Abstract
It is now recognised that a part of the inherited risk of colorectal cancer (CRC) can be explained by the co-inheritance of low-penetrance genetic variants. The accumulated experience to date in identifying these variants has served to highlight difficulties in conducting statistically and methodologically rigorous studies and follow-up analyses. The COGENT (COlorectal cancer GENeTics) consortium includes 20 research groups in Europe, Australia, the Americas, China and Japan. The overarching goal of COGENT is to identify and characterise low-penetrance susceptibility variants for CRC through association-based analyses. In this study, we review the rationale for identifying low-penetrance variants for CRC and our proposed strategy for establishing COGENT.
Keywords: colorectal cancer, association, polymorphism
Background
Although inherited susceptibility underlies ∼35% of variance in colorectal cancer (CRC) risk (Lichtenstein et al, 2000), high-penetrance germline mutations account for <6% of cases (Aaltonen et al, 2007). Much of the remaining variation in genetic risk is likely to be a consequence of the co-inheritance of multiple low-penetrance variants, some of which are common.
The ‘common-disease common-variant’ model of CRC implies that association analyses based on scans of polymorphic variants should be a powerful strategy for identifying low-penetrance susceptibility alleles. This assertion has recently been vindicated by genome-wide association (GWA) studies, which have provided robust evidence for several common low-risk variants influencing CRC risk (Tomlinson et al, 2005, 2007; Broderick et al, 2007; Zanke et al, 2007; Houlston et al, 2008; Jaeger et al, 2008; Tenesa et al, 2008). Although the risk of CRC associated with each of these common variants is individually modest, they make a significant contribution to the overall disease burden by virtue of their high frequencies in the population. Moreover, by acting in concert with each other, they have the potential to significantly affect an individual's risk of developing CRC. Hence, this class of susceptibility alleles is potentially of public health importance, allowing risk stratification within populations. One benefit for risk prediction between population subgroups is that it could enable tailoring of the invasiveness or frequency of large bowel screening, eventually leading to a reduction in mortality and even incidence through secondary prevention. Finally, the identification of new risk variants may identify new cancer pathways that made lead, in time, to the development of new prevention or treatment strategies for CRC.
To facilitate the study of predisposition to CRC, we established COGENT (COlorectal cancer GENeTics), an international consortium with the goal of identifying and characterising low-penetrance genetic variants that predispose to CRC. In this study, we review the rationale for studying low-penetrance susceptibility to CRC and our proposed strategy for COGENT.
Difficulties in conducting methodologically rigorous association studies
To date, most association studies based on the candidate gene approach have only evaluated a restricted number of polymorphisms, primarily in genes implicated in the metabolism of dietary carcinogens and protection of DNA from carcinogen-induced damage. Reports from these studies have largely been disappointing, with numerous positive associations initially from analyses of small case–control series being unconfirmed by subsequent analyses. Only a minority of studies have reported case–control data on the same variants, allowing pooling of data (Table 1). Although P-values from meta-analyses of such studies provide limited support for the role of variants in MTHFR (Huang et al, 2007; Hubner and Houlston, 2007), CCND1 (Tan et al, 2008), GSTT1 (de Jong et al, 2002), XPC (Zhang et al, 2008), NQO1 (Chao et al, 2006) and NAT2 (Chen et al, 2005), such analyses should be interpreted with caution even if publication bias is ignored. Use of false-positive report probability value (FPRP) (Wacholder et al, 2004), which integrates the earlier probability for association and statistical power, provides one method for assessing the robustness of summary estimates derived from pooled analyses. Although earlier probabilities are partly subjective, influenced by previous findings and experimental evidence with regard to the known impact of variants, the earlier probability for variants in candidate genes is unlikely to be better than 1 in 1000 (or 0.001) (Thomas and Clayton, 2004). Imposing a ‘best case’ value less than 0.001 and stipulating an odds ratio of 1.2 for associations, it is noteworthy that the likelihood of any of the variants being associated with CRC risk is not high (i.e., FPRP >0.2 suggested to be appropriate for summary analyses (Wacholder et al, 2004)). Hence, despite much research, until the advent of GWA studies, few, if any, definitive susceptibility alleles for CRC have been unequivocally identified through association studies. The accumulated experience to date has served to highlight the difficulties in conducting statistically and methodologically rigorous association studies to identify new cancer predisposition loci. The main issues are summarised below:
The increase in CRC risk conferred by any common polymorphic variant is almost certainly small (i.e., typical relative risk ∼1.2). The inherent statistical uncertainty of case–control studies involving just a few hundred cases and controls severely constrains study power to reliably identify genetic determinants conferring modest, but potentially important, risks.
As of the large number of polymorphisms in the genome, false-positive associations are inevitably more frequent than true-positive associations when testing large numbers of generic markers (especially when using off-the-shelf SNP arrays), even if studies are conducted in a scientifically rigorous manner. Hence, associations need to attain a high level of statistical significance to be established beyond reasonable doubt. For this reason, in GWA studies, a P-value threshold of 5.0 × 10−7 has been advocated and is generally considered to be appropriate for genome-wide significance.
Positive associations need to be replicated in independent case–control series to further limit the type 1 error rate. However, to increase power, the allelic architecture of the population from which these case–control series are ascertained needs to have similar ancestry and, ideally, the same linkage disequilibrium (LD) structure.
It should be recognised that cancers such as CRC are somewhat heterogeneous with respect to aetiology and biology. Specifically for CRC, colonic and rectal disease may have different risk factors and have a varied spectrum of somatic mutations and epimutations. It must thus be recognised that a given variant may not affect the risk of all histological forms of CRC. The power of any analysis stratified by histology is therefore limited because of the smaller numbers of cases in each group.
Careful attention must be paid to population stratification as a source of confounding, because cancer rates and allele frequencies vary with race/ethnicity. This is one possible explanation for some of the false-positive associations reported in literature.
