Abstract
There are few good surgical options that allow for continued spinal growth in patients with early-onset scoliosis. The “Shilla” is a growth guidance system that does not require repeated surgical lengthenings. The Shilla system guides growth at the ends of dual rods with the apex of the curve corrected, fused, and fixed to the rods. The growth occurs through the extraperiosteally implanted pedicle screws that slide along the rods at either end of the construct. We implanted 11 2-month-old immature goats with the dual rod system and euthanized all 11 goats 6 months postoperatively. We evaluated plain radiographs, regular computed tomography, microcomputed tomography, physical and histologic examinations, and a microscopic wear analysis. All of the goat spines grew with the implants in place; growth occurred in both the thoracic and lumbar ends of the rods for a total average of 48 mm. None of the implants failed, although we observed minor wear at the rod/screw interface. Growth guidance with the Shilla rod system allowed for continued growth in this goat model.
Introduction
There are usually very few effective treatment options for patients with early-onset scoliosis. Bracing may help prevent spinal curve progression in curves under 40° but is ineffective in controlling larger curves [1, 3, 8]. To correct the deformity, surgery with fusion is usually performed. Anterior or posterior procedures or combinations of both are used to correct the curvature, but fusion stops growth. Inhibition of spinal growth can lead to a disproportionate posture, a shortened trunk, and hinder lung development [8].
An alternative to standard fusion techniques is the use of growing rod systems that correct the deformity but allow for spinal growth. Harrington ratcheted single rods were the first used for growth with scheduled lengthening; these devices required external support with a brace to prevent dislodgement of the hooks [4]. The Luque system used two rods and sublaminar wires but did not consistently allow growth [7]. The most commonly used dual rod systems have been described by Akbarnia and Thompson with moderate success [1, 2, 8]. The vertical expandable prosthetic titanium rib (VEPTR) device used primarily for chest wall deformities and congenital scoliosis has at times been used for early-onset deformities [5, 6]. Unfortunately, these systems require a scheduled operative lengthening every 6 months and ultimately are treated with spinal fusion in most instances.
An alternative is a new growth guidance system (Shilla) that corrects the scoliotic apex of a curve with limited fusion and allows for continued guided growth at distal portions of the spine avoiding the need for operative lengthenings. The name derives from the location of its inception in Seoul, Korea. Previous unpublished in vitro studies using an MTS test machine demonstrate that the Shilla implants cycled through 1 million cycles without failure, and the only consequence was the production of metallic frettings at the unconstrained parts of the system (medtronic internal test report TR04-331, 2006). The compression fatigue produced in 1 million cycles is roughly equivalent to 1 year of walking in a child. Application for this device has been made for an TI–HDE designation through the FDA in the United States.
We asked whether this system (1) would permit continued growth; (2) would be associated with comorbidities such as spinal stenosis or changes at adjacent facet joints; (3) would exhibit wear of the system components; and (4) would be associated with tissue effects from metal debris.
Materials and Methods
In a pilot study, we tested the Shilla growth guidance rod system in 11 goats followed for 6 months. The technique (described in detail subsequently) is designed to directly treat the apex of a deformity with fusion limited to four vertebral levels while allowing growth in the other areas of the spine. The uniqueness of the Shilla growth guidance system is based on the continued growth that occurs when (1) polyaxial Shilla screws are placed with radiographic guidance without exposing the bone. This prevents periosteal stripping that would inhibit growth; and (2) the locking cap for the Shilla screw tightens to the screw, not the rod, capturing the rod but allowing it to slide.
The Shilla system was designed with stainless steel 5.5-mm or 4.5-mm diameter rods and both fixed-head and polyaxial bone screws. The system is designed to be strong enough to withstand normal physiological childhood activities while promoting fusion of the apical vertebrae. The construct allows the ends of the rods to guide growth in the vertebral column. The success of this implant design depends on continued growth at both the cephalad and caudad ends of the spine as a result of the extraperiosteal placement of polyaxial screws, which fit loosely on the rod. The extraperiosteal screw placement is implemented from the subfascial layer with the aid of radiographic control to place the polyaxial screws into the pedicles. This allows the sagittally contoured rod to be captured by the screw head with a securing cap that locks onto the screw and not the rod. The Shilla allows for a loose-fitting construct at the ends but does not prevent growth of the vertebral bodies.
