritional intrauterine programming has been studied particularly in the case of metabolic diseases, such as diabetes (Wolf, 2003). The effect of poor nutrition during gestation on overall lifespan has also been explored in model organisms, including mice. Interestingly, although calorie restriction is known to extend lifespan in various organisms (Koubova & Guarente, 2003), it reduces the life expectancy of the descendants by up to 30% (Ozanne & Hales, 2004) when applied to pregnant mice.
The effect of other types of stress on the intrauterine programming of adult diseases has not been studied in depth. Needless to say, the effects of prenatal exposure to DNA damage are largely unexplored in humans. Most of the available data comes from studying the side effects of nuclear accidents or from the survivors of the atomic bombs dropped over Hiroshima and Nagasaki that were in utero at the time of radiation exposure (Otake & Schull, 1998). As mentioned, the main effect was on brain size, which is also significantly reduced in Seckel patients and mice. However, no human study has focused on the longitudinal effect of prenatal exposure to DNA damage on ageing. Some work on the prenatal effects of DNA-damaging drugs has been done in model organisms, but these studies evaluated mostly the teratological effects of compounds used for cancer treatment—such as congenital abnormalities—and failed to explore the long-term effects of drug exposure. Notably, most of the anomalies that were observed on an intrauterine exposure of rats to hydroxyurea—a potent Replicative stress-inducing agent—were head malformations, which, as mentioned above, are a characteristic of Seckel syndrome (Chaube & Murphy, 1966). It should be kept in mind that these studies focused on the effects of an acute prenatal exposure to DNA damage; a more persistent stress would probably have more lasting effects. Taking all the available data into consideration, I propose to include ageing in the list of processes that can be modulated by intrauterine programming.
How could ageing be programmed in utero?
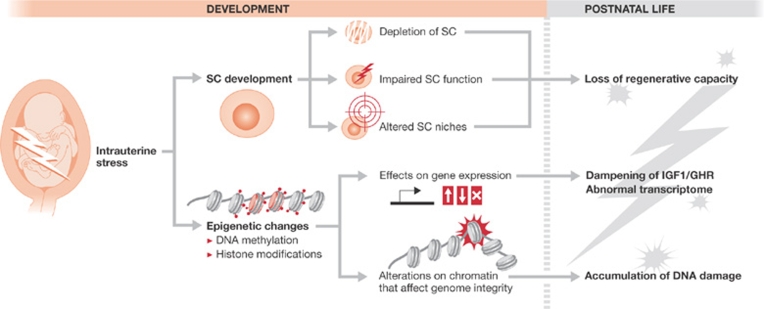
In this section, I discuss two possible mechanisms of programming ageing during development: SC dysfunction and epigenetic alterations (Fig 2).
Figure 2.
Proposed mechanisms to explain an intrauterine contribution to ageing. First, stem cell (SC) pools or their niches can be compromised during development, leading to a permanent limitation on the capacity of the tissues to regenerate in adult life. Second, the epigenome of embryonic cells can be altered by stress signals, which might permanently alter their transcriptome in both a stochastic and a programmed manner (that is, by dampening the IGF1/GHR axis, which is a charcateristic of ageing tissues). Moreover, the altered epigenetic landscape might have a lasting effect that renders chromatin more susceptible to breakage. GHR, growth hormone receptor; IGF1, insulin-like growth factor 1.
Ageing arises with the exhaustion of SC pools, with respect to both the amount of SCs and, importantly, SC functionality (Rossi et al, 2008). Hence, one explanation for the prenatal effect on ageing could be that the stress to which the embryos are exposed limits SC function, thereby also limiting the regenerative capacity of the tissues of the offspring. In this manner, the path to ageing would be shortened ab initio. The stress could directly affect the SCs, SC niches or both. In the particular case of replicative damage, it is probable that the niches would be the affected target, rather than the SCs, owing to the low cycling activity of SCs—a feature which is frequently used as a characteristic to identify them in vivo (Fuchs, 2009). Accordingly, the bone marrow of Seckel animals can reconstitute the haematopoietic pool of irradiated wild-type animals to a large extent, whereas the opposite is not true. This means that there is an inherent dysfunction of the Seckel haematopoietic SC niche, which does not support proper SC function. In summary, I suggest that the intrauterine programming of ageing in the context of Seckel syndrome might be, in part, a consequence of the mutant animals being born with poor SC niches, thereby compromising SC function for the rest of their lives.
Alternatively—or concomitantly—epigenetic changes that modulate gene expression could provide another explanation for the long-term effects of embryonic stress. Recent results have shown that aged tissues and tissues from progeroid animals present a specific transcriptional signature (Schumacher et al, 2008), which is characterized by inducing a dampening of the metabolic activity. This transcriptional state has been suggested to change the activity of the cells from a growth to a maintenance mode, allowing for an extension of life under conditions of stress. Importantly, exposing mice to sub-lethal doses of DNA damaging agents has been shown to trigger the same response (Niedernhofer et al, 2006), which reinforces the possibility that DNA damage is the cause of ageing. Interestingly, such a transcriptional signature is also observed in adult Seckel tissues, but in the absence of any concomitant DNA damage. Hence, this would be a case of a transcriptional output without its corresponding input. One possibility to explain this phenomenon is to consider it a case of “transcriptional memory” (Francis & Kingston, 2001): the DNA damage to which the tissues were exposed during development would have permanently altered the expression of ageing-related genes by the induction of epigenetic changes on their promoters. This response would then become independent of the original input—DNA damage—and remain active for the rest of the life of the mutant animals.