Epidemiological risk factor data should ideally be taken into consideration to allow the examination of interactions between known aetiological factors (e.g., dietary risk factors) and genetic risk variants. As very large sample sizes are probably needed to detect interactions, the power of these types of analyses in the association studies reported to date has been extremely limited.
Rare germline polymorphisms may be more highly penetrant and have significance for individuals, although the population-attributable risk may be low. Extreme examples include the previously identified mutations in DNA repair enzymes and Lynch Syndrome. Only through genotyping and sequencing of large numbers of individuals can additional rare variants that confer important individual risk be identified. Advances in sequencing technology make this feasible.
Table 1. Polymorphisms reported to be statistically significant in meta-analyses.
| Polymorphism | Risk group | MAF/at risk frequency | OR (95% CI) | P-value | No studies/cases | Power, OR=1.2 | FPRP @ prior of 0.001 |
|---|---|---|---|---|---|---|---|
| CCND1-G870A | GA vs GG | 0.12–0.64 | 1.18 (1.06–1.32) | 0.0031 | 12/4614 | 61% | 0.86 |
| GSTT1-null | Null vs present | 0.21–0.44 | 1.37 (1.17–1.60) | 8.1 × 10−5 | 11/1490 | 5% | 0.60 |
| MTHFR-V222A | TT vs CC | 0.32–0.40 | 0.83 (0.75–0.93) | 0.0007 | 25/12 261 | 47% | 0.74 |
| MTHFR A1298C | CC vs CA+AA | 0.29–0.22 | 0.81 (0.69–0.96) | 0.012 | 14/4764 | 37% | 0.98 |
| NAT-acetylator | Rapid vs slow | 0.32–0.77 | 1.08 (1.00–1.16) | 0.04 | 18/6741 | 99% | 0.97 |
| NQO1-Pro187Ser | CT+TT vs CC | 0.11–0.23 | 1.18 (1.02–1.35) | 0.02 | 5/1637 | 60% | 0.96 |
| XPC lys939Gl | CA vs AA | 0.65–0.61 | 1.32 (1.11–1.56) | 0.001 | 2/1060 | 13% | 0.90 |
Abbreviations: CI=confidence interval; FPRP=false-positive report probability; MAF=minor allele frequency; OR=odds ratio.
Adapted from Dong et al (2008) and Hubner and Houlston.
Characteristics of low-penetrance variants
Most studies aimed at identifying low-penetrance alleles for cancer susceptibility have been based on a candidate gene approach formulated on preconceptions of pathology pertaining to the role of specific genes in the development of CRC. However, without a clear understanding of the biology of predisposition, the choice of suitable genes for the disease is inherently problematic, and very few susceptibility loci for CRC have been identified that adopt this strategy. An unbiased approach to genetic analysis is therefore required.
The availability of high-resolution LD maps and hence of comprehensive sets of tagging SNPs that capture most of the common sequence variation allows GWA studies for disease associations to be efficiently conducted. This approach is agnostic in that it does not depend on previous knowledge of function or presumptive involvement of any gene in disease causation. Moreover, it minimises the probability of failing to identify important common variants in hitherto unstudied loci (i.e., genes and regulatory regions).
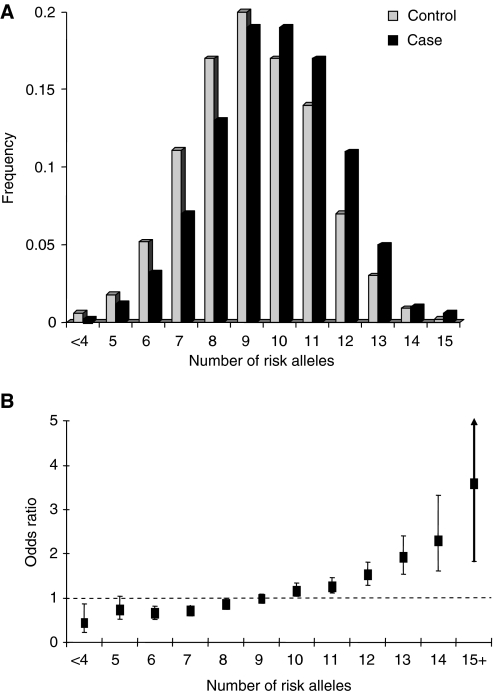
Three GWA studies of CRC have so far been reported and 10 independent loci shown conclusively to be associated with CRC risk: 8q24.21, 11q23, 18q21.1 (SMAD7), 8q23.1 (EIF3H), 15q (GREM1), 19q13.1 (RHPN2), 20q12.3, 14q22.2 (BMP4), 16q22.1 (CDH1) and 10p14 (Tomlinson et al, 2005, 2007; Broderick et al, 2007; Zanke et al, 2007; Houlston et al, 2008; Jaeger et al, 2008; Tenesa et al, 2008). Risks associated with each of the common variants at each of these loci are modest (ORs 1.1–1.3; Table 2) and there is little evidence of interactive effects. With homozygous risk variants conferring twice the heterozygote risk, the distribution of risk alleles follows a normal distribution in both case and controls, with a shift towards a higher number of risk alleles in affected individuals consistent with a polygenic model of disease predisposition (Figure 1A). Figure 1B shows the ORs relative to the median number of risk alleles. Individuals with 15+ risk alleles have at least a three-fold increase in risk compared with those with a median number of risk alleles.
