Abstract
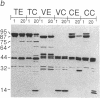
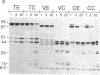
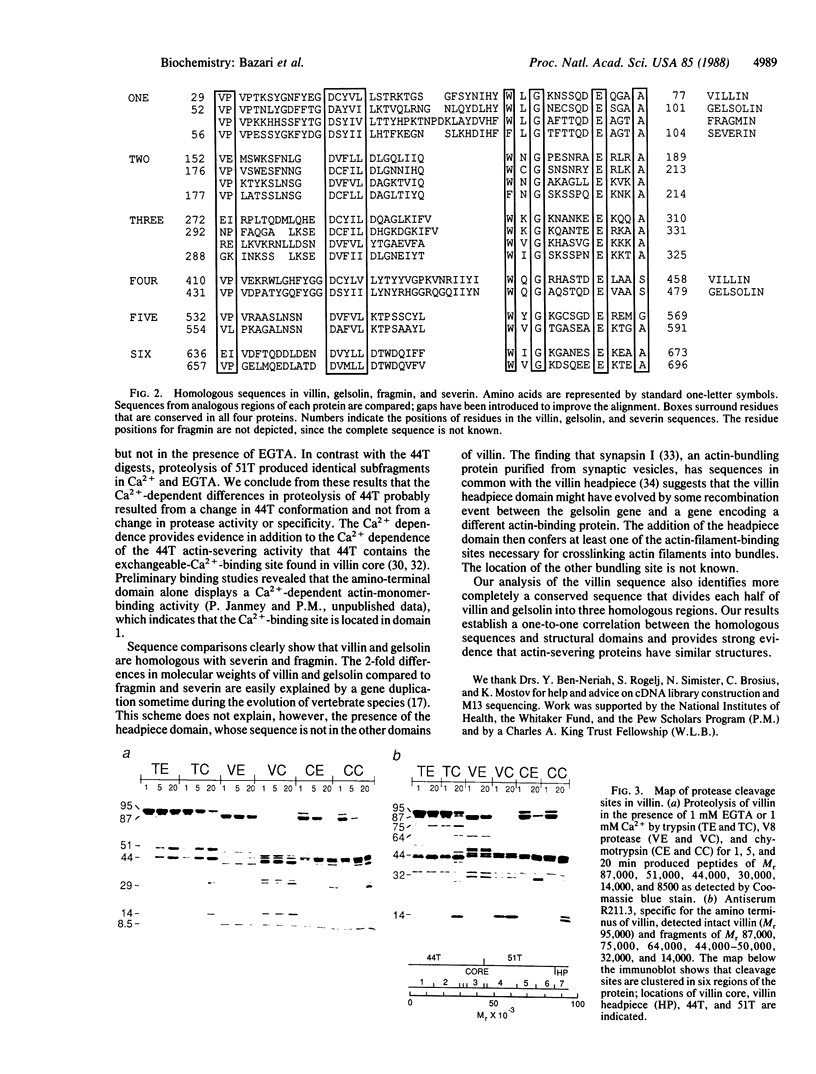
Site-specific proteases and antisera to the amino terminus of villin have been used to show that villin is organized into seven protease-resistant domains. Six are contained in the amino-terminal Mr 87,000 villin core, a Ca2+-regulated actin-severing fragment, whereas the carboxyl-terminal domain includes the villin "headpiece," a fragment involved in bundling of actin filaments. Ca2+ inhibits proteolytic cleavage between domains in the amino-terminal half of villin. The protein sequence of villin deduced from a single cDNA clone contains a conserved sequence that is repeated six times and is found in each domain of the villin core. The conserved repeats are found in other actin-severing proteins but not in the villin headpiece. Our results suggest that actin-severing proteins are organized around a common Mr 14,000-17,000 domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ampe C., Vandekerckhove J. The F-actin capping proteins of Physarum polycephalum: cap42(a) is very similar, if not identical, to fragmin and is structurally and functionally very homologous to gelsolin; cap42(b) is Physarum actin. EMBO J. 1987 Dec 20;6(13):4149–4157. doi: 10.1002/j.1460-2075.1987.tb02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André E., Lottspeich F., Schleicher M., Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988 Jan 15;263(2):722–727. [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Brown S. S., Yamamoto K., Spudich J. A. A 40,000-dalton protein from Dictyostelium discoideum affects assembly properties of actin in a Ca2+-dependent manner. J Cell Biol. 1982 Apr;93(1):205–210. doi: 10.1083/jcb.93.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Hwo S. Definition of an N-terminal actin-binding domain and a C-terminal Ca2+ regulatory domain in human brevin. J Cell Biol. 1986 Apr;102(4):1439–1446. doi: 10.1083/jcb.102.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M., Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987 Apr 16;326(6114):704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- Chaponnier C., Janmey P. A., Yin H. L. The actin filament-severing domain of plasma gelsolin. J Cell Biol. 1986 Oct;103(4):1473–1481. doi: 10.1083/jcb.103.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Geisler N., Kaulfus P., Weber K. Demonstration of at least two different actin-binding sites in villin, a calcium-regulated modulator of F-actin organization. J Biol Chem. 1981 Aug 10;256(15):8156–8161. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Calcium control of microfilaments: uncoupling of the F-actin-severing and -bundling activity of villin by limited proteolysis in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2810–2814. doi: 10.1073/pnas.78.5.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Takahashi S., Hayashi H., Hatano S. Fragmin: a calcium ion sensitive regulatory factor on the formation of actin filaments. Biochemistry. 1980 Jun 10;19(12):2677–2683. doi: 10.1021/bi00553a021. [DOI] [PubMed] [Google Scholar]