All anesthesia was administered by veterinarians with strict adherence to all Institutional Animal Care and Use Committee and Institutional Review Board requirements. The following anesthesia protocol was followed. The goats were sedated with xylazine (0.10 mg/kg intramuscularly) and then induced with ketamine (4.5 mg/kg intravenously). They were then intubated and maintained on isoflurane gas (2–3% MAC) with oxygen. They were given cefazolin (35 mg/kg intravenously) as preoperative antibiotic. Hydration was maintained with 0.9% NaCl (5 mL/hr intravenously). We made a single midline incision from the upper thoracic to the lower lumbar spine (T4-5 to L4-5) with a separate incision for the iliac crest graft harvesting. Dissection proceeded to the fascial layer and a subperiosteal approach was used for the four apical segments only with the soft tissues cleaned from these vertebral layers. The fascial incision was extended along both sides of the spinous processes 1 cm off the midline extending cephalad and caudad from this central subperiosteal exposure. The iliac crest graft was used for the apical fusion. C-arm guidance was necessary for the placement of fixed head pedicle screws at the apical four levels of each construct. Pedicle screws were placed anatomically in the center of the pedicles bilaterally at each of these apical levels. Two animals had an alternating apical pattern to determine if there was any variation in the canal diameter. The pedicle was identified radiographically and the polyaxial (Shilla) screws were placed from the muscle layer with the aid of a C-arm. An electrocautery was used to begin the approach to the pedicle followed by a gearshift placed into the pedicle. The polyaxial screw was placed without any visualization of the bony structure other than the center of the hole. Four pedicle screws were placed in the upper thoracic spine and four in the lumbar spine. Dual stainless steel 5.5-mm diameter rods were inserted with sagittal contours secured to the screws with normal locking plugs at the apical levels. These tighten to the rod. The special Shilla locking caps were used at the peripheral Shilla screws. These caps tighten to the screw leaving the rod free from constraint but captured by the screw head (Fig. 1A–B). The rods were left at least one vertebral level long at the top and bottom for growth. Routine closure was accomplished after fusion techniques were used across the apical sections.
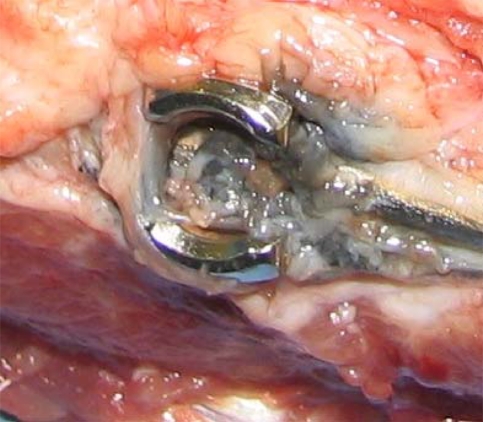
Fig. 1A–B.
(A) This photograph shows the Shilla polyaxial screw where the rod is captured but not constrained and (B) the Shilla locking cap where the top shown on the left is snapped off after tightening.
One animal with a small pedicle and canal size was rendered paraplegic at surgery and euthanized at that time with intravenous techniques (pentobarbital 200 mg/kg intravenously).
Postoperatively, the following analgesics were given: flunixin meglumine (1.5 mg/kg intramuscularly) twice a day for 2 days and butorphanol (0.05 mg/kg intramuscularly) once a day for 1 day. The following postoperative antibiotics were given: ceftiofur (2.2 mg/kg intramuscularly) twice a day for 3 days and gentamicin (6 mg/kg intramuscularly) once a day for 3 days. The animals were housed in cages with exercise routines for the 6 months before scheduled euthanasia through prescribed techniques (intravenous pentobarbital 200 mg/kg).
Spinal growth was calculated by measuring the radiographic distance between the end screws and the ends of the rods. This shortening of the rods radiographically was caused by the screws sliding along the rods during growth. Stenosis was measured looking at spinal canal diameter with a regular computed tomographic scan. We defined stenosis as a greater than 50% reduction in canal diameter.
For histologic analysis, tissue and lymph nodes from surviving goats were retrieved from the fused and growing rod regions and analyzed by one experienced histologist using hematoxylin and eosin histology. Three samples of tissue surrounding each of the Shilla screws, apical fusion screws, and control (noninstrumented) areas, along with lymph nodes near the apex and the inferior Shilla screws, were excised and stored in formalin. The samples were then cut and stained for hematoxylin and eosin analysis.