Table 2. The 10 loci associated with colorectal cancer risk identified from GWA studies (Tenesa and Dunlop, 2009).
| Gene/locus | Chromosome | SNP | Effect size (odds ratio) | Allele frequency | Population attributable risk (%) |
|---|---|---|---|---|---|
| — | 8q24 | rs6983267 | 1.21 (1.15–1.27) | 0.51 | 9.7 |
| GREM1 | 15q13 | rs4779584 | 1.26 (1.19–1.34 | 0.18 | 4.5 |
| SMAD7 | 18q21 | rs4939827 | 1.18 (1.12–1.23 | 0.52 | 8.6 |
| — | 11q23 | rs3802842 | 1.12 (1.07–1.17) | 0.29 | 3.4 |
| EIF3H | 8q23 | rs16892766 | 1.25 (1.19–1.32) | 0.07 | 1.7 |
| — | 10p14 | rs10795668 | 1.12 (1.10–1.16) | 0.67 | 7.4 |
| BMP4 | 14q21 | rs4444235 | 1.11 (1.08–1.15) | 0.46 | 4.8 |
| CDH1 | 16q22 | rs9929218 | 1.10 (1.06–1.12) | 0.71 | 6.6 |
| RHPN2 | 19q13 | rs10411210 | 1.15 (1.10–1.20) | 0.90 | 11.9 |
| BMP2 | 20q12 | rs961253 | 1.12 (1.08–1.16) | 0.35 | 4.0 |
Abbreviation: GWA=genome-wide association.
Figure 1.
Polygenic model of colorectal cancer susceptibility. (A) Distribution of risk alleles for CRC, cases (black bars) and controls (grey bars); (B) Plot of the increasing ORs for CRC with increasing number of risk alleles. The ORs are relative to the median number of risk alleles; Vertical bars correspond to 95% confidence intervals. Data from Houlston et al (2008).
Data from these GWA studies and results from similar gene discovery efforts in other tumours are proving to be highly informative with regard to the allelic architecture of cancer susceptibility in general. The number of common variants that account for more than 1% of inherited risk is very low and only a small proportion of the heritability of any cancer can be explained by currently identified loci. Estimates of the contribution of currently identified loci to excess familial risk of CRC may be conservative, as there may be imperfect tagging surrogates for true aetiological loci. Multiple causal variants may also exist at each locus, including low-frequency variants with significantly larger cumulative effects on risk. Few of the observed disease-associated variants are coding variants, with many of the loci mapping to regions bereft of genes or protein-encoding transcripts. It is likely that much of the common variation in cancer risk is mediated through sequence changes influencing gene expression, perhaps in a subtle manner, or through effects on pathway components mitigated by functional redundancy.
Future directions
Prospects for identifying additional common variants
The power of existing GWA studies to identify common alleles conferring risks of 1.2 or greater (such as the 8q24 variant) is high. Hence, there are unlikely to be many additional CRC SNPs with similar effects for alleles with frequencies >0.2 in populations of European ancestry. Recent studies have had low power to detect alleles with smaller effects and/or MAFs <0.1. By implication, variants with such profiles are likely to collectively confer substantial risk because of their multiplicity or sub-maximal LD with tagging SNPs. The tagging SNPs used for GWA studies capture on an average ∼80% of common SNPs in the European population (i.e., r2>0.8), but only ∼12% of SNPs with MAFs of 5–10% are tagged at this level, limiting the power to detect this class of susceptibility allele. GWA-based strategies are not configured optimally to identify low-frequency variants with potentially stronger effects or to identify recessively acting alleles. Nor are current arrays formatted ideally to capture copy number variants or other structural variants such as small-scale insertions or deletions, which may affect CRC risk. It is therefore highly likely that a large number of low-penetrance variants remain to be discovered. This assertion is supported by the continued excess of associations observed over those expected in studies reported to date. Further efforts to expand the scale of GWA meta-analyses, in terms of both sample size and SNP coverage, and to increase the number of SNPs taken forward to large-scale replication may identify additional variants for CRC.
Analyses of most GWA studies have so far been primarily directed towards identifying single locus SNP associations. It is possible that analyses based on haplotypes of markers may identify ‘rarer’ disease alleles that may be present on rare haplotypes missed by single SNP analyses. Under certain circumstances, especially in which interaction effects are large and main effects are small, gene–gene interactions may be detected where no locus with a main effect has been identified (Marchini et al, 2005). Multi-locus approaches may therefore be the focus of future experiments as they may yield greater power to detect associations under certain genetic models.
Identifying causal variants
Validated tagSNPs are highly unlikely to directly cause CRC. Identifying the causal variant from a tagSNP that is statistically associated with disease is difficult. Although blocks of LD allow the efficient survey of the genome, they hamper fine mapping of the disease-associated region. Different ethnic groups are likely to have different LD block patterns and they can therefore be used to refine the location of a disease susceptibility locus before resequencing and functional analyses. The usefulness of this approach depends on the size of the study and SNP allele frequencies in different ethnic groups. In some of these populations, lower environmental risk exposure with lower CRC, incomplete case ascertainment and recording tools, as well as absence of large sample sets, are other challenges.
Incorporating non-genetic risk factors into risk models
Colorectal cancer risk will probably be determined by complex interactions between the various genetic and lifestyle/dietary risk factors. Epidemiological studies have established several dietary risk factors for colorectal neoplasia; these include low vegetable and high meat consumption (especially processed meat), and micronutrient deficiency and excessive alcohol intake. There is a weaker association between CRC, smoking and lack of physical activity. Common genetic variants are likely to interact with these environmental–lifestyle risk factors to modify risk. Furthermore, common gene variants will have a role in determining the effectiveness of chemoprevention agents such as non-steroidal anti-inflammatory drugs, hormone replacement therapy and micronutrient supplementation.
In assessing the interplay between inherited and non-genetic risk factors, analyses using different population cohorts will be highly informative. Wider comparisons between the population genetics of different ethnic groups have shown that SNP allele frequencies can vary greatly among ethnic groups, principally as a result of founder effects and genetic drift. Indeed, some SNPs may be informative in one population and not in another. At least in principle and probably in practice, some variants may have stronger or weaker effects on disease, depending on environment or general genetic background, as observed in inbred lines of mice.
