Abstract
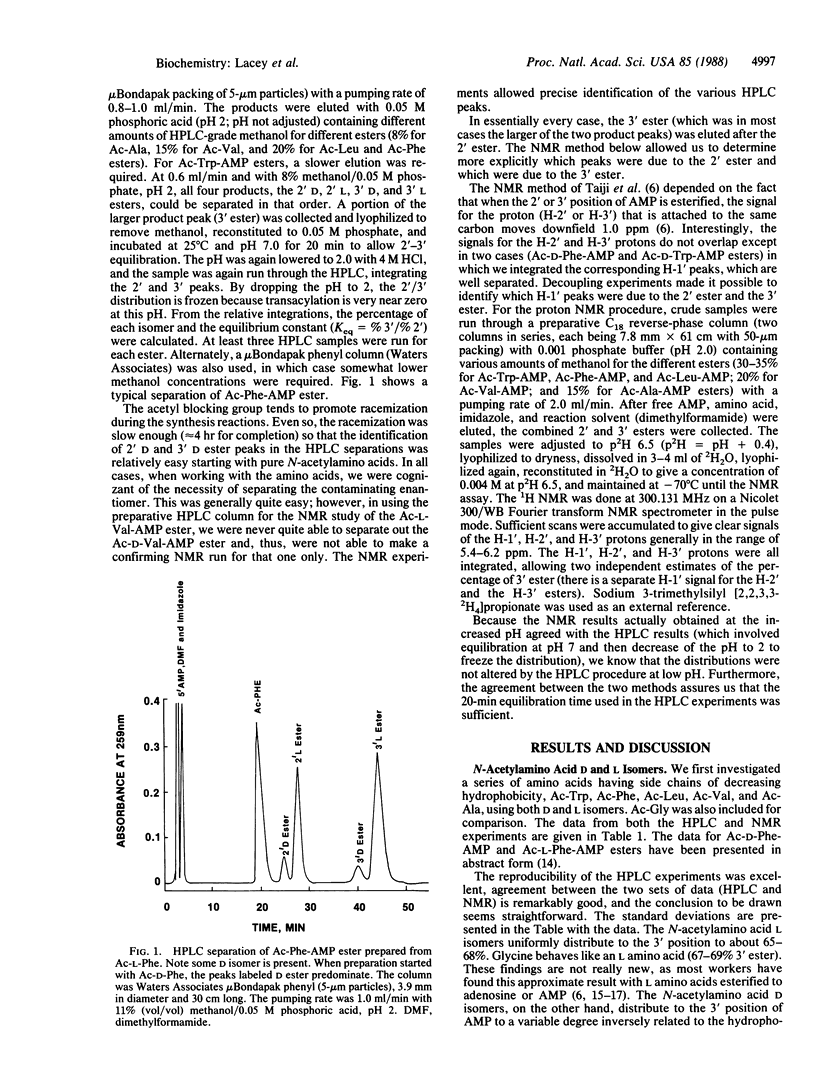
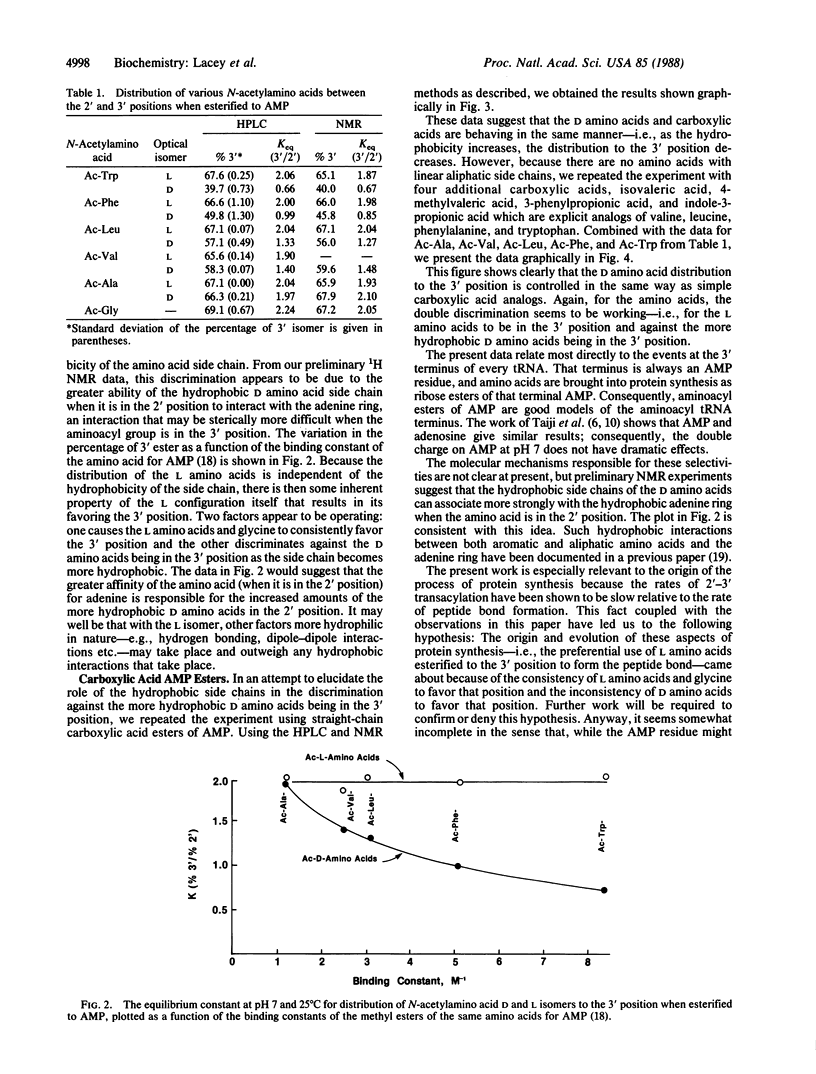
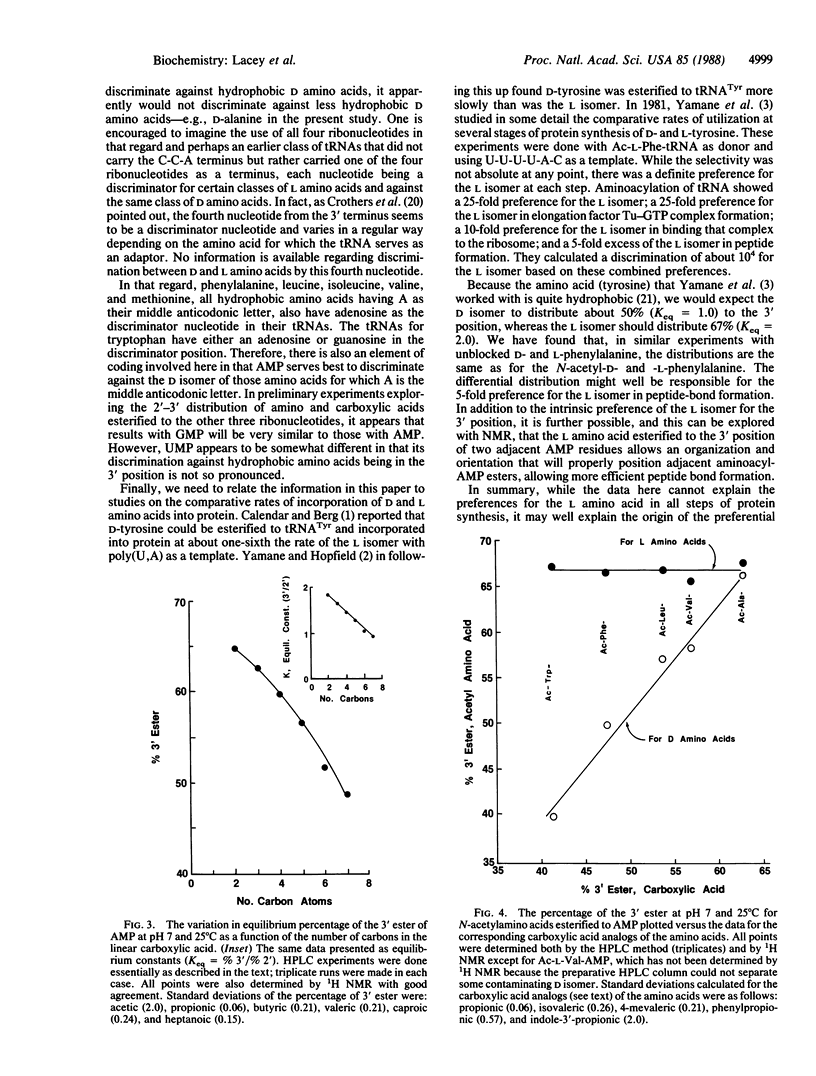
Amino acids esterified to the ribose group of 5'-adenylic acid (AMP) constantly migrate between the 2' and 3' positions of the ribose at a rate of several times per second, which is slower than the rate of peptide-bond synthesis (15-20 per sec). Because the contemporary protein-synthesizing system only incorporates amino acids into protein when they are at the 3' position of the AMP at the terminus of tRNA, the value of the equilibrium constant relative to the 2' and 3' positions is of considerable interest. Differences between D and L isomers in this regard might be especially revealing. We have used N-acetylaminoacyl esters of AMP as models for the 3' terminus of tRNA and find that glycine and the L amino acids consistently distribute predominantly to the 3' position (approximately equal to 67% 3', approximately equal to 33% 2'), but D amino acids distribute to that position generally to a lesser extent and in a manner inversely related to the hydrophobicity of the amino acid side chain. This consistency of the L amino acid preference for the 3' position, combined with the inconsistency of the D amino acid preference, may be one reason for the origin of our contemporary protein-synthesizing system, which forms the peptide bond preferentially with L amino acids and only when they are in the 3' position of the ribose moiety of the AMP residue at the 3' terminus of every tRNA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calendar R., Berg P. D-Tyrosyl RNA: formation, hydrolysis and utilization for protein synthesis. J Mol Biol. 1967 May 28;26(1):39–54. doi: 10.1016/0022-2836(67)90259-8. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Zachau H. G. Chemical evidence for the 3'-linkage of amino acids to s-RNA+. Biochem Biophys Res Commun. 1964 Feb 18;15(1):13–17. doi: 10.1016/0006-291x(64)90094-4. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- Gottikh B. P., Krayevsky A. A., Tarussova N. B., Purygin P. P., Tsilevich T. L. The general synthetic route to amino acid esters of nucleotides and nucleoside-5'-triphosphates and some properties of these compounds. Tetrahedron. 1970 Sep;26(18):4419–4433. doi: 10.1016/s0040-4020(01)93090-x. [DOI] [PubMed] [Google Scholar]
- Lacey J. C., Jr, Mullins D. W., Jr Experimental studies related to the origin of the genetic code and the process of protein synthesis--a review. Orig Life. 1983 Mar;13(1):3–42. doi: 10.1007/BF00928761. [DOI] [PubMed] [Google Scholar]
- Lacey J. C., Jr, Mullins D. W., Jr, Watkins C. L. Aliphatic amino acid side chains associate with the "face" of the adenine ring. J Biomol Struct Dyn. 1986 Feb;3(4):783–793. doi: 10.1080/07391102.1986.10508461. [DOI] [PubMed] [Google Scholar]
- Lacroute F., Stent G. S. Peptide chain growth of -galactosidase in Escherichia coli. J Mol Biol. 1968 Jul 14;35(1):165–173. doi: 10.1016/s0022-2836(68)80044-0. [DOI] [PubMed] [Google Scholar]
- Profy A. T., Usher D. A. Stereoselective aminoacylation of a dinucleoside monophosphate by the imidazolides of DL-alanine and N-(tert-butoxycarbonyl)-DL-alanine. J Mol Evol. 1984;20(2):147–156. doi: 10.1007/BF02257375. [DOI] [PubMed] [Google Scholar]
- Profy A. T., Usher D. A. Stereoselective aminoacylation of polyribonucleotides. J Am Chem Soc. 1984;106:5030–5031. doi: 10.1021/ja00329a080. [DOI] [PubMed] [Google Scholar]
