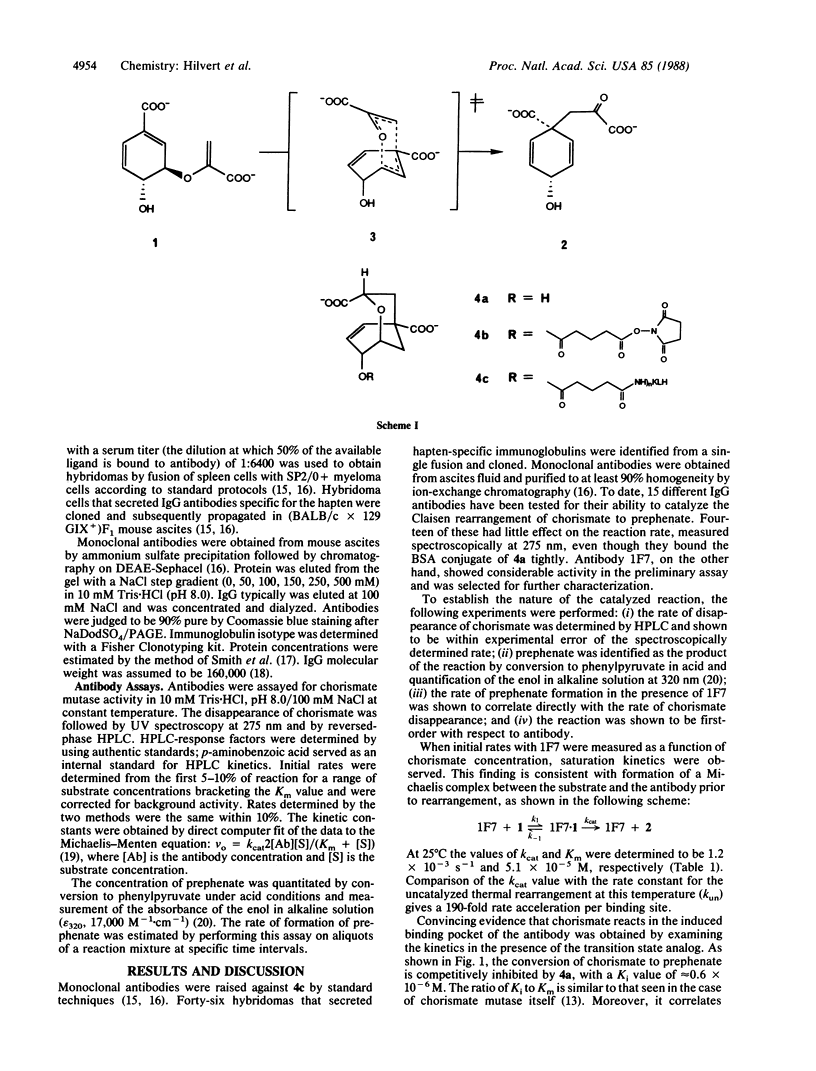
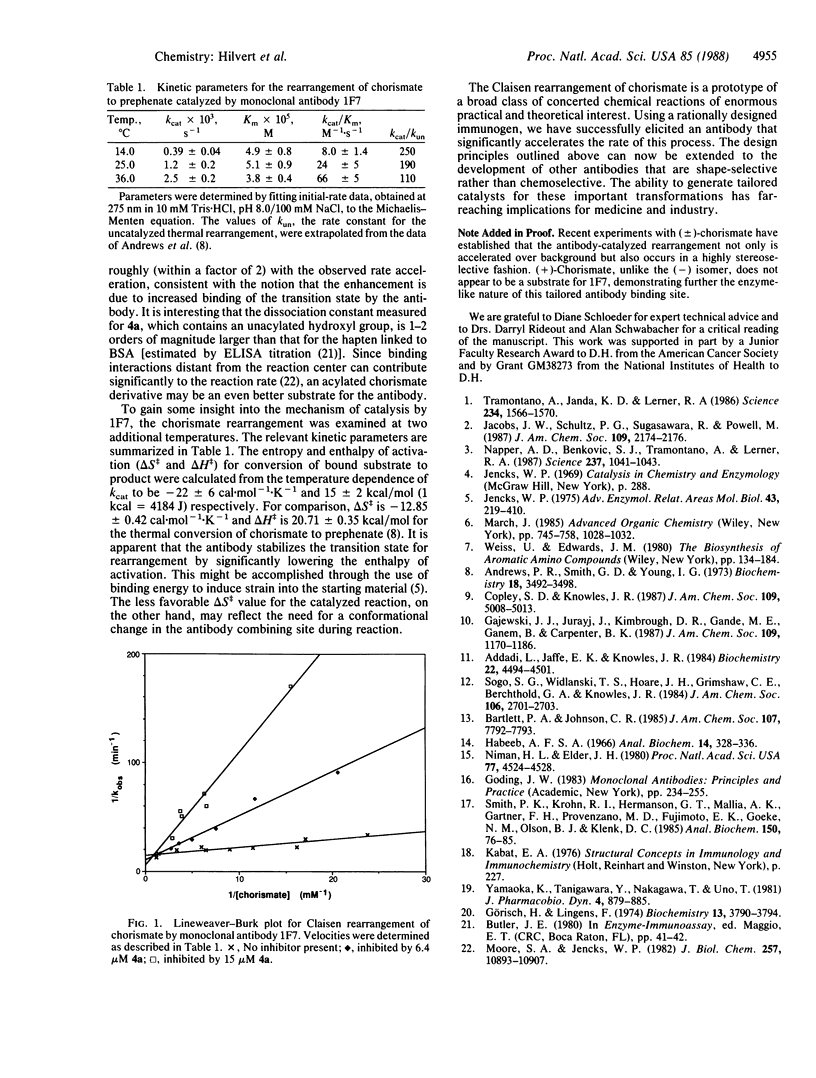
Abstract
Monoclonal antibodies were prepared against a transition state analog inhibitor of chorismate mutase (EC 5.4.99.5). One of the antibodies catalyzes the rearrangement of chorismate to prephenate with rate accelerations of more than 2 orders of magnitude compared to the uncatalyzed reaction. Saturation kinetics were observed, and at 25 degrees C the values of kcat and Km were 1.2 X 10(-3) s-1 and 5.1 X 10(-5) M respectively. The transition state analog was shown to be a competitive inhibitor of the reaction with Ki equal to 0.6 microM. These results demonstrate the feasibility of using rationally designed immunogens to generate antibodies that catalyze concerted reactions.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addadi L., Jaffe E. K., Knowles J. R. Secondary tritium isotope effects as probes of the enzymic and nonenzymic conversion of chorismate to prephenate. Biochemistry. 1983 Sep 13;22(19):4494–4501. doi: 10.1021/bi00288a022. [DOI] [PubMed] [Google Scholar]
- Andrews P. R., Smith G. D., Young I. G. Transition-state stabilization and enzymic catalysis. Kinetic and molecular orbital studies of the rearrangement of chorismate to prephenate. Biochemistry. 1973 Aug 28;12(18):3492–3498. doi: 10.1021/bi00742a022. [DOI] [PubMed] [Google Scholar]
