Abstract
Little is known about tibial bone remodeling with TKA and its clinical relevance. We performed a randomized clinical trial to compare tibial bone density changes in cemented components with different bearing designs. Bone density changes were assessed using quantitative computed tomography (qCT)-assisted osteodensitometry. Twenty-eight rotating-platform and 26 fixed-platform cemented TKAs were included. The nonoperated contralateral side was used as a control. CT scans were performed postoperatively and 1 year and 2 years after the index operation. Cancellous bone density loss (up to 12.6% at 2 years) was observed in all proximal tibial regions in both cohorts. In contrast, we found lower cortical bone density loss (up to 3.6% at 2 years). We found no differences in bone loss between fixed- and rotating-platform implants. The decrease of cancellous bone density after TKA suggests stress transfer to the cortical bone.
Introduction
Quality of proximal tibial bone mineral density (BMD) is a factor that may determine migration of implants after TKA [6, 8, 13, 25]. Dual-energy xray absorptiometry (DEXA) studies suggest proximal tibial BMD declines after TKA [1, 5, 9, 12, 17, 19–21]. However, it is not clear whether there are differences in BMD changes between cortical and cancellous bone. In contrast to DEXA, qCT-assisted osteodensitometry offers three-dimensional, volumetric analysis of cortical and cancellous regions independently and is accurate and reliable about the hip [14, 15, 18] and knee [16].
Furthermore, there are little available data regarding whether different TKA implant designs create differences in BMD changes postoperatively. Conventional fixed bearings experience shear stress at the interface between the femoral component and the tibial insert. Compression, tension, shear, and axial torque forces are transmitted through the tibial baseplate to the proximal tibia. Rotating-platform bearings decouple multidirectional motion patterns at two interfaces, thus reducing maximum shear stress at each interface. This exposes the tibial baseplate to less axial torque and more evenly distributes compressive force, thus more closely reproducing normal knee kinematics [26]. Loading conditions after fixed platform TKA have been explored in finite element models, which show substantial BMD loss compared with the normal knee [2, 10].
We tested three hypotheses: (1) proximal tibial BMD after TKA would decrease; (2) cancellous bone would show a greater BMD loss than cortical bone; and (3) the rotating-platform TKA would have less BMD loss than a fixed-platform design.
Materials and Methods
We randomized 46 selected patients (54 knees) with degenerative knee disease undergoing TKA to receive either a PFC Sigma® fixed-platform (fixed-bearing) or a PFC Sigma® rotating-platform (mobile-bearing) total knee system (DePuy International, Leeds, UK). Knees for patients undergoing bilateral surgery were randomized independently. We excluded patients with severe deformity (requiring femoral or tibial augment), with inflammatory arthritis, who were younger than 45 years or older than 85 years, who refused consent, who had previous failed TKA or unicompartmental arthroplasty, who had previous high tibial osteotomy, and who had a TKA of the contralateral knee. With a two-sided 95% confidence interval, we rated a sample size of 20 knees in each cohort would have 80% power to detect a 5% difference between the two implants. At 2 years followup, complete data sets for 41 patients (48 knees; 25 rotating, 23 fixed) were available. Three patients died (three knees) and two moved overseas (three knees). Demographic data for the two groups were collected (Table 1). The study was approved by the local ethics committee. We obtained written informed consent from all patients.
Table 1.
Demographic data for patient groups
| Variable | Rotating platform | Fixed platform |
|---|---|---|
| Number of knees | 25 | 23 |
| Gender (male:female) | 15:10 | 12:11 |
| Mean age at index operation (years) | 67.2 (47–83) | 67.7 (50–79) |
| Level of activity | ||
| Sedentary | 0 | 0 |
| Semisedentary | 1 | 3 |
| Light labor | 15 | 13 |
| Moderate labor | 8 | 4 |
| Heavy labor | 0 | 1 |
The fixed platform is manufactured in titanium-aluminum-vanadium (Ti-Al-Va) alloy with a cruciform stem design. The rotating platform is manufactured in cobalt-chromium-molybdenum (Co-Cr-Mo) alloy and has a round stem. Both tibial options are designed to be used with the same Co-Cr-Mo femoral component. The fixed and rotating polyethylene inserts were GUR 1020 (Ticona LLC, Summit, NJ) ultrahigh-molecular-weight polyethylene sterilized by gamma irradiation in vacuum.