- Reuben J., Polk F. E. Nucleotide-amino acid interactions and their relation to the genetic code. J Mol Evol. 1980 May;15(2):103–112. doi: 10.1007/BF01732664. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. The -C-C-A end of tRNA and its role in protein biosynthesis. Prog Nucleic Acid Res Mol Biol. 1979;22:1–69. doi: 10.1016/s0079-6603(08)60798-9. [DOI] [PubMed] [Google Scholar]
- Taiji M., Yokoyama S., Miyazawa T. Slow transacylation of peptidyladenosine allows analysis of the 2'/3'-isomer specificity of peptidyltransferase. Biochemistry. 1985 Oct 8;24(21):5776–5780. doi: 10.1021/bi00342a013. [DOI] [PubMed] [Google Scholar]
- Taiji M., Yokoyama S., Miyazawa T. Transacylation rates of (aminoacyl)adenosine moiety at the 3'-terminus of aminoacyl transfer ribonucleic acid. Biochemistry. 1983 Jun 21;22(13):3220–3225. doi: 10.1021/bi00282a028. [DOI] [PubMed] [Google Scholar]
- Wagner T., Cramer F., Sprinzl M. Activity of the 2' and 3' isomers of aminoacyl transfer ribonucleic acid in the in vitro peptide elongation on Escherichia coli ribosomes. Biochemistry. 1982 Mar 30;21(7):1521–1529. doi: 10.1021/bi00536a009. [DOI] [PubMed] [Google Scholar]
- Yamane T., Hopfield J. J. Experimental evidence for kinetic proofreading in the aminoacylation of tRNA by synthetase. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2246–2250. doi: 10.1073/pnas.74.6.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane T., Miller D. L., Hopfield J. J. Discrimination between D- and L-tyrosyl transfer ribonucleic acids in peptide chain elongation. Biochemistry. 1981 Dec 8;20(25):7059–7064. doi: 10.1021/bi00528a001. [DOI] [PubMed] [Google Scholar]