Identification of the interaction between genetic variants and environmental risk factors is contingent on very large data sets ideally from different population cohorts, something only achievable through multi-centre collaborations. Even with such collaborative efforts, incorporating environmental risk factor data into models of predisposition is likely to be a serious challenge as, although ethnicity can be defined through genotype, environmental background is harder to standardise.
Inherited prognostic and predictive variants
In addition to influencing the risk of developing CRC, inherited genetic host factors are likely to influence the natural course of the disease. As a potential prognostic factor, the concept of germline variation imparting inter-individual variability in tumour development, progression and metastasis is receiving increasing attention. Compared with breast cancer, studies of the impact of germline variation on CRC prognosis have been more limited. Prognostic studies have generally examined the same candidate genes that are considered to have a role in predisposition. Genetic variation affecting inter-individual disease expression may influence the later stages of malignancy rather than early events associated with an inherited predisposition. Variants in growth factor, apoptosis or immune surveillance signalling pathways, for instance, might not cause CRC initiation but could have a substantial effect on the outcome of established disease. Chemotherapy response and toxicity may be related to germline genotype. Linking GWA data to patient outcome provides an attractive strategy for identifying new prognostic markers. It is essential to impose appropriate statistical thresholds and conduct replication analyses to avoid reporting false positives.
Rationale for the cogent consortium
The recognition that low-penetrance alleles contribute to the inherited risk of CRC represents a major advance in our understanding. In view of the above-noted issues, over the last few years, collaborations have been steadily developing between groups in the United Kingdom, Canada, the Americas, Holland, Germany, Finland, Spain and Australia that are engaged in ongoing searches for low-penetrance CRC variants through association-based studies. What initially began from relatively loose affiliations centred around work on specific projects has begun to crystallise into a more formal collaborative network after replication analyses of two published GWA studies. To continue and expand collaboration, a meeting was held at the University of Leiden, the Netherlands, in January 2009 to review ongoing association studies. There assembled an international team of researchers with expertise encompassing genetic epidemiology, statistical genetics, gene mapping, biology, molecular genetics, pathology and diagnosis and clinical management of CRC.
There was a consensus among participants that many of the challenges inherent in this field can best be addressed by international cooperative efforts, and the group unanimously decided to establish a CRC association consortium. An invitation to join COGENT that was subsequently extended to other groups known to be performing CRC association studies was well received. At present, 20 groups that are performing case–control genetic association studies have joined COGENT (Table 3). The eligibility criterion for inclusion is the involvement in a case–control study based on at least 500 cases and 500 controls sampled from the same population. The sample size limit aims to ameliorate the potential statistical, biological and technological/methodological confounding effects of small sample sizes (Moonesinghe et al, 2008). Collectively, over 48 000 cases and 43 000 controls have so far been accrued by COGENT researchers (Table 3).
Table 3. Number of CRC cases and controls currently established by COGENT consortium members.
|
Number of subjects
|
||||
|---|---|---|---|---|
| European | Study name | General setting | Cases | Controls |
| Institute of Cancer Research, UK | NSCCG (National Study of Colorectal Cancer) (Penegar et al, 2007) | Population-based UK study. Spouse controls from NSCCG (Penegar et al, 2007) and GELCAPS (Genetic Lung Cancer Predisposition Study) (Eisen et al, 2008) | 12 976 | 6000 |
| Edinburgh University, UK | COGS (Colorectal Cancer Genetics Susceptibly Study) (Porteous et al, 2003) | Population-based incident case series aged <55 at diagnosis. Population-based controls | 1012 | 1012 |
| SOCCS (Scottish Colorectal Cancer Study) (Theodoratou et al, 2008) | Population-based incident case series; Scotland, UK | 3000 | 3000 | |
| Oxford University, UK | CORGI (Colorectal Tumour Gene Identification Consortium) (Kemp et al, 2006) | Cases with family history of CRC ascertained through clinical genetics centres in the UK. Spouse controls with no personal or family history of CRC | 940 | 965 |
| VICTOR – post-treatment stage of a Phase III, randomised double-blind, placebo-controlled study of rofecoxib (VIOXX) in colorectal cancer patients after potentially curative therapy (Kerr et al, 2007) | Samples from a closed clinical trial | 910 | — | |
| QUAZAR2 – multicentre international study of capecitbine+/−bevacizumab as adjuvant treatment of CRC | UK blood donors | 139 | 376 | |
| Cambridge University, UK | SEARCH (Studies of Epidemiology and Risk Factors in Cancer Hereditary) | Population-based case–control study; Cambridge, UK | 3000 | 3000 |
| Barcelona and Santiago, Spain | EPICOLON Consortium (Castells and Andreu, 2007) | Population-based case–control study; Spain | 2000 | 2000 |
| Barcelona, Spain | ENTERICOS (Disinfection by-products and other Environmental, genetic and molecular determinants of colorectal cancer – subproductos de la desinfección y otros determinantes ambientales, genéticos y moleculares del cáncer colorectal en España) | Case–control study of CRC to evaluate the increased risk associated with chronic DBP exposure through ingestion, inhalation and dermal absorption | 500 | 500 |
| Bellvitge Case–Control Study | 370 | 325 | ||
| University of Helsinki, Finland | FCCPS (Finnish Colorectal Cancer Predisposition Study) (Salovaara et al, 2000) | Population-based study; South-eastern Finland | 1440 | 2000 |
| Karolinska Institute, Sweden | Unselected cases ascertained through 12 hospitals serving the Stockholm-Gotland and Uppsala-Örebro health-care regions in Sweden. Blood donor controls | 3000 | 3000 | |
| German Cancer Research Centre (DKFZ): on behalf of German HNPCC consortium | German HNPCC consortium (Frank et al, 2008) | Familial non-HNPCC cases recruited through German HNPCC consortium, principally through six hospitals of Bochum, Bonn, Dresden, Düsseldorf, Heidelberg and Munich/Regensburg. Controls: unrelated and ethnicity- and age-matched blood donors recruited by the Institute of Transfusion Medicine and Immunology, Faculty of Mannheim, Germany | 1000 | 1000 |
| University of Keil and Greifswald, Germany | POPGEN (Population Genetic Cohort) from Schleswig-Holstein, north Germany (Krawczak et al, 2006; Schafmayer et al, 2007). SHIP (Survey of Health in Pommerania) from east and north-east Germany (Volzke et al, 2005) | Population-based biobank projects | 2720 | 2720 |
| German Cancer Research Centre | ESTHER (Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten Therapie chronischer Erkrankungen in der älteren Bevölkerung) | Population-based biobank project | 670 | 670 |