Randomization was performed by opening a sealed envelope containing the allocation derived from a computer-generated random number sequence. Surgery was performed at two institutions by one of two experienced arthroplasty surgeons (RPP, MC). A medial parapatellar approach was performed with an intramedullary guide used to align the femoral cutting block and an extramedullary guide used for the tibial block. A ligament balancing tool (DePuy) was used to create symmetric flexion and extension gaps. The tibial component was sized to provide maximum cortical contact without excessive overhang. Antibiotic-impregnated cement (Cemex® Genta; Tecres, Verona, Italy) was used to fix femoral and tibial components. The patella was resurfaced as the surgeon believed necessary. Physiotherapy was initiated on the first postoperative day with instructions to weightbear as tolerated on two crutches. Unrestricted flexion was initiated with return of active quadriceps contraction.
We followed patients clinically at 6 weeks, 1 year, and 2 years after the index operation. Clinical outcome was evaluated with the WOMAC, SF-12, Oxford Knee Score, Knee Society score, and visual analog pain scale. Evaluation was performed by research staff (TH) not involved in the surgery and blinded to the implant type. Weightbearing anteroposterior and lateral radiographs were taken at each followup and assessed by the consultant surgeons involved (RPP, MC). Lucency was defined as the appearance or progression of lucent areas greater than 2 mm in the zones described in the radiographic criteria of the Knee Society score [3]. Implant migration was defined by angulation of either component relative to the postoperative axis.
CT scans were performed during the first 5 days after surgery, before patient discharge, to establish baseline BMD. Followup CT scans were conducted at 1 and 2 years postsurgery. CT scanning was performed in a standard scanner (Siemens Somatom Plus; Siemens, Erlangen, Germany) with a standardized protocol (140 kV, 206 mAs, 150-mm × 150-mm field). The patients were positioned in a standardized foot holder to control for rotation. Coronal and sagittal scout views were performed to align the knee in the scanner. Sequential axial scans then were performed with 2-mm slice thickness starting 10 mm below the tip of the prosthesis and proceeding proximally in 5-mm increments (Fig. 1). A phantom with a hydroxyapatite (HA) core of known density (800 mg CaHA/mL) was scanned with each patient to allow conversion of radiographic density in Hounsfield units to BMD. Each scan was downloaded and analyzed using a unique software tool (CAPPA postOP; CAS Innovations, Erlangen, Germany). Slices were subdivided into four quadrants corresponding to anteromedial, anterolateral, posteromedial, and posterolateral. This was achieved by creating a line connecting the posterior border of the tibia to the posterior border of the fibula. A perpendicular line then was taken through the center of the implant to establish medial and lateral halves. A parallel line was taken again through the center of the implant to establish anterior and posterior halves. A final line perpendicular to this, again through the center of the implant, allowed division of the slice into anteromedial, anterolateral, posteromedial, and posterolateral quadrants (Fig. 2). Cortical and cancellous bone density regions were assessed applying a threshold of 800 HU to define the corticocancellous border [24]. All bone within the margins of the cortex was defined as cancellous bone. All bone outside the corticocancellous border was defined as cortical. Cement and the prosthesis in the cancellous region were excluded from analysis with a threshold greater than 1000 HU. All images were visually checked.
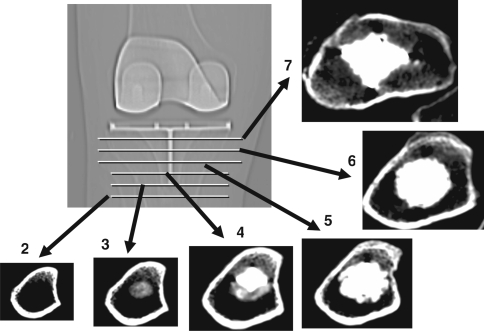
Fig. 1.
A CT scout image shows the levels (horizontal lines) used to establish slices for analysis. The first scan slice was aligned through the tip of the stem with slices taken proximally and distally at 5-mm increments. This image shows representative slices of cortical and cancellous bone along with the implant and cement in the proximal scans. Slices are numbered with 2 the most distal and 7 the most proximal. Slice 1, 5 mm below Slice 2, has been omitted in this diagram.
