Abstract
Background
Long-term survival of uncemented total joint replacements relies on osseointegration. With reduced bone stock impacted morselized allograft enhances early implant fixation but is subject to resorption.
Purpose
We therefore asked whether soaking morselized allograft in different concentrations of bisphosphonate before impaction would enhance fixation.
Methods
In each of 10 dogs, we implanted four unloaded titanium implants surrounded by a 2.5-mm gap into the proximal humerus, two implants in each humerus. The gap was filled with impacted morselized allograft soaked in saline or a low-, middle-, or high-dose bisphosphonate solution (0.005, 0.05, or 0.5 mg zoledronate/mL). At 4 weeks, the implants were evaluated by histomorphometric analysis and mechanical pushout test.
Results
The low dose of zoledronate increased new bone formation in the allograft but the high dose decreased new bone formation. The high dose of zoledronate resulted in the greatest inhibition of allograft resorption, whereas the low dose of zoledronate resulted in the lowest inhibition of allograft resorption. Implants surrounded allograft soaked in the low dose of zoledronate or saline had better fixation for all three mechanical parameters compared with implants surrounded by allograft soaked in the middle or high dose of zoledronate.
Conclusions
These data suggest bisphosphonate may enhance osseointegration of allografted implants and emphasize the need for preclinical testing of antiresorptive therapies.
Introduction
Long-term survival of hip arthroplasties depends on early and secure fixation to bone [18, 22]. In situations with reduced bone stock at the implantation site, the use of morselized allograft is a well-established way of enhancing early implant fixation [11, 23]. The objective of bone grafting is to achieve stability of an implant with the use of compacted, morselized bone graft and subsequently allow restoration of living bone stock by bone ingrowth [25]. Therapies that can facilitate graft incorporation potentially could increase early implant stability and thereby long-term implant survival.
One approach to enhance graft incorporation could be with the use of bisphosphonates. These compounds have strong affinity to bone and can inhibit bone resorption [10]. Several clinical studies suggest the use of bisphosphonates as oral and local adjuvants in total joint arthroplasties increase periimplant bone density or reduce implant migration [19, 26, 27]. The effect of soaking morselized allograft in bisphosphonate before impacting it around an experimental implant has been described [6, 14]. Bisphosphonate impaired the biomechanical implant fixation and blocked new bone formation and resorption. Other preclinical studies showed bisphosphonate can increase new bone formation while preserving the allograft [2–4]. These studies used a lower local dose compared with the dose used our previous study [6, 14], and the allograft was rinsed in saline after soaking it in bisphosphonate or the bisphosphonate was administered systemically. One explanation for the apparently contradictory findings and decreased implant fixation in our previous studies [6, 14] could be the use of too high a dose of bisphosphonate and omission of rinsing away unbound bisphosphonate.
We therefore hypothesized impacting morselized allograft around an experimental implant after it had been soaked in bisphosphonate and subsequently rinsed would increase the mechanical implant fixation, enhance new bone formation, and preserve the allograft in a dose-dependent manner.
Materials and Methods
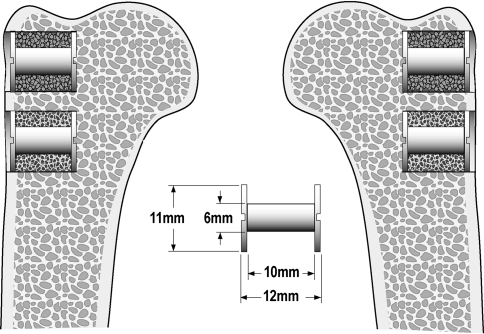
We used 10 skeletally mature female American Foxhounds with a median weight of 21 kg (range, 19–25 kg). Before conducting the study, we made a sample size calculation. Given the standardized and simple conditions in this experimental model, the minimal relevant difference was arbitrarily set to a 50% relative change in the biomechanical fixation as described by shear stiffness, strength, and energy absorption. Based on a previous study, we assumed the standard deviation on the relative change to be 50% [12]. The standard deviation was calculated as an average of the change in stiffness, strength, and energy absorption. Two-sided α and β were set to 5% and 20%, respectively. Two extra animals were added to the calculated sample size of eight to counteract decreased power if implants from one or two animals were lost for followup and subsequent analysis. We inserted four porous-coated titanium cylindrical implants (one of each of three bisphosphonate doses or saline control) into each dog. Two implants were inserted into the proximal part of each humerus. Each implant was surrounded by a 2.5-mm gap filled with impacted, morselized allograft (Fig. 1). We rinsed the allograft in saline or a low-, middle-, or high-dose (0.005, 0.05, or 0.5 mg zoledronate/mL saline) of zoledronate and subsequently rinsed it in saline before impaction. The different treatment groups were rotated systematically between proximal and distal implantation sites and between left and right humeri. Randomization determined implant placement in the first dog. We systematically rotated the four implant doses to obtain uniform distribution of all treatment groups between all implantation sites and thereby avoid a potential bias. The observation time was 4 weeks. This study was approved by our institution’s Animal Care and Use Committee. Institutional guidelines for treatment and care of experimental animals were followed.
