Abstract
Osteochondritis dissecans is a lesion of subchondral bone with subsequent involvement of the overlying cartilage. Although the etiology of the disease is unknown, mechanical, traumatic, and ischemic etiologies have been suggested, in addition to developmental and genetic factors. There are several treatment options depending on the stage of the disease and surgeon preference. The use of a fresh osteochondral allograft for treatment of a lesion of the femoral condyle is relatively new, and we report its use in a unique situation involving identical twins who both presented with osteochondritis dissecans of the same anatomic location within 2 years of each other. Since these were identical lesions in identical twins, this commonality supports the suggestion that some genetic component may be present in the etiology, especially in this situation where a genetic connection existed. We recommend genetic studies to determine the extent of genetic influence on the disease.
Introduction
Osteochondritis dissecans (OCD) is a lesion of subchondral bone with subsequent involvement of the overlying cartilage. The disease occurs at the physes of long bones, in the talus, and most commonly in the knee, particularly the medial femoral condyle [9, 12, 16]. Diagnosis is generally possible with conventional radiographs, but surgical treatment is best guided by proper staging via MRI [7]. Although the etiology of the disease is unknown, mechanical [18], traumatic [3], and ischemic [11] etiologies have been suggested, in addition to developmental and genetic factors [4, 9, 15]. Mechanical and trauma occur in areas that are easily damaged [3, 18]. Ischemia, on the other hand, has been suggested for areas that are not susceptible to injury [11]. Genetic and developmental factors are still relatively unstudied, but it has been reported as a cause by Woods and Harris [23] in identical twins and by Kenniston et al. [14] in fraternal twins. Our two cases add to the literature to help support the idea that there may be a genetic predisposition to OCD.
Case Report
We report identical twins who both presented with OCD of the left medial femoral condyles. The condition in both patients was treated with the use of a fresh allograft. Both twins agreed to be included in the study.
The first twin (hereafter referred to as T1) was an active and healthy 17-year-old boy who complained of left knee pain, swelling, and occasional locking. He reported experiencing these symptoms after sustaining a loading and twisting injury playing rugby. Upon further questioning, he admitted to intermittent discomfort in his left knee since middle school.
Radiographs and MRI were obtained for T1 when he presented to our clinic, which revealed an osteochondral defect of the medial femoral condyle and multiple loose bodies (Fig. 1). Arthroscopy was performed and the defect was 2.5 × 2.5 cm (Fig. 2). The loose bodies were fragmented and well worn and the surgeon believed they were not appropriate for reimplantation into the defect. Instead, because the defect appeared to be filled with fibrocartilage at the time of surgery, simple débridement and loose body removal were performed.
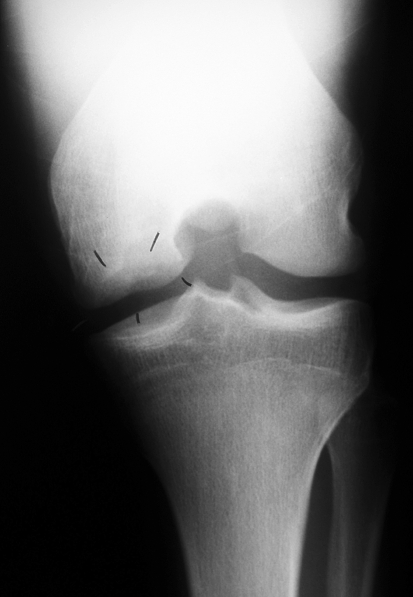
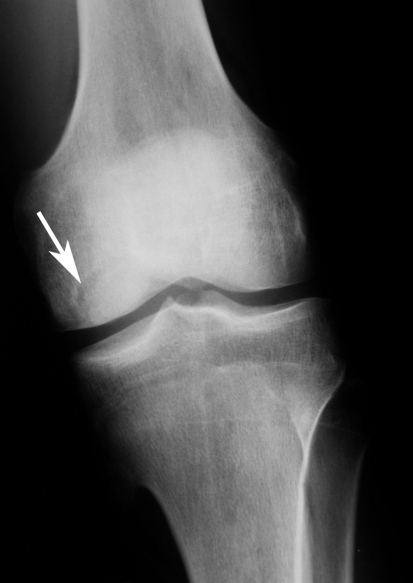
Fig. 1.
A radiograph shows an osteochondral lesion of the left knee in the first twin. The black marks indicate the area of the lesion.
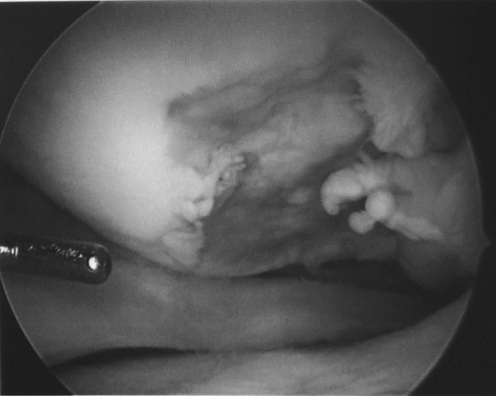
Fig. 2.