| Institute of Experimental Medicine, Academy of Science, Czech Republic | — | Unselected CRC cases mainly recruited from nine oncological departments (Prague, Benesov, Brno, Liberec, Ples, Pribram, Usti nad Labem and Zlin) (Tulupova et al, 2008). Controls hospital patient and blood donors | 1300 | 3000 |
| University of Groningen, the Netherlands | SCOPE study (de Jong et al, 2005) | Unselected CRC cases, hospital patient controls from the Netherlands | 774 | 1000 |
| University of Leiden, the Netherlands | Unselected CRC cases. Controls ascertained through genetic testing programmes for non-cancer-related conditions | 1500 | 1500 | |
| Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy | Unselected CRC cases, population controls | 700 | 1200 | |
| Australia | ||||
| Ludwig Institute for Cancer Research, Melbourne | Victorian Cancer Biobank | Population-based biobank project | 1000 | 500 |
| The University of Newcastle, New South Wales | Hunter Family Cancer Service | Population-based collection of cases and controls from the Hunter Region of New South Wales | 600 | 3000 |
| The Americas | ||||
| Ibague, Colombia. Universidad del Tolima | Unselected CRC cases, population-based controls | 500 | 700 | |
| Toronto, Canada | OFCCR (Ontario Familial Colorectal Cancer Registry) (Cotterchio et al, 2000) | Population-based case–control study; Ontario | 1257 | 1336 |
| Asia | ||||
| University Hong Kong Medical Centre, China | UHKMC series | Unselected CRC cases, hospital patient controls | 2000 | 2000 |
| University of Tokyo, Japan | Biobank Japan | Population-based biobank project | 6000 | 6000 |
| Total | 49 308 | 46 804 | ||
Abbreviations: COGENT=COlorectal cancer GENeTics; CRC=colorectal cancer.
In each of the study centres, collection of samples and of clinico-pathological information has been undertaken with informed consent and relevant ethical review board approval in accordance with the tenets of the Declaration of Helsinki. Material transfer agreements have already been used between partners to allow for sharing of individualised data, and similar procedures will be adopted for future collaborative work.
Data pooling provides a very cost-effective approach to achieve an adequate power for subgroup analyses, which are unlikely to have sufficient sample sizes in a single study. Several potential problems need to be considered at the stage of data pooling. Given that individual studies have different data formats, covariates from individual studies will be agreed upon and compiled into a common set of variables relevant to specific projects. Study data sets sent from different centres will be checked for outliers, aberrant distribution, inadmissible values and inconsistencies before pooling to ensure data accuracy. Systematic variation between centres in terms of genotyping will be assessed globally using principal components and on a per-SNP basis. Discrepancies can be cross-verified with study centres.
COGENT represents the first international collaborative study seeking to comprehensively understand the impact of low-penetrance susceptibility to CRC and to describe the genetic landscape of the disease. The immediate goal of the group is to work together collaboratively to study polymorphisms that were previously associated with CRC risk and to plan for future high-quality studies. Past productive collaboration has laid the groundwork for these future studies centred initially on the expansion of discovery and replication of GWA studies, with biological analyses of variants and epidemiological studies as longer-term aims.
Acknowledgments
The work of UK groups is supported by grants from Cancer Research UK and the European Union. In Spain, work is supported by grants from the Instituto de Salud Carlos III, grants FIS 05/1006, 08/1359, 08/1635 and European Commission FP6 Food-CT-2006-036224 (VM). In Canada, work is supported by grants from Genome Canada (the ARCTIC Project), the National Cancer Institute of Canada (the CaRE Project) and the Ontario Institute for Cancer Research. In Finland, work is supported by grants from Academy of Finland (Finnish Centre of Excellence Program 2006-2011), the Sigrid Juselius Foundation, the Finnish Cancer Societies and by the European Union. The work of DFKS is supported by the German Genome Network (NGFNplus). In the Czech Republic, work is supported by GACR 310/07/1430. In Sweden, work is supported by grants from the Swedish Cancer Foundation and the Swedish Research Council. In Barcelona and Santiago (Epicolon), work is supported by grants from Fondo de Investigación Sanitaria (FIS 04/1126, 05/2031, 05/0071, 08/0025, 08/1276), Xunta de Galicia (PGIDIT07PXIB9101209PR), Fundación de Investigación Médica Mutua Madrileña (CRP and SCB), Ministerio de Educación y Ciencia (SAF 07-64873), Asociación Española contra el Cáncer, Fundación Olga Torres (SCB) and Acción Transversal contra el Cáncer (Instituto de Salud Carlos III). CIBERER and CIBEREHD are funded by the Instituto de Salud Carlos III. SCB is supported by a contract from the Fondo de Investigación Sanitaria (CP 03-0070, Ministerio de Sanidad. Provision of genotyping is gratefully provided by Santiago de Compostela node of the Spanish National Genotyping Center (CeGen). In The Netherlands, work of RH is supported by the Dutch Cancer Society and the European Community, and the work of JTW, TvW, HM and PD is supported by the Dutch Cancer Society (UL2005-3247) and Fonds NutsOhra. In Hong Kong, work is supported by grants from the Michael and Betty Kadoorie Cancer Genetics Research Programme II and by the Bobby Moore Fund of Cancer Research UK. Work in Australia is conducted under the auspices of the Hilton Ludwig Cancer Metastasis Initiative and supported by a grant from the NHMRC (Project Grant 489418). In Japan, this work was conducted as a part of the BioBank Japan Project that was supported by the Ministry of Education, Culture, Sports, Sciences and Technology of the Japanese government. The ESTHER and VERDI studies are supported by grants from the Baden Württemberg Ministry of Research, Science and Arts and the German Cancer Aid (Deutsche Krebshilfe, Grant M24/95/BR I). The Kiel cohort (POPGEN) is funded through the German Ministry of Education and Reserach through the POPGEN Biobank grant and the Colon Cancer Network of the German National Genome Research Network. SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research, the Ministry of Cultural Affairs, as well as by the Social Ministry of the Federal State of Mecklenburg-West Pomerania. Genome-wide data have been supported by the Federal Ministry of Education and Research (Grant no. 03ZIK012) and by a joint grant from Siemens Healthcare, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania. The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of Siemens AG. MME, AV and LC-C have received funding from Cancer Research UK and Universidad del Tolima. In Italy, collection of samples and clinical data of individuals recruited at Fondazione IRCCS, Istituto Nazionale Tumori was possible because of efforts by Lucio Bertario, Paola Sala, Silvia Veneroni, Manuela Gariboldi, Fernando Ravagnani, Tiziana Camerini, Maria Gaetana Di Mauro, Maria Grazia Daidone and Marco A Pierotti. This study is supported by grants from Associazione/Fondazione Italiana per la Ricerca sul Cancro (AIRC/FIRC) and Lega Italiana per la Lotta ai Tumori.