- Görisch H., Lingens F. Chorismate mutase from Streptomyces. Purification, properties, and subunit structure of the enzyme from Streptomyces aureofaciens Tü 24. Biochemistry. 1974 Aug 27;13(18):3790–3794. doi: 10.1021/bi00715a027. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Jencks W. P. Binding energy, specificity, and enzymic catalysis: the circe effect. Adv Enzymol Relat Areas Mol Biol. 1975;43:219–410. doi: 10.1002/9780470122884.ch4. [DOI] [PubMed] [Google Scholar]
- Moore S. A., Jencks W. P. Formation of active site thiol esters of CoA transferase and the dependence of catalysis on specific binding interactions. J Biol Chem. 1982 Sep 25;257(18):10893–10907. [PubMed] [Google Scholar]
- Napper A. D., Benkovic S. J., Tramontano A., Lerner R. A. A stereospecific cyclization catalyzed by an antibody. Science. 1987 Aug 28;237(4818):1041–1043. doi: 10.1126/science.3616626. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Elder J. H. Molecular dissection of Rauscher virus gp70 by using monoclonal antibodies: localization of acquired sequences of related envelope gene recombinants. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4524–4528. doi: 10.1073/pnas.77.8.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tramontano A., Janda K. D., Lerner R. A. Catalytic antibodies. Science. 1986 Dec 19;234(4783):1566–1570. doi: 10.1126/science.3787261. [DOI] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]



