Abstract
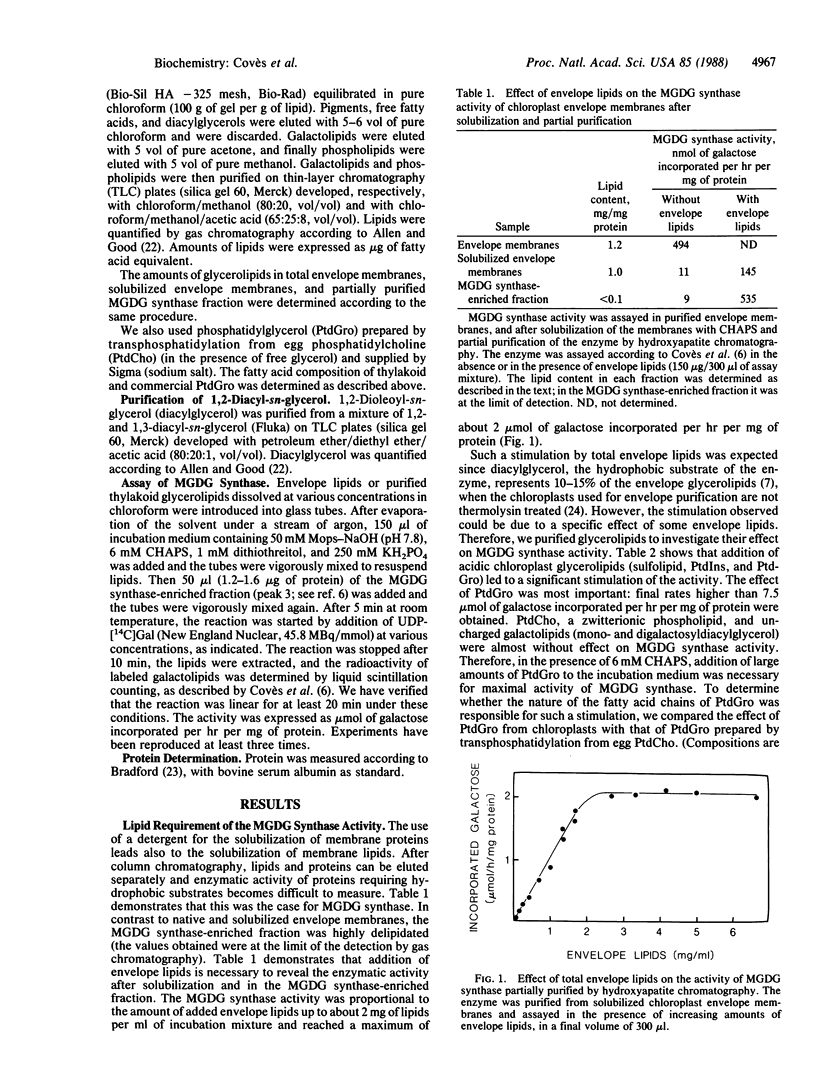
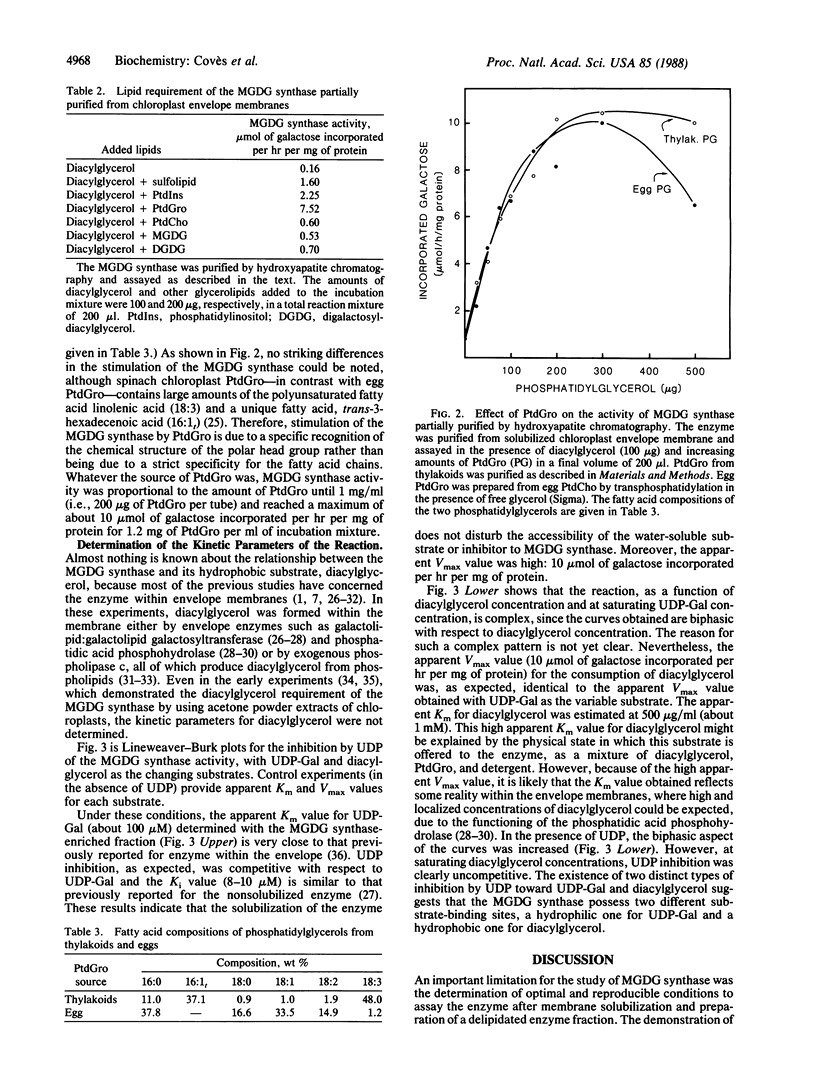
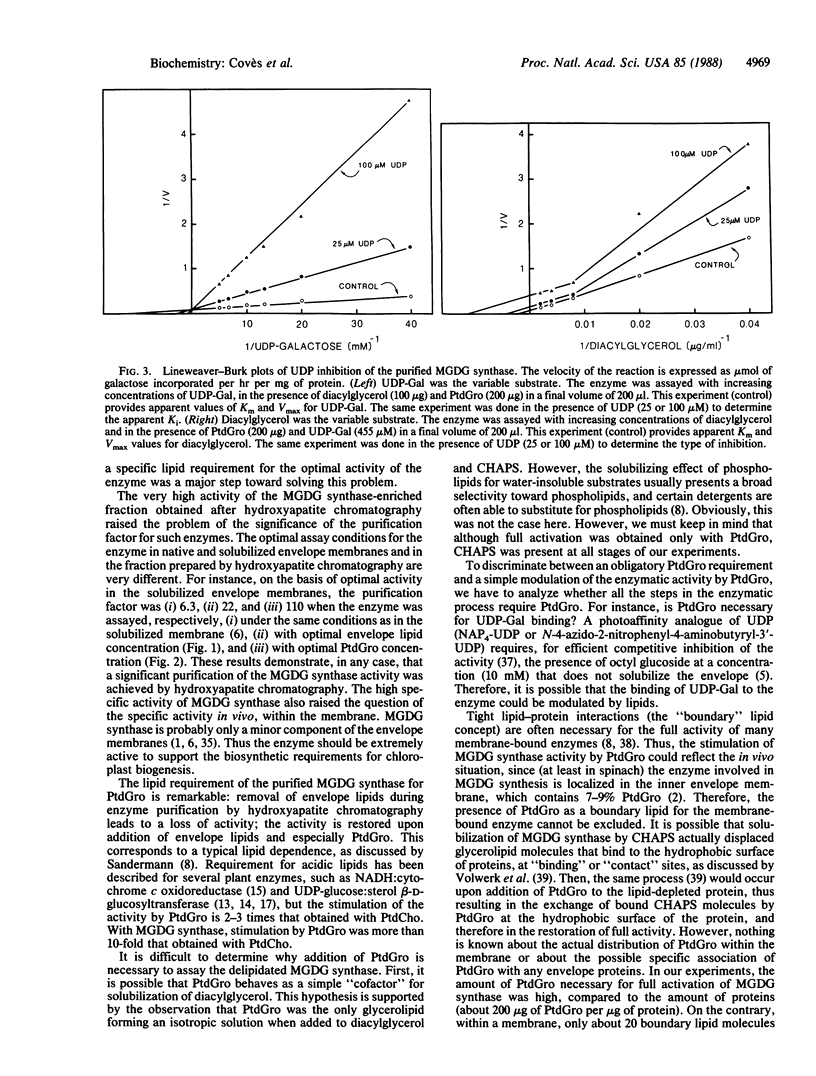
We have demonstrated a lipid requirement for the UDPgalactose:1,2-diacylglycerol 3-β-D-galactosyl-transferase (or monogalactosyldiacylglycerol synthase; EC 2.4.1.46), an enzyme involved in the biosynthesis of monogalactosyldiacylglycerol, solubilized from chloroplast envelope membranes and partially purified by hydroxyapatite chromatography. The enzyme fraction was highly delipidated (<0.1 mg of lipid per mg of protein), and addition of lipids extracted from chloroplast membranes was necessary to reveal the activity. Acidic glycerolipids, and especially phosphatidylglycerol, were the best activators of the enzyme. The preparation of a delipidated enzyme fraction and the development of optimal assay conditions were prerequisites for the determination of the kinetic parameters for the hydrophobic substrate of the enzyme, diacylglycerol. In addition, we have demonstrated the existence of two substrate-binding sites: a hydrophobic one for diacylglycerol and a hydrophilic one for UDP-galactose.