The link between epigenetics and ageing is not new. For example, overall DNA methylation levels were shown to decrease during normal ageing more than two decades ago (Wilson & Jones, 1983). A broader study recently revealed that the epigenetic landscape of histone and DNA modifications is significantly altered during the life of monozygotic twins (Fraga et al, 2005). One of the discoveries that attracted most attention in this regard is the role of sirtuins in regulating longevity (Finkel et al, 2009). The overexpression of sirtuins—which belong to a family of histone deacetylases—has been shown to extend lifespan in various organisms. Conversely, the elimination of sirtuin 2 in mice leads to progeria. However, the way in which sirtuins regulate longevity remains unknown. One possibility is that sirtuins have an active role in DNA repair, again linking their role in ageing to DNA damage. Yet, recent evidence has shown that sirtuins also coordinate the transcriptional response associated with ageing (Oberdoerffer et al, 2008). Finally, it is also possible that the chromatin changes associated with ageing not only affect gene expression, but also render DNA more susceptible to damage (Pegoraro et al, 2009). In summary, the epigenetic alterations that are established during embryogenesis could also contribute to the intrauterine programming of adult disease.
Conclusions
I propose that ageing can be influenced by intrauterine stress. This effect could be linked mechanistically to epigenetic changes initiated in utero, a permanent alteration of SCs and/or SC niches, or a combination of both. The implications of this concept are enormous. If something is programmed, it follows that it could be deprogrammed. If the ageing-linked chromatin modifications were known, one could try to erase them in adult animals as a means to promote longevity. In addition, this hypothesis raises the possibility of exploring life-extending approaches by altering the conditions during gestation. Future research will hopefully resolve the validity of these ideas (Sidebar A). Concepts aside, now that ageing studies have come to the forefront of biomedical research and ageing is sometimes considered as a pathological process, I would want to end by saying that healthy ageing should never be understood as a disease; as Mark Twain reportedly said, “Age is an issue of mind over matter. If you don't mind, it doesn't matter”.
Sidebar A | In need of answers.
Do ageing-related epigenetic changes modulate lifespan? The connection between epigenetics and ageing is an emergent topic, and several DNA or histone modifications have been found to accumulate during a lifetime. However, whether these changes are neutral bystanders or have an active role in triggering ageing remains unknown. If epigenetic manipulation can be shown to extend mammalian lifespan, it will put the spotlight of ageing research on epigenetics.
What is the contribution of stem cell (SC) niches to lifespan? SC research is an important topic in the ageing field. However, the extent to which the SC niches—rather than SCs themselves—contribute to the phenotypes found in the elderly remains largely unknown. Tools that allow a proper study of SC niches—and even their identification—should be developed to provide insight into their effect on ageing.
To what extent is ageing normally affected by development? The study of Seckel mice was a significant example, showing that large amounts of embryonic DNA damage exert a phenotype in the adults. However, this is an extreme case and might not apply to normal situations. Do other types of intrauterine stress have a similar effect? Is normal development devoid of stress or is there significant variation among individuals? These and other questions remain open for future study.
Oscar Fernandez-Capetillo
Acknowledgments
Work in the author's laboratory is supported by grants from the Spanish Ministry of Science (RYC-2003-002,731, CSD2007-00017 and BFU2005-09,429), European Research Council (ERC-210,520) and the EMBO Young Investigator Programme.