Fig. 1.
Implants surrounded by impacted allograft soaked in bisphosphonate or saline were inserted in the proximal part of the humerus.
We used 40 custom-made titanium alloy (Ti-6Al-4 V) implants with a plasma-sprayed, porous-coated Ti-6Al-4 V surface. The rough surface coating was applied by Biomet, Inc (Warsaw, IN). The implants were cylindrical with a height of 10 mm and a mean diameter of 5.85 mm (SD, 0.31). The manufacturer of the implant coating determined mean pore diameter to be 480 μm, porosity 44%, and coating thickness 1.6 mm.
We prepared the cancellous allograft under sterile conditions from the humeral head and femoral head harvested from two dogs not included in this study. All cartilage was removed, and a bone mill (Biomet Bone Mill System; Biomet Inc) made fine grains with a length between 1 mm and 3 mm. We mixed the allograft from both dogs not included in this study before we divided it into small portions and stored it at −80°C for less than 1 week. After thawing for 1 hour, a portion of allograft with a mean weight of 1.17 g (range, 1.15–1.19 g) was soaked in either 1 mL saline or 1 mL zoledronate solution (low, middle, or high dose) for 3 minutes, as in our previous study [14]. The zoledronate solution was made by diluting Zometa® (Novartis, Basel, Switzerland), obtained through our hospital pharmacy, with saline. One vial of Zometa® contains 4.0 mg zoledronate, 220 mg mannitol, and 24 mg sodium citrate in 5 mL distillated water. After removing excess saline or zoledronate solution, we rinsed the allograft in saline by gentle agitation for 3 minutes. The soaking and rinsing procedure removed bone marrow from the allograft. Excess fluid was removed again and the allograft was impacted around the implants. Grafting was performed manually with a specially designed instrument that could fit over the implant while impacting the allograft in the 2.5-mm gap. To standardize the impaction process, one investigator (BE) performed all impacting of the allograft.
All surgery was performed using sterile conditions and with the dogs under general anesthesia induced with 5% thiopental before intubation and maintained with 1.5% isoflurane. Buprenorphine hydrochloride was used for analgesia. The proximal part of the humerus was exposed through a lateral extraarticular approach. Two Kirschner wires were inserted perpendicular to the surface with a 17.0-mm distance between them. The most proximal Kirschner wire was inserted at the level of the greater tubercle. Over the Kirschner wires, we made a 12.0-mm deep hole with an 11.0-mm cannulated drill. All drilling was performed at two revolutions per second to minimize thermal trauma to the bone. After removing bone debris and irrigating the bone cavity, we inserted a 6.0-mm implant with a footplate. Morselized allograft was impacted into the 2.5-mm gap around the implants. A top washer was screwed onto the implant to contain the graft and maintain concentric implant placement. The first implant inserted in each dog was the implant surrounded with allograft soaked in saline, and the second implant inserted was the implant surrounded with allograft soaked in the low-dose zoledronate. The third implant inserted in each dog was the implant surrounded with allograft soaked in the middle-dose zoledronate. The last implant inserted in each dog was the implant surrounded with allograft soaked in the high-dose zoledronate. This sequence of implant insertion was chosen so as not to transfer zoledronate from allograft containing a relatively high dose of zoledronate to allograft containing a relatively lower dose. Two additional allografted implants were inserted in a canine cadaver humerus to estimate the allograft volume and surface fraction at time zero. The allograft was impacted by one surgeon (BE).