- Hesterberg L. K., Weber K. Demonstration of three distinct calcium-binding sites in villin, a modulator of actin assembly. J Biol Chem. 1983 Jan 10;258(1):365–369. [PubMed] [Google Scholar]
- Hesterberg L. K., Weber K. Isolation of a domain of villin retaining calcium-dependent interaction with G-actin, but devoid of F-actin fragmenting activity. Eur J Biochem. 1986 Jan 2;154(1):135–140. doi: 10.1111/j.1432-1033.1986.tb09368.x. [DOI] [PubMed] [Google Scholar]
- Hinssen H. An actin-modulating protein from Physarum polycephalum. I. Isolation and purification. Eur J Cell Biol. 1981 Feb;23(2):225–233. [PubMed] [Google Scholar]
- Kwiatkowski D. J., Janmey P. A., Mole J. E., Yin H. L. Isolation and properties of two actin-binding domains in gelsolin. J Biol Chem. 1985 Dec 5;260(28):15232–15238. [PubMed] [Google Scholar]
- Kwiatkowski D. J., Stossel T. P., Orkin S. H., Mole J. E., Colten H. R., Yin H. L. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986 Oct 2;323(6087):455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P., Jakes R., Cameron L., Atherton E. Mapping the cysteine residues and actin-binding regions of villin by using antisera to the amino and carboxyl termini of the molecule. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6788–6792. doi: 10.1073/pnas.82.20.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P., Jakes R., Walker J. E. A gelsolin-like Ca2+-dependent actin-binding domain in villin. Nature. 1985 May 16;315(6016):248–250. doi: 10.1038/315248a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McCaffery C. A., DeGennaro L. J. Determination and analysis of the primary structure of the nerve terminal specific phosphoprotein, synapsin I. EMBO J. 1986 Dec 1;5(12):3167–3173. doi: 10.1002/j.1460-2075.1986.tb04625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop J., Weber A., Mooseker M. S., Franzini-Armstrong C., Bishop M. F., Dubyak G. R., Tucker M., Walsh T. P. Different calcium dependence of the capping and cutting activities of villin. J Biol Chem. 1986 Jul 15;261(20):9274–9281. [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Pringault E., Arpin M., Garcia A., Finidori J., Louvard D. A human villin cDNA clone to investigate the differentiation of intestinal and kidney cells in vivo and in culture. EMBO J. 1986 Dec 1;5(12):3119–3124. doi: 10.1002/j.1460-2075.1986.tb04618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouayrenc J. F., Fattoum A., Méjean C., Kassab R. Characterization of the Ca2+-induced conformational changes in gelsolin and identification of interaction regions between actin and gelsolin. Biochemistry. 1986 Jul 1;25(13):3859–3867. doi: 10.1021/bi00361a018. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L., Spudich J. A. A 45,000-mol-wt protein from unfertilized sea urchin eggs severs actin filaments in a calcium-dependent manner and increases the steady-state concentration of nonfilamentous actin. J Cell Biol. 1984 Sep;99(3):844–851. doi: 10.1083/jcb.99.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]