Footnotes
URLs
Epidemiologische Studie zu Chancen der Verhütung, Früherkennung und optimierten Therapie chronischer Erkrankungen in der älteren Bevölkerung (ESTHER) – http://www.esther.dkfz.org/esther/).
Studies of Epidemiology and Risk Factors in Cancer Heredity (SEARCH) – http://www.srl.cam.ac.uk/search/Homepage.htm
QUASAR – http://www.octo-oxford.org.uk/alltrials/trials/q2.html).
Biobank Japan – http://www.src.riken.go.jp/english/project/person/index.html
Victorian cancer biobank – http://www.viccancerbiobank.org.au/
National Study of Colorectal Cancer Genetics (NSCCG) – http://www.icr.ac.uk/research/research_sections/cancer_genetics/cancer_genetics_teams/molecular_and_population_genetics/nsccg/index.shtml
References
- Aaltonen L, Johns L, Jarvinen H, Mecklin JP, Houlston R (2007) Explaining the familial colorectal cancer risk associated with mismatch repair (MMR)-deficient and MMR-stable tumors. Clin Cancer Res 13: 356–361 [DOI] [PubMed] [Google Scholar]
- Broderick P, Carvajal-Carmona L, Pittman AM, Webb E, Howarth K, Rowan A, Lubbe S, Spain S, Sullivan K, Fielding S, Jaeger E, Vijayakrishnan J, Kemp Z, Gorman M, Chandler I, Papaemmanuil E, Penegar S, Wood W, Sellick G, Qureshi M, Teixeira A, Domingo E, Barclay E, Martin L, Sieber O, Kerr D, Gray R, Peto J, Cazier JB, Tomlinson I, Houlston RS (2007) A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet 39: 1315–1317 [DOI] [PubMed] [Google Scholar]
- Castells A, Andreu M (2007) EPICOLON project: contribution to the knowledge of Lynch syndrome and other familial or hereditary colorectal cancer. Med Clin (Barc) 128: 55–60 [DOI] [PubMed] [Google Scholar]
- Chao C, Zhang ZF, Berthiller J, Boffetta P, Hashibe M (2006) NAD(P)H:quinone oxidoreductase 1 (NQO1) Pro187Ser polymorphism and the risk of lung, bladder, and colorectal cancers: a meta-analysis. Cancer Epidemiol Biomarkers Prev 15: 979–987 [DOI] [PubMed] [Google Scholar]
- Chen K, Jiang QT, He HQ (2005) Relationship between metabolic enzyme polymorphism and colorectal cancer. World J Gastroenterol 11: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterchio M, McKeown-Eyssen G, Sutherland H, Buchan G, Aronson M, Easson AM, Macey J, Holowaty E, Gallinger S (2000) Ontario familial colon cancer registry: methods and first-year response rates. Chronic Dis Can 21: 81–86 [PubMed] [Google Scholar]
- de Jong MM, Nolte IM, te Meerman GJ, van der Graaf WT, de Vries EG, Sijmons RH, Hofstra RM, Kleibeuker JH (2002) Low-penetrance genes and their involvement in colorectal cancer susceptibility. Cancer Epidemiol Biomarkers Prev 11: 1332–1352 [PubMed] [Google Scholar]
- de Jong MM, Nolte IM, Te Meerman GJ, van der Graaf WT, Mulder MJ, van der Steege G, Bruinenberg M, Schaapveld M, Niessen RC, Berends MJ, Sijmons RH, Hofstra RM, de Vries EG, Kleibeuker JH (2005) Colorectal cancer and the CHEK2 1100delC mutation. Genes Chromosomes Cancer 43: 377–382 [DOI] [PubMed] [Google Scholar]
- Dong LM, Potter JD, White E, Ulrich CM, Cardon LR, Peters U (2008) Genetic susceptibility to cancer: the role of polymorphisms in candidate genes. JAMA 299: 2423–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen T, Matakidou A, Consortium G, Houlston R (2008) Identification of low penetrance alleles for lung cancer: the GEnetic Lung CAncer Predisposition Study (GELCAPS). BMC Cancer 8: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank B, Burwinkel B, Bermejo JL, Forsti A, Hemminki K, Houlston R, Mangold E, Rahner N, Friedl W, Friedrichs N, Buettner R, Engel C, Loeffler M, Holinski-Feder E, Morak M, Keller G, Schackert HK, Kruger S, Goecke T, Moeslein G, Kloor M, Gebert J, Kunstmann E, Schulmann K, Ruschoff J, Propping P (2008) Ten recently identified associations between nsSNPs and colorectal cancer could not be replicated in German families. Cancer Lett 271: 153–157 [DOI] [PubMed] [Google Scholar]
- Houlston RS, Webb E, Broderick P, Pittman AM, Di Bernardo MC, Lubbe S, Chandler I, Vijayakrishnan J, Sullivan K, Penegar S, Carvajal-Carmona L, Howarth K, Jaeger E, Spain SL, Walther A, Barclay E, Martin L, Gorman M, Domingo E, Teixeira AS, Kerr D, Cazier JB, Niittymaki I, Tuupanen S, Karhu A, Aaltonen LA, Tomlinson IP, Farrington SM, Tenesa A, Prendergast JG, Barnetson RA, Cetnarskyj R, Porteous ME, Pharoah PD, Koessler T, Hampe J, Buch S, Schafmayer C, Tepel J, Schreiber S, Volzke H, Chang-Claude J, Hoffmeister M, Brenner H, Zanke BW, Montpetit A, Hudson TJ, Gallinger S, Campbell H, Dunlop MG (2008) Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet 40: 1426–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Han S, Li Y, Mao Y, Xie Y (2007) Different roles of MTHFR C677T and A1298C polymorphisms in colorectal adenoma and colorectal cancer: a meta-analysis. J Hum Genet 52: 73–85 [DOI] [PubMed] [Google Scholar]