We administered antibiotics (Rocephin; Sandoz GmbH, Kundl, Austria) immediately before surgery and 3 days postoperatively. Analgesics (Buprenox; Hospira Inc, Lake Forest, IL) were used for the first 3 postoperative days. The dogs were allowed full weightbearing immediately postoperatively. The dogs were kept in cages pair-wise and allowed social interaction outside the cages once a day with the other study dogs.
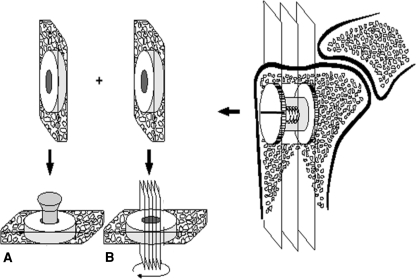
All dogs were euthanized 4 weeks after surgery using intravenous sodium pentobarbital (10 mL Beuthanasia-d Special; Schering-Plough, Summit, NJ). The proximal part of the humerus was cleaned of soft tissue and stored at −20°C. After removing the top washer, we cut two specimens perpendicular to the long axis of each implant with surrounding bone using a water-cooled band saw (Exact Apparatebau, Nordenstedt, Germany) (Fig. 2). The outermost specimen with a thickness of approximately 3.5 mm was stored at −20°C and used for later pushout testing (Fig. 2A). The innermost specimen with a thickness of approximately 6.5 mm was fixed in 70% ethanol and used for later histomorphometric analysis. The implants in both samples were at least surrounded by an 8-mm zone of bone including the allografted gap. Preparation of specimens and subsequent evaluation were performed blinded.
Fig. 2A–B.
A schematic diagram shows specimen preparation. Each bone-implant specimen is cut into two pieces: (A) a 3.5-mm specimen for the mechanical pushout test and (B) a 6.5-mm specimen for histomorphometric analyses. The 6.5-mm specimen is rotated randomly around the long axis of the implant after which four parallel sections are cut parallel to the long axis of the implant.
We tested 39 specimens containing the outermost 3.5-mm part of the implants to failure with an axial pushout test on an MTS Bionics Test Machine (MTS, Eden Prairie, MN). The specimens were placed on a metal support jig with a 7.4-mm diameter central opening. Centering the implant over the opening assured a 0.7-mm distance between the implant and support jig as recommended by Dhert et al. [9]. Before conducting the mechanical pushout test, it was ensured that both faces of the specimen were parallel. The direction of loading was from the cortical surface inward. A preload of 3 N was applied to standardize contact conditions before initiating loading. We used a displacement rate of 5 mm per minute, and continuous load versus displacement data were recorded. A 2.5-kN load cell was used. Because of variation in implant sizes, all pushout parameters were normalized by the cylindrical surface area of the transverse implant section tested. Maximum shear strength (MPa) was determined from the maximum force applied until failure of the bone-implant interface. Maximum shear stiffness (MPa/mm) was obtained from the slope of the linear section of the load versus displacement curve. Total energy absorption (kJ/m2) was calculated as the area under the load displacement curve until failure.
Forty histomorphometric specimens containing the 6.5-mm innermost part of the implants and surrounding bone were dehydrated gradually in ethanol (70%–100%) containing basic fuchsin and embedded in methylmethacrylate. Four vertical, uniform, random sections were cut with a hard-tissue microtome (KDG-95; MeProTech, Heerhugowaard, The Netherlands) parallel to the long axis of the implant as described by Overgaard et al. [20] (Fig. 2B). This provides highly reproducible results with a negligible bias [5]. Before making the sections, the implant was rotated randomly around its long axis. The sections were cut parallel to this axis. The 50-μm thick sections were counterstained with 2% light-green (BDH Laboratory Supplies, Poole, UK) and then cut with a distance of 400 μm between each section (Fig. 2). This resulted in bone being stained green and nonmineralized tissue red.
Blind histomorphometric analysis was performed using a stereologic software program that superimposes sine-weighted lines or points on microscopic fields captured on a monitor (CASTgrid; Olympus Denmark A/S, Ballerup, Denmark). Bone-to-implant contact was defined as the implant surface covered with new bone or allograft and estimated by manually counting intercepts between sine-weighted lines and surface covered with bone. Bone volume fraction was estimated by manual point counting in a gap 0 to 2000 μm around the implants. The specimen preparation method and use of sine-weighted lines made it possible to obtain unbiased estimates [8]. Allograft and new bone were distinguished based on the presence of osteocytes, lamellar or woven appearance, and trabecular shape. We used the areal and volume fractions of allograft and new bone for statistical analysis. Previous studies suggest these morphologic characteristics result in intraindividual and interindividual reproducibility of maximal 8% for new bone and maximal 6% for allograft [5, 6, 14].