Three of us (JT, HZ, MH) graded the rods, set screws, and pedicle screws for surface wear at the sites where the rods slide inside the growing screws with the following subjective scale: 0 = absence of wear, 1 = minimal wear, 2 = moderate wear, and 3 = extensive wear. The rods are shot peened and have a slightly rough surface. “Absence of wear” is self-explanatory. “Minimal wear” was the visual indication of contact with little or no material loss. Evidence could be a shiny section, a halo, or other discoloration. “Moderate wear” for the rod was where the shot peened surface of the rod was worn halfway through the rough texture to the core of the rod. For the set screw, it was a 1- to 4-μm depression from the rod measured using white-light interferometry (Zygo, Middlefield, CT) and extended across the full diameter. For the pedicle screws, it was a rough texture on 50% or less of the crown component and obvious localized material loss on the head component. “Extensive wear” for the rod was denoted by the shot peened surface being worn through to the core (smooth surface) and wear patterns around more than 30% of the local surface. For the set screw, it was a greater than 4-μm depression from the rod extending across the full diameter. For the pedicle screws, extensive wear was a rough texture on more than 50% of the surface of the crown component and obvious localized material loss on the head component. Moderate and extensive wear was also classified by how much area was worn. Larger sections of moderate wear could be classified as extensive wear just as small sections of extensive wear could be considered moderate. The pedicle screws and rods were classified by macro- and microexaminations. Worn sections of the rods were measured in millimeters with a 15-cm ruler, whereas the surface was examined by these criteria with a standard microscope. We recorded how many sides of the rod the wear lines were present on and obtained three-dimensional images and profile of the wear with the Zygo. Pedicle screws were examined under optical microscopy to determine location and severity. Location was the crown component, the head component, or both. Location was also noted in terms of where on the crown and where on the head. Severity was classified from 0 to 3 according to these criteria. We used white-light interferometry (Zygo) in combination with microscopic examinations to classify set screw wear patterns. The set screws were labeled with a black dot at one side to denote position in the screw before removal, fixtured on the Zygo table, and analyzed to obtain images of the three-dimensional surface. Depth calculations were performed and wear was classified from 0 to 3 according to these criteria.
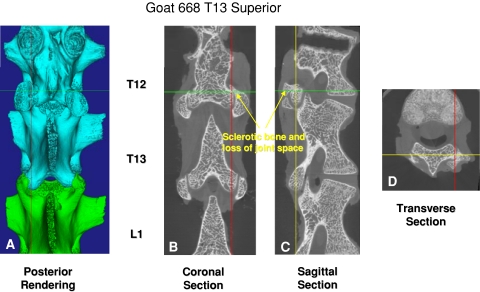
Three of us (RM, DS, HZ) assessed the fusion of the superior articulating surfaces of the T13 facet joints using microcomputed tomographic analysis. T13 was the vertebra adjacent caudally to the fused apical instrumentation. We (RM, DS, JT, KM) also manually and visually evaluated the spines for fusion by assessing the flexibility of each motion segment of concern and noting the presence of bony ingrowth between the facets. With direct visualization of the observers, a consensus was reached concerning the stiffness of the spine with manual movements.
Results
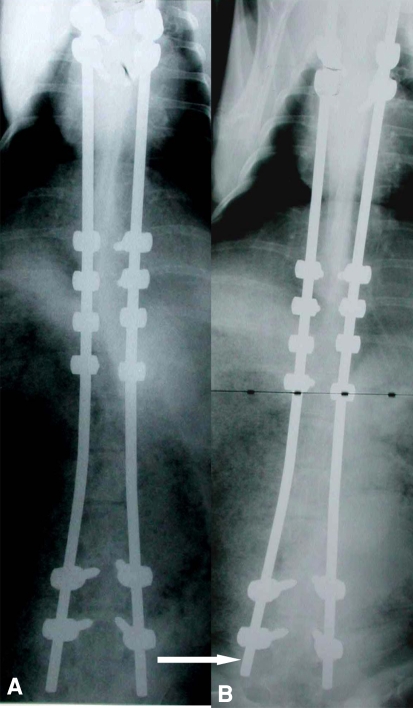
At 6-month followup, all the animals had evidence of growth radiographically with sliding of the Shilla screws along the rods towards the ends at an average of 48 mm (range, 37–62 mm) (Fig. 2A–B). Manual testing revealed movement of the unconstrained Shilla screws at the top and bottom of the construct.