- Hubner R, Houlston R. Polymorphic variation and risk of colorectal cancer. In: Tomlinson IPM (ed). Hereditary Colorectal Cancer
- Hubner RA, Houlston RS (2007) MTHFR C677T and colorectal cancer risk: a meta-analysis of 25 populations. Int J Cancer 120: 1027–1035 [DOI] [PubMed] [Google Scholar]
- Jaeger E, Webb E, Howarth K, Carvajal-Carmona L, Rowan A, Broderick P, Walther A, Spain S, Pittman A, Kemp Z, Sullivan K, Heinimann K, Lubbe S, Domingo E, Barclay E, Martin L, Gorman M, Chandler I, Vijayakrishnan J, Wood W, Papaemmanuil E, Penegar S, Qureshi M, Farrington S, Tenesa A, Cazier JB, Kerr D, Gray R, Peto J, Dunlop M, Campbell H, Thomas H, Houlston R, Tomlinson I (2008) Common genetic variants at the CRAC1 (HMPS) locus on chromosome 15q13.3 influence colorectal cancer risk. Nat Genet 40: 26–28 [DOI] [PubMed] [Google Scholar]
- Kemp Z, Carvajal-Carmona L, Spain S, Barclay E, Gorman M, Martin L, Jaeger E, Brooks N, Bishop DT, Thomas H, Tomlinson I, Papaemmanuil E, Webb E, Sellick GS, Wood W, Evans G, Lucassen A, Maher ER, Houlston RS (2006) Evidence for a colorectal cancer susceptibility locus on chromosome 3q21–q24 from a high-density SNP genome-wide linkage scan. Hum Mol Genet 15: 2903–2910 [DOI] [PubMed] [Google Scholar]
- Kerr DJ, Dunn JA, Langman MJ, Smith JL, Midgley RS, Stanley A, Stokes JC, Julier P, Iveson C, Duvvuri R, McConkey CC (2007) Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med 357: 360–369 [DOI] [PubMed] [Google Scholar]
- Krawczak M, Nikolaus S, von Eberstein H, Croucher PJ, El Mokhtari NE, Schreiber S (2006) PopGen: population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genet 9: 55–61 [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K (2000) Environmental and heritable factors in the causation of cancer – analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 343: 78–85 [DOI] [PubMed] [Google Scholar]
- Marchini J, Donnelly P, Cardon LR (2005) Genome-wide strategies for detecting multiple loci that influence complex diseases. Nat Genet 37: 413–417 [DOI] [PubMed] [Google Scholar]
- Moonesinghe R, Khoury MJ, Liu T, Ioannidis JP (2008) Required sample size and nonreplicability thresholds for heterogeneous genetic associations. Proc Natl Acad Sci USA 105: 617–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penegar S, Wood W, Lubbe S, Chandler I, Broderick P, Papaemmanuil E, Sellick G, Gray R, Peto J, Houlston R (2007) National study of colorectal cancer genetics. Br J Cancer 97: 1305–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous M, Dunckley M, Appleton S, Catt S, Dunlop M, Campbell H, Cull A (2003) Is it acceptable to approach colorectal cancer patients at diagnosis to discuss genetic testing? A pilot study. Br J Cancer 89: 1400–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salovaara R, Loukola A, Kristo P, Kaariainen H, Ahtola H, Eskelinen M, Harkonen N, Julkunen R, Kangas E, Ojala S, Tulikoura J, Valkamo E, Jarvinen H, Mecklin JP, Aaltonen LA, de la Chapelle A (2000) Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol 18: 2193–2200 [DOI] [PubMed] [Google Scholar]
- Schafmayer C, Buch S, Egberts JH, Franke A, Brosch M, El Sharawy A, Conring M, Koschnick M, Schwiedernoch S, Katalinic A, Kremer B, Folsch UR, Krawczak M, Fandrich F, Schreiber S, Tepel J, Hampe J (2007) Genetic investigation of DNA-repair pathway genes PMS2, MLH1, MSH2, MSH6, MUTYH, OGG1 and MTH1 in sporadic colon cancer. Int J Cancer 121: 555–558 [DOI] [PubMed] [Google Scholar]
- Tan XL, Nieters A, Kropp S, Hoffmeister M, Brenner H, Chang-Claude J (2008) The association of cyclin D1 G870A and E-cadherin C-160A polymorphisms with the risk of colorectal cancer in a case control study and meta-analysis. Int J Cancer 122: 2573–2580 [DOI] [PubMed] [Google Scholar]
- Tenesa A, Dunlop MG (2009) New insights into the aetiology of colorectal cancer from genome-wide association studies. Nat Rev Genet (e-pub ahead of print) [DOI] [PubMed]