We determined differences in biomechanical implant fixation as defined by shear strength, shear stiffness, and energy absorption between the four different dosages group using ANOVA. All three variables passed ANOVA and subsequently were analyzed pair wise with a Student’s paired t-test. All biomechanical data were tested to be normally distributed using Shapiro-Wilk test for normality and q-norm plots. We determined differences in amount of newly formed bone on and around the implant between the four different dosages group using Friedman’s ANOVA. All variables passed Friedman’s ANOVA and subsequently were analyzed pair wise with Wilcoxon Signed rank test. Data were not normally distributed. Finally, we determined differences in amount of allograft on and around the implant between the four different dosages group using Friedman’s ANOVA. All variables passed ANOVA and subsequently were analyzed pair wise with Wilcoxon Signed rank test. Data were not normally distributed. We used Intercooled Stata 9.0 (Stata Inc, College Station, TX) for statistical analysis.
Results
All dogs were fully weightbearing within 3 days postoperatively and completed the 4-week observation period. We observed no clinical signs of infection around the implants at the time of autopsy. One specimen for biomechanical testing from the low-dose group was excluded because of technical error in preparation.
We found a dose-dependent difference in the mechanical fixation of the implants as defined by shear strength, shear stiffness, and energy absorption (Table 1). The best mechanical implant fixation was in the low-dose and control groups (Table 2). The high-dose group had the lowest mechanical fixation as compared with the three other groups (Table 2).
Table 1.
Biomechanical implant fixation
| Dose (mg zoledronate/mL) | Maximum shear stiffness (MPa/mm) | Maximum shear strength (MPa) | Total energy absorption (kJ/m2) |
|---|---|---|---|
| Control (0 mg/mL) | 8.9 (5.6–12.2) | 1.9 (1.2–2.7) | 0.4 (0.2–0.6) |
| Low dose (0.005 mg/mL) | 10.5 (5.6–15.4) | 2.6 (1.4–3.7) | 0.7 (0.4–1.0) |
| Middle dose (0.05 mg/mL) | 4.0 (2.1–5.8) | 1.0 (0.6–1.5) | 0.4 (0.2–0.6) |
| High dose (0.5 mg/mL) | 0.8 (0.6–1.1) | 0.4 (0.3–0.4) | 0.3 (0.2–0.4) |
Values are expressed as means with 95% confidence intervals in parentheses.
Table 2.
P values for paired comparisons of biomechanical parameters between treatment groups
| Maximum shear stiffness | ||||
|---|---|---|---|---|
| Group | Control | Low dose | Middle dose | High dose |
| Control | — | — | — | — |
| Low dose | 0.55 | — | — | — |
| Middle dose | 0.023 | 0.015 | — | — |
| High dose | 0.0008 | 0.0035 | 0.0053 | — |
| Maximum shear strength | ||||
|---|---|---|---|---|
| Control | Low dose | Middle dose | High dose | |
| Control | — | — | — | — |
| Low dose | 0.30 | — | — | — |
| Middle dose | 0.024 | 0.038 | — | — |
| High dose | 0.0007 | 0.0015 | 0.0053 | — |
| Total energy absorption | ||||
|---|---|---|---|---|
| Control | Low dose | Middle dose | High dose | |
| Control | — | — | — | — |
| Low dose | 0.15 | — | — | — |
| Middle dose | 0.99 | 0.13 | — | — |
| High dose | 0.48 | 0.0022 | 0.41 | — |
Analysis of variance followed by Student’s paired t-test.