Fig. 2A–B.
Shown here is an example of anteroposterior radiographs of a goat showing implants (A) immediately after insertion and (B) 6 months postoperatively. Note the arrow showing the rods are shorter 6 months later denoting growth of the spine.
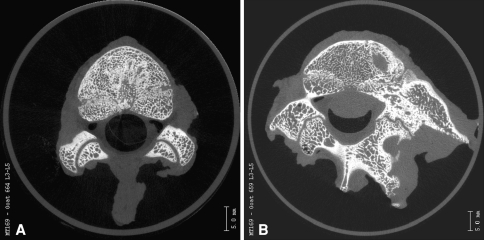
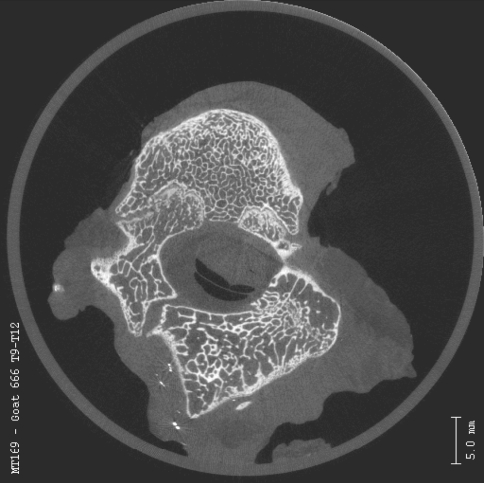
We observed no evidence of apical stenosis with either the bilateral screw pattern or alternating screw insertion in any of our specimens when compared with controls. The microcomputed tomographic analysis showed the transitional facets adjacent to the fused segment exhibited degenerative changes. However, normal articular cartilage was evident visually on microcomputed tomography in the intervening facet joints between the transitional level and the unconstrained screws. These mobile facets were within the instrumented area but not fused or severely degenerated. The facet joints at the level of the Shilla screws reflected degenerative changes secondary to the abutment of the screw head against the edge of the pedicle and superior process of the facet. The control facets possessed orderly, rounded articulating surfaces with normal trabecular structure (Fig. 3A–D). Fusion of the apical facets was evident as well (Figs. 4A–B, 5).
Fig. 3A–D.
Microcomputed tomographic analysis of facet wear is illustrated here.
Fig. 4A–B.
An axial microcomputed tomographic scan of the L3-L4 facets of a (A) normal control goat and (B) a Shilla-instrumented goat is pictured. At the instrumented level, the ipsilateral facet to the screw is degenerated, whereas the contralateral facet is slightly narrowed.
Fig. 5.
This is the axial microcomputed tomographic scan of the T11-T12 facets of a goat instrumented with an alternating apical screw pattern. Note the right facet is fused.
The wear analysis of the unconstrained instrumentation (Shilla screws) demonstrated wear patterns on the set screws and caps implanted at the distal levels of the thoracic and lumbar spine. Moreover, the screws and rods at the unconstrained lumbar levels exhibited more wear patterns than levels in the thoracic spine. Observations revealed the locking caps were dented as a result of movement at the interface between the rod and screw during growth. Rods exhibited patterns matching the set screws and locking caps (Fig. 6). Wear of the rods, pedicle screws, and set screws did not cause any structural failures. Manual testing revealed movement of the unconstrained screws and a solid fusion without movement at the fused apex.
Fig. 6.
This photograph shows average locking cap wear with rod dent.
Metallic wear debris was observed in the soft tissues adjacent to the Shilla growth guidance screws. After excision of the vertebral column, the para-aortic nodes anterior to the spine, which were adjacent to the Shilla screws, were dark in color as a result of metallic debris. Microscopic analysis of these lymph nodes revealed the presence of metallic debris in the lymphoid cells. The para-aortic lymph nodes adjacent to the fused apical nonmoving segment were analyzed for comparison and did not have any of this metallic debris present. Tissue retrieved around the cephalad and caudal nonlocking (Shilla) set screws exhibited a moderate to extensive inflammatory reaction in local tissue (Fig. 7). Metallic debris was observed in lymph nodes located around unconstrained screws with particles up to 45 μm in size. No metallic debris was observed at the fused regions.