Keywords: monogalactosyldiacylglycerol biosynthesis, phosphatidylglycerol requirement, protein-lipid interactions, UDP inhibition, Spinacia oleracea
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Block M. A., Dorne A. J., Joyard J., Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. I. Electrophoretic and immunochemical analyses. J Biol Chem. 1983 Nov 10;258(21):13273–13280. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cocucci M., Ballarin-Denti A. Effect of polar lipids on ATPase activity of membrane preparations from germinating radish seeds. Plant Physiol. 1981 Aug;68(2):377–381. doi: 10.1104/pp.68.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne A. J., Joyard J., Block M. A., Douce R. Localization of phosphatidylcholine in outer envelope membrane of spinach chloroplasts. J Cell Biol. 1985 May;100(5):1690–1697. doi: 10.1083/jcb.100.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Haverkate F., van Deenen L. L. Isolation and chemical characterization of phosphatidyl glycerol from spinach leaves. Biochim Biophys Acta. 1965 Jul 7;106(1):78–92. doi: 10.1016/0005-2760(65)90097-4. [DOI] [PubMed] [Google Scholar]
- Jolliot A., Demandre C., Mazliak P. Regulation by Lipids of Plant Microsomal Enzymes: II. LIPID DEPENDENCE OF THE NADH-CYTOCHROME c REDUCTASE OF POTATO TUBERS. Plant Physiol. 1981 Jan;67(1):9–11. doi: 10.1104/pp.67.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliot A., Justin A. M., Bimont E., Mazliak P. Regulation by Lipids of Plant Microsomal Enzymes: III. Phospholipid Dependence of the Cytidine-Diphospho-Choline Phosphotransferase of Potato Microsomes. Plant Physiol. 1982 Jul;70(1):206–210. doi: 10.1104/pp.70.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Douce R. Characterization of phosphatidate phosphohydrolase activity associated with chloroplast envelope membranes. FEBS Lett. 1979 Jun 1;102(1):147–150. doi: 10.1016/0014-5793(79)80947-3. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Mise en évidence et rôle des diacylglycerols de l'enveloppe des chloroplastes d'épinard. Biochim Biophys Acta. 1976 Jan 22;424(1):125–131. doi: 10.1016/0005-2760(76)90057-6. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Mudd J. B., van Vliet H. H., van Deenen L. L. Biosynthesis of galactolipids by enzyme preparations from spinach leaves. J Lipid Res. 1969 Nov;10(6):623–630. [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Siebertz H. P., Heinz E., Linscheid M., Joyard J., Douce R. Characterization of lipids from chloroplast envelopes. Eur J Biochem. 1979 Nov;101(2):429–438. doi: 10.1111/j.1432-1033.1979.tb19736.x. [DOI] [PubMed] [Google Scholar]
- Ullmann P., Bouvier-Navé P., Benveniste P. Regulation by Phospholipids and Kinetic Studies of Plant Membrane-Bound UDP-Glucose Sterol beta-d-Glucosyl Transferase. Plant Physiol. 1987 Sep;85(1):51–55. doi: 10.1104/pp.85.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volwerk J. J., Mrsny R. J., Patapoff T. W., Jost P. C., Griffith O. H. Multiple equilibria binding treatment of lipid and detergent interactions with membrane proteins. Application to cytochrome c oxidase solubilized in cholate. Biochemistry. 1987 Jan 27;26(2):466–475. doi: 10.1021/bi00376a019. [DOI] [PubMed] [Google Scholar]
- van Besouw A., Wintermans J. F. Galactolipid formation in chloroplast envelopes. I. Evidence for two mechanisms in galactosylation. Biochim Biophys Acta. 1978 Apr 28;529(1):44–53. doi: 10.1016/0005-2760(78)90102-9. [DOI] [PubMed] [Google Scholar]
- van Besouw A., Wintermans J. F. The synthesis of galactosydiacylglycerols by chloroplast envelopes. FEBS Lett. 1979 Jun 1;102(1):33–37. doi: 10.1016/0014-5793(79)80922-9. [DOI] [PubMed] [Google Scholar]