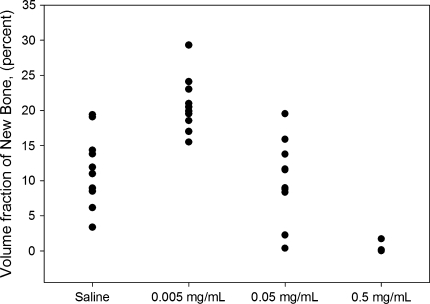
We found a dose-dependent difference in the amount of new bone formed around the implants (Table 3). The amount of new bone formation was greatest around the implants in the low-dose group (Table 3). No new bone formation was observed around the implants in the high-dose group (Fig. 3). We observed no difference in the amount of new bone in contact with the implant surface between the control, low-dose, and the middle-dose groups. Formation of new bone on the surface of the implants in the high-dose group was blocked as compared with all other groups.
Table 3.
Fractions of bone in gap and on the implant surface
| Dose (mg zoledronate/mL) | Gap (%) | Surface (%) | ||
|---|---|---|---|---|
| New bone | Allograft | New bone | Allograft | |
| Control (0 mg/mL) | 12 (8–14) | 19 (16–21) | 3 (1–12) | 1 (0–1) |
| Low dose (0.005 mg/mL) | 20 (19–23) | 35 (31–39) | 2 (0–2) | 1 (0–1) |
| Middle dose (0.05 mg/mL) | 11 (8–14) | 44 (40–46) | 1 (0–3) | 5 (3–7) |
| High dose (0.5 mg/mL) | 0 (0–0) | 43 (40–47) | 0 (0–0) | 6 (5–8) |
Values are expressed as medians with interquartile ranges in parentheses.
Fig. 3.
A graph shows the volume fractions of new bone in the gap around implants.
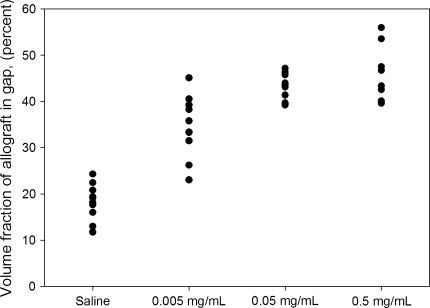
Addition of increasing concentrations of zoledronate resulted in a dose-dependent increase in the amount of allograft around the implants from the control group to the middle-dose group. From the middle- to the high-dose groups, we observed no difference in amount of allograft around the implants (Fig. 4; Table 4). We observed increased preservation in the amount of allograft in contact with the implant surface in the control and low-dose groups compared with the middle- and high-dose groups. The mean volume and mean surface fraction of allograft from the time zero implants were, respectively, 47% and 8%. The new bone formation and antiresorptive effect of the bisphosphonate treatment were restricted histologically to the gap around the implants (Fig. 5). We observed no histologic differences with respect to new bone formation or allograft resorption between the host-allograft interface and the gap around the implants. No excess bone formation or other signs of allograft rejection were observed.
Fig. 4.
A graph shows the volume fractions of allograft in the gap around implants.
Table 4.
P values for paired comparisons of histomorphometry between treatments groups
| Group | New bone in gap | |||
|---|---|---|---|---|
| Control | Low dose | Middle dose | High dose | |
| Control | — | — | — | — |
| Low dose | 0.013 | — | — | — |
| Middle dose | 0.54 | 0.0050 | — | — |
| High dose | 0.0076 | 0.0076 | 0.013 | — |
| Allograft in gap | ||||
|---|---|---|---|---|
| Control | Low dose | Middle dose | High dose | |
| Control | — | — | — | — |
| Low dose | 0.0050 | — | — | — |
| Middle dose | 0.0050 | 0.0059 | — | — |
| High dose | 0.0076 | 0.0075 | 0.21 | — |
| New bone on implant surface | ||||
|---|---|---|---|---|
| Control | Low dose | Middle dose | High dose | |
| Control | — | — | — | — |
| Low dose | 0.079 | — | — | — |
| Middle dose | 0.15 | 1.00 | — | — |
| High dose | 0.0089 | 0.012 | 0.048 | — |
| Allograft on implant surface | ||||
|---|---|---|---|---|
| Control | Low dose | Middle dose | High dose | |
| Control | — | — | — | — |
| Low dose | 0.74 | — | — | — |
| Middle dose | 0.012 | 0.011 | — | — |
| High dose | 0.0075 | 0.0075 | 0.23 | — |
Friedman’s analysis followed by Wilcoxon signed rank test.