Fig. 7.
Shown here is a photograph demonstrating blackened tissue adjacent to a nonlocking (Shilla) set screw.
Discussion
Early-onset scoliosis currently is difficult to treat. Most surgical options, including fusions, are problematic in one way or another owing to the desirability of continued spinal growth. The “Shilla” is a pedicle screw system allowing growth at nonfused segments that does not require repeated surgical lengthenings. The Shilla system guides growth at the ends of dual rods with the apex of the curve corrected, fused, and fixed to the rods. In a pilot animal study, we asked whether this system (1) would permit continued growth; (2) would be associated with comorbidities such as spinal stenosis or changes at adjacent facet joints; (3) would exhibit wear of the system components; and (4) would be associated with tissue effects from metal debris.
The limitations of this study include the small number of animals studied and the small size of pedicles encountered in some of the animals leading to paraplegia, further reducing the number of subjects. Safe use of pedicle screws in small subjects necessitates spinal cord monitoring that was not available with this study. The information gained from the animals completing the study was carefully studied and supplied valuable information for potential use in children with early-onset scoliosis. The rapid growth seen in the goats between 6 weeks and 8 months parallels roughly the growth seen in children between early childhood and adolescence (maturity). The animal model supplied sufficient material to prove the efficacy of the system with regard to continued growth and minimal untoward effects.
The Shilla construct successfully allowed for vertebral column growth to occur in this caprine model at 6-month followup. Additionally, the facet joints that were instrumented but not fused survived with maintenance of motion and preservation of the articular surface.
Wear and histologic evaluations indicated expected results. Higher wear and wear particles were observed at the unconstrained junctions. Little to no wear was evident around the fused apex.
The results from this animal study help support the use of the Shilla system in humans by allowing for continued guided growth without the need for operative rod lengthening.
Acknowledgments
We thank Rex Armstrong and Tracy Wassell for their assistance.
Footnotes
One of the authors (REM) is a consultant for and has received funding from Medronic (Minneapolis, MN). One or more of the authors (JLT, MAWH) is employed by Medtronic.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akbarnia BA, Marks DS, Boachie-Adjei O, Thompson AG, Asher MA. Dual growing rod technique for the treatment of progressive early-onset scoliosis: a multicenter study. Spine. 2005;30(Suppl):s46–s57. doi: 10.1097/01.brs.0000175190.08134.73. [DOI] [PubMed] [Google Scholar]
- 2.Blakemore LC, Scoles PV, Poe-Kochert C, Thompson GH. Submuscular Isola rod with or without limited apical fusion in the management of severe spinal deformities in young children: preliminary report. Spine. 2001;26:2044–2048. doi: 10.1097/00007632-200109150-00021. [DOI] [PubMed] [Google Scholar]
- 3.Braun JT, Akyuz E, Ogilvie JW. The use of animal models in fusionless scoliosis investigations. Spine. 2005;30(Suppl):s35–s45. doi: 10.1097/01.brs.0000175187.61474.9a. [DOI] [PubMed] [Google Scholar]
- 4.Harrington PR. Treatment of scoliosis. Correction and internal fixation by spine instrumentation. J Bone Joint Surg Am. 1962;44:591–610. [PubMed] [Google Scholar]
- 5.Hell AK, Campbell RM, Hefti F. The vertical expandable prosthetic titanium rib implant for the treatment of thoracic insufficiency syndrome associated with congenital and neuromuscular scoliosis in young children. J Pediatr Orthop B. 2005;14:287–293. doi: 10.1097/01202412-200507000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Hell AK, Hefti F, Campbell RM., Jr Treatment of congenital scoliosis with the vertical expandable prosthetic titanium rib implant [in German] Orthopade. 2004;33:911–918. doi: 10.1007/s00132-004-0694-4. [DOI] [PubMed] [Google Scholar]
- 7.Luque ER. Paralytic scoliosis in growing children. Clin Orthop Relat Res. 1982;163:202–209. [PubMed] [Google Scholar]
- 8.Thompson GH, Akbarnia BA, Kostial P, Poe-Kochert C, Armstrong DG, Roh J, Lowe R, Asher MA, Marks DS. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine. 2005;30:2039–2044. doi: 10.1097/01.brs.0000179082.92712.89. [DOI] [PubMed] [Google Scholar]