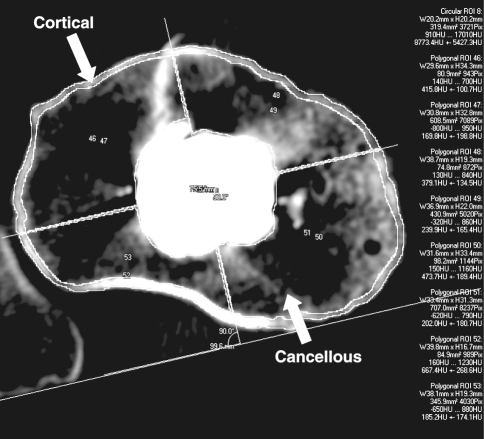
Fig. 2.
Analysis of an individual CT slice shows division of the slice into quadrants and separate analysis of cortical and cancellous structures excluding the stem and cement.
Owing to the dependent nature of the data, the hypotheses were tested using generalized least-squares models, each with an autoregressive AR(1) error structure in the individuals. In all cases, the estimated correlation parameter for the AR(1) error structure was significantly higher than 0 and thus necessary in the model. For Hypothesis 1, the model included bone type, scan position, and followup time as covariates. The response was BMD as a proportion of the BMD at baseline. The coefficient for the followup time covariate at 1 year and 2 years was used to estimate the change in BMD through time. For Hypothesis 2, the model included the same covariates, but the response was different in BMD by bone type at 1-year and 2-year followups as a proportion of the BMD at baseline. The coefficient for the bone type covariate was used to estimate the difference in change in BMD between cortical and cancellous bone. For Hypothesis 3, the model was the same as that fitted for Hypothesis 2, with the addition of a covariate for implant type. The coefficient for this additional covariate was used to estimate the difference in change in BMD between the two implant types. For all hypotheses, second-order interaction terms also were considered as a covariate, but their coefficients were not significant in any of the models. Analysis was performed using the nlme library inside the R statistical package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Overall there was evidence of BMD loss at 1 year (p < 0.001) and 2 years (p < 0.001) when compared with the baseline BMD. However, there was no evidence of a difference in BMD loss when comparing Year 1 with Year 2 (p = 0.117). Overall BMD loss was estimated to be between 5.2% and 9.5% (95% confidence interval [CI]) at 1 year and between 5.7% and 10.5% (95% CI) at 2 years when considered as a proportion of original baseline BMD.
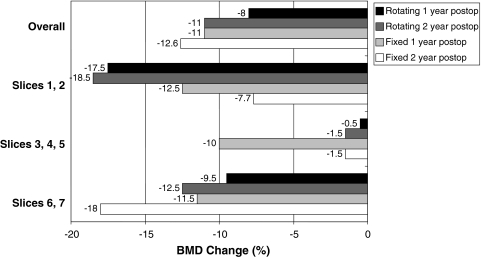
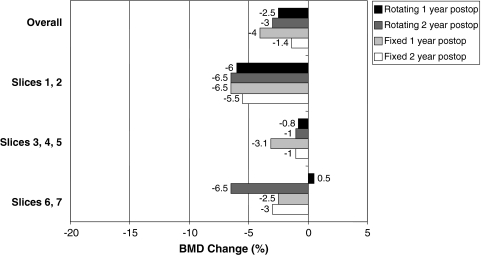
Loss of BMD was between 5.3% and 10.8% (95% CI) greater (p < 0.001) in cancellous bone than in cortical bone when considered as loss proportionate to baseline BMD. Cancellous BMD loss was estimated at 10.3% at 1 year and 12.6% at 2 years (Fig. 3). Cortical BMD loss was estimated at 3% at 1 year and 3.6% at 2 years (Fig. 4).
Fig. 3.
A graph shows cancellous BMD change for fixed and rotating platforms at 1 and 2 years postoperatively (postop), grouped for overall change (top) and by slice levels.
Fig. 4.
A graph shows cortical BMD change for fixed and rotating platforms at 1 and 2 years postoperatively (postop), grouped for overall change (top) and by slice levels.
We found no evidence of a difference in BMD loss between the two implant groups (p = 854). At 1-year and 2-year followups, we found no difference in clinical or radiographic outcome between the two implant groups (Table 2). There were no major complications, no revisions, and no loose implants according to the criteria of the Knee Society score [3] in either group.
Table 2.