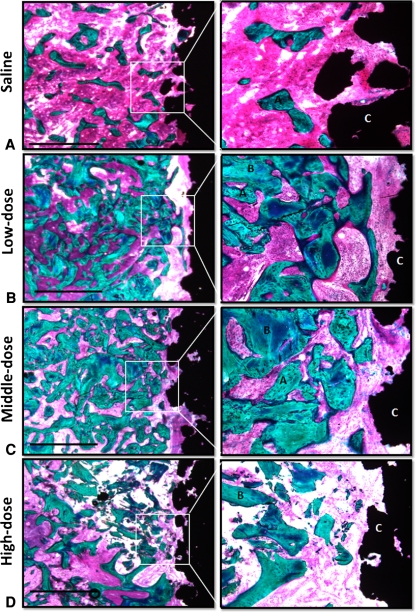
Fig. 5A–D.
Representative histologic sections of the (A) saline, (B) low-dose, (C) middle-dose, and (D) high-dose treatment groups are shown. The sections are from four implants inserted in the same animal. Enhanced photomicrographs of the histologic sections are shown on the right. Increased amounts of bone are seen around the implants from the low- and middle-dose treatment groups. The allograft chips are not surrounded by any new bone in the high-dose treatment group. A = new bone; B = allograft, C = implant. Bar = 1 mm (Stain, basic fuchsin and light green; magnification, objective ×1.25, ocular ×10 (left), objective ×10, ocular ×10 (right).
Discussion
Long-term survival of total joint replacements relies on initial mechanical stability and sustained osseointegration to prevent migration and implant loosening [18, 22]. In situations with reduced bone stock at the implantation site, the use of morselized allograft is a well-established way of enhancing early implant fixation [11, 23]. Therapies that can facilitate graft incorporation potentially could increase early implant stability and thereby long-term implant survival. The purpose of this study was to investigate whether implant fixation could be increased by impacting morselized allograft soaked in different concentrations of zoledronate around experimental titanium-coated implants.
Our experimental hip implant model is subject to limitations. First, the data are limited to the portion of a cementless joint replacement that is surrounded by impacted, morselized allograft and placed in cancellous bone [7, 17]. We did this to imitate the calcar portion of an uncemented hip implant where osseointegration takes place. Second, the model is simple and has a high degree of variance control, but does not require direct load transfer through the implant-bone interface. The lack of direct load transfer stress shields the implant and promotes resorption, and thereby makes it easier to detect a potential difference in allograft resorption between the dosages groups. Third, the model has no access to the joint space. The lack of joint fluid in the bone-implant interface makes the model less complex and the results easier to interpret. Fourth, the model is uncemented, which does not replicate those of the clinical revision arthroplasties that are cemented [25]. Therefore, caution should be taken when extrapolating data to clinical cemented arthroplasties. Fifth, to compare the data with those from our previous studies using the same model, we chose to use the outermost section (Fig. 2) for mechanical testing and the innermost section for histomorphometry. This uniform choice potentially could introduce bias when correlating biomechanical results to histomorphometric results. Sixth, the observation period of 4 weeks was chosen to compare the data with that in previous studies using the same implant model [6, 14]. Seventh, the canine was chosen as the test animal because its bone closely resembles human bone [1].
The observed changes in biomechanical implant fixation reflected the changes in new bone formation. We observed an enhancing effect on new bone formation with our low dose of zoledronate but an inhibitory effect with our high dose of zoledronate. This is in accordance with other studies in which an increase in biomechanical fixation correlated to an increase in amount of bone [7, 15, 16]. However, an increase in bone volume fraction resulting from preservation of allograft with a high dose of zoledronate did not correlate with an increase in biomechanical fixation.
Our low dose of zoledronate was able to increase new bone formation. One explanation for this augmenting effect could be the preserving effect of zoledronate on the allograft, thus prolonging its osteoconductive effect and thereby increasing new bone formation. This has been observed in other studies [2, 3, 15, 16]. The amount of new bone in the low-dose group was similar in magnitude as reported by others [3]. Another explanation for the increased bone formation in the low-dose group could be a direct anabolic effect of zoledronate on new bone formation. However, this effect has been observed only in vitro [13, 21]. The decreased new bone formation in the middle- and high-dose groups could be the result of a toxic effect of a too high dose of zoledronate [12].