References
- Almond D, Mazumder B (2008) Health capital and the prenatal environment: the effect of maternal fasting during pregnancy. NBER Work Pap Ser 14428 [Google Scholar]
- Arnold SR, Spicer D, Kouseff B, Lacson A, Gilbert-Barness E (1999) Seckel-like syndrome in three siblings. Pediatr Dev Pathol 2: 180–187 [DOI] [PubMed] [Google Scholar]
- Barker DJP (1994) Mothers, Babies and Disease. London, UK: BMJ [Google Scholar]
- Barlow C et al. (1996) Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171 [DOI] [PubMed] [Google Scholar]
- Boscherini B, Iannaccone G, La Cauza C, Mancuso G, Girotti F, Finocchi G, Pasquino AM (1981) Intrauterine growth retardation. A report of two cases with bird-headed appearance, skeletal changes and peripheral GH resistance. Eur J Pediatr 137: 237–242 [DOI] [PubMed] [Google Scholar]
- Brown EJ, Baltimore D (2000) ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev 14: 397–402 [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Hall BD, Maclean RN, Lozzio CB (1987) Do some patients with Seckel syndrome have hematological problems and/or chromosome breakage? Am J Med Genet 27: 645–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D (2008) ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 9: 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaube S, Murphy ML (1966) The effects of hydroxyurea and related compounds on the rat fetus. Cancer Res 26: 1448–1457 [PubMed] [Google Scholar]
- de Klein A, Muijtjens M, van Os R, Verhoeven Y, Smit B, Carr AM, Lehmann AR, Hoeijmakers JH (2000) Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr Biol 10: 479–482 [DOI] [PubMed] [Google Scholar]
- Fathizadeh A, Soltani K, Medenica M, Lorincz AL (1979) Pigmentary changes in Seckel's syndrome. J Am Acad Dermatol 1: 52–54 [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R (2009) Recent progress in the biology and physiology of sirtuins. Nature 460: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Giussani DA, Forhead AJ (2006) Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21: 29–37 [DOI] [PubMed] [Google Scholar]
- Fraga MF et al. (2005) Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 102: 10604–10609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NJ, Kingston RE (2001) Mechanisms of transcriptional memory. Nat Rev Mol Cell Biol 2: 409–421 [DOI] [PubMed] [Google Scholar]
- Fuchs E (2009) The tortoise and the hair: slow-cycling cells in the stem cell race. Cell 137: 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis GA, van der Horst GT, Vijg J, Hoeijmakers JH (2008) DNA damage and ageing: new-age ideas for an age-old problem. Nat Cell Biol 10: 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman P, Hanson M (2005) The Fetal Matrix: Evolution, Development and Disease. Cambridge, UK: Cambridge University Press [Google Scholar]
- Harper JW, Elledge SJ (2007) The DNA damage response: ten years after. Mol Cell 28: 739–745 [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L (2003) How does calorie restriction work? Genes Dev 17: 313–321 [DOI] [PubMed] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) DNA repair, genome stability, and aging. Cell 120: 497–512 [DOI] [PubMed] [Google Scholar]
- Murga M, Buenting S, Montaña MF, Soria R, Lee YS, McKinnon PJ, Nussenzweig A, Fernandez-Capetillo O (2009) A mouse model of ATR-Seckel shows embryonic DNA replicative stress and accelerated ageing. Nat Genet 41: 891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DP (1928) Ovarian radiation—its effect on the health of subsequent children. Review of the literature, experimental and clinical, with a report of 320 human pregnancies. Surg Gynecol Obstet 47: 201–221 [Google Scholar]
- Niedernhofer LJ et al. (2006) A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444: 1038–1043 [DOI] [PubMed] [Google Scholar]
- O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA (2003) A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33: 497–501 [DOI] [PubMed] [Google Scholar]
- Oberdoerffer P et al. (2008) SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135: 907–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake M, Schull WJ (1998) Radiation-related brain damage and growth retardation among the prenatally exposed atomic bomb survivors. Int J Radiat Biol 74: 159–171 [DOI] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN (2004) Lifespan: catch-up growth and obesity in male mice. Nature 427: 411–412 [DOI] [PubMed] [Google Scholar]
- Ozgenc A, Loeb LA (2005) Current advances in unraveling the function of the Werner syndrome protein. Mutat Res 577: 237–251 [DOI] [PubMed] [Google Scholar]
- Pegoraro G, Kubben N, Wickert U, Gohler H, Hoffmann K, Misteli T (2009) Ageing-related chromatin defects through loss of the NURD complex. Nat Cell Biol 11: 1261–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J, Bhaumik D (2007) Two faces of p53: aging and tumor suppression. Nucleic Acids Res 35: 7475–7484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447: 725–729 [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL (2008) Stems cells and the pathways to aging and cancer. Cell 132: 681–696 [DOI] [PubMed] [Google Scholar]
- Ruzankina Y, Schoppy DW, Asare A, Clark CE, Vonderheide RH, Brown EJ (2009) Tissue regenerative delays and synthetic lethality in adult mice after combined deletion of Atr and Trp53. Nat Genet 41: 1144–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher B, Garinis GA, Hoeijmakers JH (2008) Age to survive: DNA damage and aging. Trends Genet 24: 77–85 [DOI] [PubMed] [Google Scholar]
- Seckel H (1960) Bird-Headed Dwarfs: Studies in Developmental Anthropology Including Human Proportions. Springfield, IL, USA: Charles C Thomas [Google Scholar]
- Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC (2004) Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 6: 168–170 [DOI] [PubMed] [Google Scholar]
- Shiloh Y (2003) ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3: 155–168 [DOI] [PubMed] [Google Scholar]
- Wilson VL, Jones PA (1983) DNA methylation decreases in aging but not in immortal cells. Science 220: 1055–1057 [DOI] [PubMed] [Google Scholar]
- Wolf G (2003) Adult type 2 diabetes induced by intrauterine growth retardation. Nutr Rev 61: 176–179 [DOI] [PubMed] [Google Scholar]
- Xu Y, Baltimore D (1996) Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev 10: 2401–2410 [DOI] [PubMed] [Google Scholar]
- Yu CE et al. (1996) Positional cloning of the Werner's syndrome gene. Science 272: 258–262 [DOI] [PubMed] [Google Scholar]