Preoperative and postoperative (Year 2) clinical and radiographic scores
| Score | Rotating platform | Fixed platform | p Value |
|---|---|---|---|
| SF-12 Mental Component score | 0.30 | ||
| Preoperative | 52 (30–69) | 58 (35–71) | |
| Postoperative | 54 (35–67) | 57 (49–66) | |
| SF-12 Physical Component score | 0.41 | ||
| Preoperative | 28 (20–39) | 27 (19–56) | |
| Postoperative | 46 (25–59) | 44 (24–57) | |
| Knee Society score | 0.10 | ||
| Preoperative | 54 (19–94) | 58 (29–79) | |
| Postoperative | 88 (62–99) | 89 (79–100) | |
| Knee Society functional score | 0.48 | ||
| Preoperative | 51 (10–100) | 54 (10–100) | |
| Postoperative | 81 (45–100) | 79 (55–100) | |
| WOMAC total score | 0.40 | ||
| Preoperative | 55 (21–77) | 62 (27–84) | |
| Postoperative | 96 (73–100) | 97 (91–100) | |
| Range of motion (°) | 0.10 | ||
| Preoperative | 106 (65–145) | 114 (90–145) | |
| Postoperative | 114 (95–130) | 117 (100–135) | |
| Oxford Knee Score | 0.23 | ||
| Preoperative | 40 (28–49) | 35 (18–52) | |
| Postoperative | 18 (12–33) | 17 (12–25) | |
| Visual analog pain score | 0.77 | ||
| Preoperative | 5 (0–9) | 4 (1–8) | |
| Postoperative | 0 (0–3) | 0 (0–2) | |
| Progressive radiolucent zones tibia | |||
| Year 1 | 0 | 0 | |
| Year 2 | 0 | 0 | |
Values are reported as means, with ranges in parentheses.
We observed no BMD loss in the nonoperated knee for either cancellous bone (p = 0.385) or cortical bone (p = 0.765) for patients who had unilateral arthroplasty.
Discussion
The process of load transfer and bone remodeling in the proximal tibia after TKA is an area of ongoing research. Based on current literature, we expected to see a loss of proximal tibial BMD within 2 years after arthroplasty [1, 2, 7, 9, 11, 12, 21]. We also expected the implant to alter physiologic stress transfer at the plateau, specifically reducing cancellous BMD to a greater degree than cortical BMD by shielding the cancellous bone and transferring stress to the cortical bone. Finally, it has been suggested the tibiofemoral loading condition greatly contributes to stress shielding [2]. Thus, we have proposed the more physiologic stress transfer of the rotating-platform design would result in less BMD loss than the fixed-platform design.
Our study has some limitations. The implants involved have differences in design aside from the polyethylene insert, most notably the cruciform peg of the fixed platform and the round peg of the rotating platform. Lonner et al. [9] reported greater bone loss around long-stem tibial components compared with those with short pegs. To our knowledge, there is no published literature comparing stems of the same length but different geometry. There are also differences in metallurgy, the fixed platform being Ti-Al-Va alloy and the rotating platform being Co-Cr-Mo alloy. Abu-Rajab et al. [1] were unable to detect a difference in bone loss comparing cemented and uncemented tibial platforms, suggesting relatively similar modulus of the tray and its interface may not make a major difference to the degree of early remodeling. We did not subgroup our patients according to preoperative and postoperative leg alignment; accordingly, BMD changes observed at followup are not correlated to the frontal plane weightbearing settings. A finite element study of TKA predicted lowered strain beneath the tibial tray, which, applied to current knowledge of bone remodeling, predicts bone resorption [2]. Furthermore, according to the finite element model of Perillo-Marcone and Taylor [10], alterations in position of the implant into varus or valgus alignment may create higher strain in the medial or lateral regions, respectively. This suggests regional changes in BMD are affected by implant position and load transmission, so we assume a varus or valgus knee configuration would cause BMD changes independent of the implant design. To answer this question, we would require a markedly larger number of subjects. However, all TKA implants included in this study were inserted within a ± 3° frontal plane varus/valgus alignment. This factor should reduce the risk that our BMD findings are flawed by abnormal leg alignment. Proximal tibia bone remodeling may be dependent on implant size and positioning. The tibial tray was selected to contact as much cortical margin as possible. In situations in which the tray is entirely within the cortical margin, bone remodeling may be quite different.