Our data suggest soaking allograft in bisphosphonate and subsequently rinsing away unbound bisphosphonate can either increase or decrease new bone formation depending on the concentration of bisphosphonate. Bisphosphonate was reported to decrease the biomechanical implant fixation and almost block allograft resorption and new bone formation [6, 14]. The decreased bone formation could be explained by the relatively high graft density, which might have acted as a hindrance for ingrowth of new bone [24]. Another explanation for the decreased implant fixation could be the use of a too high a dose of bisphosphonate or the presence of unbound, potentially toxic bisphosphonate [12]. As we observed in this study, the ability of bisphosphonate to increase new bone formation indicates that the totally blocked bone formation previously observed could be the effect of a too high a dose of bisphosphonate and the omission of rinsing away unbound potential toxic bisphosphonate. This also could explain the contradictory findings in new bone formation between our previous studies and other studies investigating the effect of allograft and bisphosphonate [2, 3, 6, 14]. However, our data also indicate rinsing away bisphosphonate is not a guarantee for increased new bone formation and thereby emphasizes the need for preclinical testing of new antiresorptive therapies.
Our findings suggest there may be a clinical advantage in soaking allograft in bisphosphonate before impacting it around an uncemented total joint arthroplasty. Furthermore, the positive influence of the low-dose zoledronate on implant fixation allows combination of bisphosphonate with bone morphogenetic proteins (BMPs) as adjuvants in uncemented allografted total joint arthroplasties. The antiresorptive effect of bisphosphonate may be able to eliminate the potential drawbacks of increased allograft resorption induced by BMPs without affecting the osteoinductive properties [17]. Soaking allograft in bisphosphonate may increase new bone formation while inhibiting allograft resorption. However, the data also emphasize the importance of preclinical testing, because too high a concentration of bisphosphonate can inhibit new bone formation and potentially impair the biomechanical implant fixation.
Acknowledgments
We thank Jane Pauli and Anette Milton, Orthopaedic Research Laboratory, Aarhus University Hospital, for technical expertise.
Footnotes
One or more of the authors (TJ) have received funding from The Danish Rheumatism Association, Denmark. Biomet Inc coated the implants.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Orthopaedic Research Laboratory, Aarhus University Hospital, Aarhus, Denmark (specimen preparation, testing, and analysis), and Orthopaedic Biomechanics Laboratory, Midwest Research Foundation, Minneapolis, MN, USA (surgery).
References
- 1.Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139:663–670. doi: 10.1210/en.139.2.663. [DOI] [PubMed] [Google Scholar]
- 2.Aspenberg P, Astrand J. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand. 2002;73:20–23. doi: 10.1080/000164702317281350. [DOI] [PubMed] [Google Scholar]
- 3.Astrand J, Aspenberg P. Systemic alendronate prevents resorption of necrotic bone during revascularization: a bone chamber study in rats. BMC Musculoskelet Disord. 2002;3:19–23. doi: 10.1186/1471-2474-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astrand J, Harding AK, Aspenberg P, Tagil M. Systemic zoledronate treatment both prevents resorption of allograft bone and increases the retention of new formed bone during revascularization and remodelling: a bone chamber study in rats. BMC Musculoskelet Disord. 2006;7:63–69. doi: 10.1186/1471-2474-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baas J. Adjuvant therapies of bone graft around non-cemented experimental orthopedic implants stereological methods and experiments in dogs. Acta Orthop Suppl. 2008;79:1–43. [PubMed] [Google Scholar]
- 6.Baas J, Elmengaard B, Jensen TB, Jakobsen T, Andersen NT, Soballe K. The effect of pretreating morselized allograft bone with rhBMP-2 and/or pamidronate on the fixation of porous Ti and HA-coated implants. Biomaterials. 2008;29:2915–2922. doi: 10.1016/j.biomaterials.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Baas J, Lamberg A, Jensen TB, Elmengaard B, Soballe K. The bovine bone protein lyophilisate Colloss improves fixation of allografted implants: an experimental study in dogs. Acta Orthop. 2006;77:791–798. doi: 10.1080/17453670610013015. [DOI] [PubMed] [Google Scholar]