- Tenesa A, Farrington SM, Prendergast JG, Porteous ME, Walker M, Haq N, Barnetson RA, Theodoratou E, Cetnarskyj R, Cartwright N, Semple C, Clark AJ, Reid FJ, Smith LA, Kavoussanakis K, Koessler T, Pharoah PD, Buch S, Schafmayer C, Tepel J, Schreiber S, Volzke H, Schmidt CO, Hampe J, Chang-Claude J, Hoffmeister M, Brenner H, Wilkening S, Canzian F, Capella G, Moreno V, Deary IJ, Starr JM, Tomlinson IP, Kemp Z, Howarth K, Carvajal-Carmona L, Webb E, Broderick P, Vijayakrishnan J, Houlston RS, Rennert G, Ballinger D, Rozek L, Gruber SB, Matsuda K, Kidokoro T, Nakamura Y, Zanke BW, Greenwood CM, Rangrej J, Kustra R, Montpetit A, Hudson TJ, Gallinger S, Campbell H, Dunlop MG (2008) Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet 40: 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E, Farrington SM, Tenesa A, McNeill G, Cetnarskyj R, Barnetson RA, Porteous ME, Dunlop MG, Campbell H (2008) Modification of the inverse association between dietary vitamin D intake and colorectal cancer risk by a FokI variant supports a chemoprotective action of Vitamin D intake mediated through VDR binding. Int J Cancer 123: 2170–2179 [DOI] [PubMed] [Google Scholar]
- Thomas DC, Clayton DG (2004) Betting odds and genetic associations. J Natl Cancer Inst 96: 421–423 [DOI] [PubMed] [Google Scholar]
- Tomlinson I, Webb E, Carvajal-Carmona L, Broderick P, Kemp Z, Spain S, Penegar S, Chandler I, Gorman M, Wood W, Barclay E, Lubbe S, Martin L, Sellick G, Jaeger E, Hubner R, Wild R, Rowan A, Fielding S, Howarth K, Silver A, Atkin W, Muir K, Logan R, Kerr D, Johnstone E, Sieber O, Gray R, Thomas H, Peto J, Cazier JB, Houlston R (2007) A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet 39: 984–988 [DOI] [PubMed] [Google Scholar]
- Tomlinson IP, Webb E, Carvajal-Carmona L, Broderick P, Howarth K, Pittman AM, Spain S, Lubbe S, Walther A, Sullivan K, Jaeger E, Fielding S, Rowan A, Vijayakrishnan J, Domingo E, Chandler I, Kemp Z, Qureshi M, Farrington SM, Tenesa A, Prendergast JG, Barnetson RA, Penegar S, Barclay E, Wood W, Martin L, Gorman M, Thomas H, Peto J, Bishop DT, Gray R, Maher ER, Lucassen A, Kerr D, Evans DG, Schafmayer C, Buch S, Volzke H, Hampe J, Schreiber S, John U, Koessler T, Pharoah P, van Wezel T, Morreau H, Wijnen JT, Hopper JL, Southey MC, Giles GG, Severi G, Castellvi-Bel S, Ruiz-Ponte C, Carracedo A, Castells A, Forsti A, Hemminki K, Vodicka P, Naccarati A, Lipton L, Ho JW, Cheng KK, Sham PC, Luk J, Agundez JA, Ladero JM, de la Hoya M, Caldes T, Niittymaki I, Tuupanen S, Karhu A, Aaltonen L, Cazier JB, Campbell H, Dunlop MG, Houlston RS (2008) A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet 40: 623–630 [DOI] [PubMed] [Google Scholar]
- Tulupova E, Kumar R, Hanova M, Slyskova J, Pardini B, Polakova V, Naccarati A, Vodickova L, Novotny J, Halamkova J, Hemminki K, Vodicka P (2008) Do polymorphisms and haplotypes of mismatch repair genes modulate risk of sporadic colorectal cancer? Mutat Res 648: 40–45 [DOI] [PubMed] [Google Scholar]
- Volzke H, Werner A, Wallaschofski H, Friedrich N, Robinson DM, Kindler S, Kraft M, John U, Hoffmann W (2005) Occupational exposure to ionizing radiation is associated with autoimmune thyroid disease. J Clin Endocrinol Metab 90: 4587–4592 [DOI] [PubMed] [Google Scholar]
- Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N (2004) Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 96: 434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke BW, Greenwood CM, Rangrej J, Kustra R, Tenesa A, Farrington SM, Prendergast J, Olschwang S, Chiang T, Crowdy E, Ferretti V, Laflamme P, Sundararajan S, Roumy S, Olivier JF, Robidoux F, Sladek R, Montpetit A, Campbell P, Bezieau S, O’Shea AM, Zogopoulos G, Cotterchio M, Newcomb P, McLaughlin J, Younghusband B, Green R, Green J, Porteous ME, Campbell H, Blanche H, Sahbatou M, Tubacher E, Bonaiti-Pellie C, Buecher B, Riboli E, Kury S, Chanock SJ, Potter J, Thomas G, Gallinger S, Hudson TJ, Dunlop MG (2007) Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet 39: 989–994 [DOI] [PubMed] [Google Scholar]
- Zhang D, Chen C, Fu X, Gu S, Mao Y, Xie Y, Huang Y, Li Y (2008) A meta-analysis of DNA repair gene XPC polymorphisms and cancer risk. J Hum Genet 53: 18–33 [DOI] [PubMed] [Google Scholar]