Our data support a net loss of proximal tibial BMD after TKA. Several studies to date have used DEXA to show the reduction in BMD beneath implants [1, 7, 9, 11, 12, 21]. Li and Nilsson [7], in their series of 28 patients implanted with a flat-pegged tibial tray, reported an initial BMD loss of 13.1%, which recovered by 2 years. The recovery may reflect an increase in cortical BMD, secondary to stress transfer, masking cancellous BMD loss. Petersen et al. [11, 12] reported mean proximal tibial bone loss of 22% beneath a stemmed tibial tray at 3 years. Soininvaara et al. [21] reported BMD loss at 1 year in a stemmed tibial tray, ranging from 6.6% in the medial metaphysis to 4.7% in the diaphysis. Lateral metaphyseal bone was not affected in their cohort. Hvid et al. [4] adapted a qCT program for measuring BMD in the spine to measure cancellous BMD in the proximal tibia after TKA. They reported a mean decrease of cancellous BMD of 11% in patients with arthrosis and 32% in patients with rheumatoid arthritis across all levels at 2 years. They also reported a decrease in BMD with time in the preoperatively more loaded condyle, ie, varus knees lost BMD on the medial side after realignment during TKA. Although they presumed this to be secondary to correction of the mechanical alignment, a corresponding increase in BMD on the preoperatively less loaded condyle was not seen. The same effect occurred in valgus knees. Studies with controls [5, 6, 9, 20] have found no evidence of BMD change either in the contralateral knee or spine after TKA. This implies a local effect attributable to the implant and changes in mechanical alignment as opposed to a global BMD change. In our cohort, there was no change in the BMD of the nonoperated side.
We observed a substantially greater loss of cancellous BMD compared with cortical BMD. This is not readily apparent on radiography. Surgeons performing early revision surgery should be aware of this loss in terms of the tibial construct, notably in the availability of bone graft and in the use of cement and long stems to ensure early stability. Although the BMD decline in our patients has not resulted in clinical failure, longer-term remodeling is less clear. Some authors have reported stabilization of BMD by 2 years [7, 8], with Saari et al. [17] finding stable BMD loss out to 5 years. Others have reported ongoing loss at 3 years [4, 11, 12]. Although stress shielding after TKA contributes to early bone loss, the process of BMD decline is multifactorial [22, 23]. The progression to aseptic loosening is variable and it is not clear when and to what degree certain factors influence bone remodeling. Although polyethylene wear and osteolysis have been linked in the hip, the relationship is not as defined after TKA [23]. Although speculative, a lower initial BMD brought about by stress shielding may lead to earlier symptomatic loosening when particulate debris increases with time. This is important, as there is evidence that initial BMD may be maintained by intervention with bisphosphonates [27].
At 2 years, there was no difference in BMD change between the rotating- and fixed-platform knees. This would suggest the transmission of stress to the proximal tibia is the same between these implant designs in terms of resultant BMD remodeling. Longer-term followup is required to establish if this relationship persists. We do not know of another study comparing fixed and rotating platforms with regard to tibial bone remodeling. Our results suggest the stem on the tibial tray is important in terms of stress transfer. Other authors have examined changes in baseplate design and fixation. Lonner et al. [9] compared a flat tibial tray with four 0.5-cm pegs with a tray with a single 4-cm stem and reported a major difference in tibial BMD at 2 years. The stemmed design had bone loss of as much as 60% in some metaphyseal regions (average 40%) persisting at 2 years, whereas the pegged tray approximated the BMD of the nonoperated side. This supports the theory of stress transfer occurring through the stem to the more distal bone regions. It is not clear why there was such large bone loss in their study compared with others, although only 12 older female patients were involved [9]. Abu-Rajab et al. [1] examined BMD change between cemented and noncemented implants with stemmed tibial trays. They reported mean tibial BMD loss of as much as 10% at 2 years but found no difference between groups. Saari et al. [17] examined tibial BMD change in stemmed components with either posterior-stabilized or cruciate-retaining polyethylene inserts. Loading on the proximal tibia was theorized to differ between designs as a result of increased constraint in the posterior-stabilized knee. Although they also reported decreased BMD at 5 years varying from 5% to 23% depending on region of interest, they did not find a difference between the implant designs.
Our qCT data concur with the current literature in showing substantial tibial BMD loss after TKA. Furthermore, we found tibial cancellous BMD loss is more pronounced than cortical BMD loss, a phenomenon that may not be apparent on conventional radiographic imaging. We were unable to detect a difference in tibial BMD change between rotating and fixed TKA platforms.