- 8.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 9.Dhert WJ, Verheyen CC, Braak LH, Wijn JR, Klein CP, Groot K, Rozing PM. A finite element analysis of the push-out test: influence of test conditions. J Biomed Mater Res. 1992;26:119–130. doi: 10.1002/jbm.820260111. [DOI] [PubMed] [Google Scholar]
- 10.Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4:30–34. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gie GA, Linder L, Ling RS, Simon JP, Slooff TJ, Timperley AJ. Impacted cancellous allografts and cement for revision total hip arthroplasty. J Bone Joint Surg Br. 1993;75:14–21. doi: 10.1302/0301-620X.75B1.8421012. [DOI] [PubMed] [Google Scholar]
- 12.Idris AI, Rojas J, Greig IR, Van’t Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 13.Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004;25:4105–4115. doi: 10.1016/j.biomaterials.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen T, Baas J, Bechtold JE, Elmengaard B, Soballe K. Soaking morsellized allograft in bisphosphonate can impair implant fixation. Clin Orthop Relat Res. 2007;463:195–201. doi: 10.1097/BLO.0b013e31813c6696. [DOI] [PubMed] [Google Scholar]
- 15.Jakobsen T, Baas J, Kold S, Bechtold JE, Elmengaard B, Soballe K. Local bisphosphonate treatment increases fixation of hydroxyapatite-coated implants inserted with bone compaction. J Orthop Res. 2009;27:189–194. doi: 10.1002/jor.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobsen T, Kold S, Bechtold JE, Elmengaard B, Soballe K. Local alendronate increases fixation of implants inserted with bone compaction: 12-week canine study. J Orthop Res. 2007;25:432–441. doi: 10.1002/jor.20276. [DOI] [PubMed] [Google Scholar]
- 17.Jensen TB, Overgaard S, Lind M, Rahbek O, Bunger C, Soballe K. Osteogenic protein 1 device increases bone formation and bone graft resorption around cementless implants. Acta Orthop Scand. 2002;73:31–39. doi: 10.1080/000164702317281378. [DOI] [PubMed] [Google Scholar]
- 18.Karrholm J, Borssen B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4–7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg Br. 1994;76:912–917. [PubMed] [Google Scholar]
- 19.Kesteris U, Aspenberg P. Rinsing morcellised bone grafts with bisphosphonate solution prevents their resorption: a prospective randomised double-blinded study. J Bone Joint Surg Br. 2006;88:993–996. doi: 10.1302/0301-620X.88B8.17457. [DOI] [PubMed] [Google Scholar]
- 20.Overgaard S, Soballe K, Jorgen H, Gundersen H. Efficiency of systematic sampling in histomorphometric bone research illustrated by hydroxyapatite-coated implants: optimizing the stereological vertical-section design. J Orthop Res. 2000;18:313–321. doi: 10.1002/jor.1100180221. [DOI] [PubMed] [Google Scholar]
- 21.Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000;60:6001–6007. [PubMed] [Google Scholar]
- 22.Ryd L, Albrektsson BE, Carlsson L, Dansgard F, Herberts P, Lindstrand A, Regner L, Toksvig-Larsen S. Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg Br. 1995;77:377–383. [PubMed] [Google Scholar]
- 23.Slooff TJ, Huiskes R, Horn J, Lemmens AJ. Bone grafting in total hip replacement for acetabular protrusion. Acta Orthop Scand. 1984;55:593–596. doi: 10.3109/17453678408992402. [DOI] [PubMed] [Google Scholar]
- 24.Tagil M, Aspenberg P. Impaction of cancellous bone grafts impairs osteoconduction in titanium chambers. Clin Orthop Relat Res. 1998;352:231–238. [PubMed] [Google Scholar]
- 25.Toms AD, Barker RL, Jones RS, Kuiper JH. Impaction bone-grafting in revision joint replacement surgery. J Bone Joint Surg Am. 2004;86:2050–2060. doi: 10.2106/00004623-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 26.Venesmaa PK, Kroger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhav EM. Alendronate reduces periprosthetic bone loss after uncemented primary total hip arthroplasty: a prospective randomized study. J Bone Miner Res. 2001;16:2126–2131. doi: 10.1359/jbmr.2001.16.11.2126. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson JM, Stockley I, Peel NF, Hamer AJ, Elson RA, Barrington NA, Eastell R. Effect of pamidronate in preventing local bone loss after total hip arthroplasty: a randomized, double-blind, controlled trial. J Bone Miner Res. 2001;16:556–564. doi: 10.1359/jbmr.2001.16.3.556. [DOI] [PubMed] [Google Scholar]