Acknowledgments
We thank Dr. Lyndon Bradley, Dr. Anubav Mital, Dr. Shaneel Deo, Dr. Andrew Graydon, Dr. Keryn Reilly, and Therese Harper for assistance in clinical evaluation. We also thank Dr. Lutz Mueller, Dr. Rainer Schmidt, Dr. Alexander Kress, and Dr. Tobias Nowak for assistance in developing qCT osteodensitometry at the University of Auckland.
Footnotes
One or more of the authors (JTM, MC, RPP) have received funding from unrestricted educational grants from the Wishbone Trust New Zealand and the Stevensons Trust New Zealand. The study was partially funded by DePuy International, Leeds, UK.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at University of Auckland.
References
- 1.Abu-Rajab RB, Watson WS, Walker B, Roberts J, Gallacher SJ, Meek RM. Peri-prosthetic bone mineral density after total knee arthroplasty: cemented versus cementless fixation. J Bone Joint Surg Br. 2006;88:606–613. doi: 10.1302/0301-620X.88B5.16893. [DOI] [PubMed] [Google Scholar]
- 2.Au AG, James Raso V, Liggins AB, Amirfazli A. Contribution of loading conditions and material properties to stress shielding near the tibial component of total knee replacements. J Biomech. 2007;40:1410–1416. doi: 10.1016/j.jbiomech.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Ewald FC. The Knee Society total knee arthroplasty roentgenograhic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9–12. [PubMed] [Google Scholar]
- 4.Hvid I, Bentzen SM, Jorgensen J. Remodeling of the tibial plateau after knee replacement: CT bone densitometry. Acta Orthop Scand. 1988;59:567–573. doi: 10.3109/17453678809148787. [DOI] [PubMed] [Google Scholar]
- 5.Karbowski A, Schwitalle M, Eckardt A, Heine J. Periprosthetic bone remodelling after total knee arthroplasty: early assessment by dual energy x-ray absorptiometry. Arch Orthop Trauma Surg. 1999;119:324–326. doi: 10.1007/s004020050419. [DOI] [PubMed] [Google Scholar]
- 6.Li MG, Nilsson KG. The effect of the preoperative bone quality on the fixation of the tibial component in total knee arthroplasty. J Arthroplasty. 2000;15:744–753. doi: 10.1054/arth.2000.6617. [DOI] [PubMed] [Google Scholar]
- 7.Li MG, Nilsson KG. Changes in bone mineral density at the proximal tibia after total knee arthroplasty: a 2-year follow-up of 28 knees using dual energy x-ray absorptiometry. J Orthop Res. 2000;18:40–47. doi: 10.1002/jor.1100180107. [DOI] [PubMed] [Google Scholar]
- 8.Li MG, Nilsson KG. No relationship between postoperative changes in bone density at the proximal tibia and the migration of the tibial component 2 years after total knee arthroplasty. J Arthroplasty. 2001;16:893–900. doi: 10.1054/arth.2001.24376. [DOI] [PubMed] [Google Scholar]
- 9.Lonner JH, Klotz M, Levitz C, Lotke PA. Changes in bone density after cemented total knee arthroplasty: influence of stem design. J Arthroplasty. 2001;16:107–111. doi: 10.1054/arth.2001.16486. [DOI] [PubMed] [Google Scholar]
- 10.Perillo-Marcone A, Taylor M. Effect of varus/valgus malalignment on bone strains in the proximal tibia after TKR: an explicit finite element study. J Biomech Eng. 2007;129:1–11. doi: 10.1115/1.2401177. [DOI] [PubMed] [Google Scholar]
- 11.Petersen MM, Gehrchen PM, Ostgaard SE, Nielsen PK, Lund B. Effect of hydroxyapatite-coated tibial components on changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty: a prospective randomized study using dual-energy x-ray absorptiometry. J Arthroplasty. 2005;20:516–520. doi: 10.1016/j.arth.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Petersen MM, Nielsen PT, Lauritzen JB, Lund B. Changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty: a 3-year follow-up of 25 knees. Acta Orthop Scand. 1995;66:513–516. doi: 10.3109/17453679509002305. [DOI] [PubMed] [Google Scholar]
- 13.Petersen MM, Nielsen PT, Lebech A, Toksvig-Larsen S, Lund B. Preoperative bone mineral density of the proximal tibia and migration of the tibial component after uncemented total knee arthroplasty. J Arthroplasty. 1999;14:77–81. doi: 10.1016/S0883-5403(99)90206-1. [DOI] [PubMed] [Google Scholar]
- 14.Pitto RP, Bhargava A, Pandit S, Walker C, Munro JT. Quantitative CT-assisted osteodensitometry of femoral adaptive bone remodelling after uncemented total hip arthroplasty. Int Orthop. 2008;32:589–595. doi: 10.1007/s00264-007-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pitto RP, Mueller LA, Reilly K, Schmidt R, Munro J. Quantitative computer-assisted osteodensitometry in total hip arthroplasty. Int Orthop. 2007;31:431–438. doi: 10.1007/s00264-006-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly K, Munro J, Pandit S, Kress A, Walker C, Pitto RP. Inter-observer validation study of CT-osteodensitometry in total knee arthroplasty. Arch Orthop Trauma Surg. 2007;127:729–731. doi: 10.1007/s00402-007-0351-6. [DOI] [PubMed] [Google Scholar]
- 17.Saari T, Uvehammer J, Carlsson L, Regner L, Karrholm J. Joint area constraint had no influence on bone loss in proximal tibia 5 years after total knee replacement. J Orthop Res. 2007;25:798–803. doi: 10.1002/jor.20358. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt R, Pitto RP, Kress A, Ehremann C, Nowak TE, Reulbach U, Forst R, Muller L. Inter- and intraobserver assessment of periacetabular osteodensitometry after cemented and uncemented total hip arthroplasty using computed tomography. Arch Orthop Trauma Surg. 2005;125:291–297. doi: 10.1007/s00402-005-0812-8. [DOI] [PubMed] [Google Scholar]
- 19.Soininvaara T, Kroger H, Jurvelin JS, Miettinen H, Suomalainen O, Alhava E. Measurement of bone density around total knee arthroplasty using fan-beam dual energy x-ray absorptiometry. Calcif Tissue Int. 2000;67:267–272. doi: 10.1007/s002230001111. [DOI] [PubMed] [Google Scholar]
- 20.Soininvaara TA, Miettinen HJ, Jurvelin JS, Alhava EM, Kroger HP. Bone mineral density in the proximal femur and contralateral knee after total knee arthroplasty. J Clin Densitom. 2004;7:424–431. doi: 10.1385/JCD:7:4:424. [DOI] [PubMed] [Google Scholar]
- 21.Soininvaara TA, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM, Kroger HP. Periprosthetic tibial bone mineral density changes after total knee arthroplasty: one-year follow-up study of 69 patients. Acta Orthop Scand. 2004;75:600–605. doi: 10.1080/00016470410001493. [DOI] [PubMed] [Google Scholar]
- 22.Sumner DR, Galante JO. Determinants of stress shielding: design versus materials versus interface. Clin Orthop Relat Res. 1992;274:202–212. [PubMed] [Google Scholar]
- 23.Sundfeldt M, Carlsson LV, Johansson CB, Thomsen P, Gretzer C. Aseptic loosening, not only a question of wear: a review of different theories. Acta Orthop. 2006;77:177–197. doi: 10.1080/17453670610045902. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland CJ, Gayou DE. Artifacts and thresholding in X-ray CT of a cortical bone and titanium composite. J Comput Assist Tomogr. 1996;20:496–503. doi: 10.1097/00004728-199605000-00035. [DOI] [PubMed] [Google Scholar]
- 25.Therbo M, Petersen MM, Varmarken JE, Olsen CA, Lund B. Influence of pre-operative bone mineral content of the proximal tibia on revision rate after uncemented knee arthroplasty. J Bone Joint Surg Br. 2003;85:975–979. doi: 10.1302/0301-620X.85B7.13882. [DOI] [PubMed] [Google Scholar]
- 26.Walker PS. Biomechanics of total knee replacement designs. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-biology. 3. Philadelphia, PA: Lippincott, Williams and Wilkins; 2005. pp. 657–702. [Google Scholar]
- 27.Wang CJ, Wang JW, Ko JY, Weng LH, Huang CC. Three-year changes in bone mineral density around the knee after a six-month course of oral alendronate following total knee arthroplasty: a prospective randomized study. J Bone Joint Surg Am. 2006;88:267–272. doi: 10.2106/JBJS.E.00051. [DOI] [PubMed] [Google Scholar]