An arthroscopic view shows the lesion in the left knee of the first twin.
The patient’s postoperative recovery was routine, but he received little relief from the procedure and continued to have pain and swelling in the left knee. Three months later, T1 was again examined and judged a candidate for a fresh osteochondral condylar transplant and an appropriate graft was acquired (AlloSource, Centennial, CO). A medial patellar arthrotomy was performed and exposure was obtained under tourniquet. Sharp osteotomes were used to resect the necrotic bed around the area of the OCD and a small burr was used for fine débridement of the bed down to bleeding bone. Length, width, and depth measurements of the defect were made. Bone wax was then placed in the defect to produce a model for size and shape of the fresh osteoarticular allograft. The allograft obtained from Allosource was cut to a size slightly larger than needed with a sagittal saw from a contour-matched aspect of the allograft femur. A press fit of the allograft into the femoral defect was produced by trial and error with careful débridement. Range of motion was evaluated to test allograft stability. Allomatrix® (Wright Medical Technology, Arlington, TN) was used to grout the bony interface between allograft and native bone, and five Orthosorb® pins (Johnson & Johnson, New Brunswick, NJ) were placed in a divergent pattern for fixation. Nineteen months postimplantation, he had returned to playing basketball without pain, swelling, or a decrease in function.
Two years after his twin first presented, at the age of 19, Twin 2 (hereafter referred to as T2) also presented with left knee pain and swelling. Although he had no history of acute trauma, he reported symptoms for a duration of 1 month.
T2 had radiographs and MRI performed, which revealed a 2.8- × 2.1-cm osteochondral defect of the medial femoral condyle. Arthroscopic fixation was performed at another institution and his postoperative course was routine. Five months postoperatively, his pain, swelling, and locking returned. MRI showed the osteochondral fragment had become displaced (Fig. 3). Arthroscopy was performed at this time and a loose body was removed (Fig. 4). A large defect was discovered and débrided, although no microfracture or drilling was performed. At this point, he was referred to our clinic for consideration of a fresh osteochondral allograft, which was implanted in the same fashion as T1’s graft 6 months later.
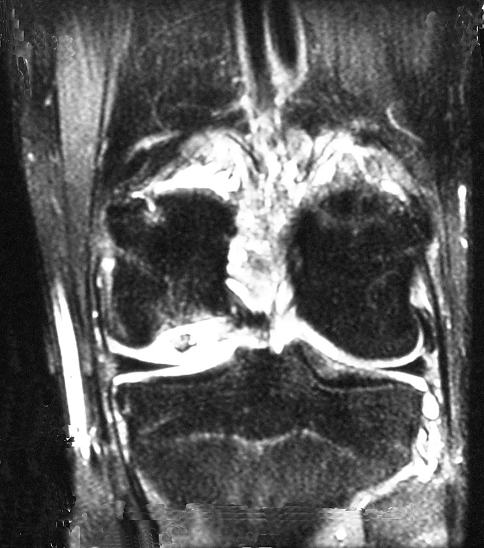
Fig. 3.
A MR image of the second twin’s knee shows the area of the lesion.
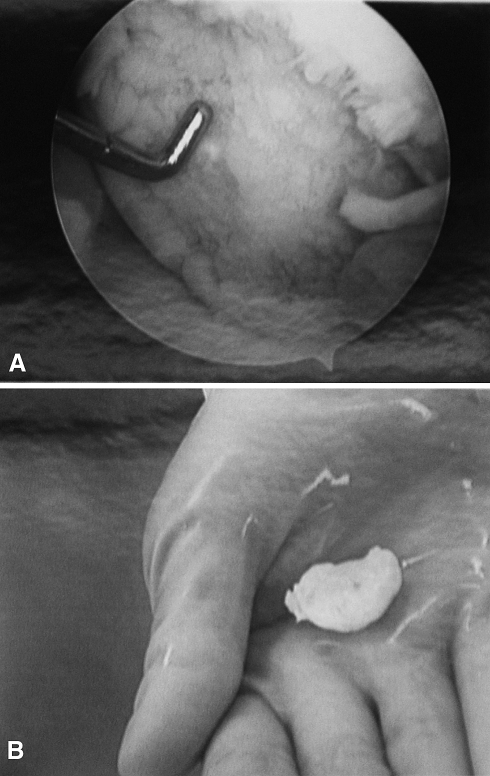
Fig. 4A–B.
(A) An arthroscopic view shows the lesion in the left knee of the second twin. (B) A photograph shows the removed loose body.
Both twins received left medial femoral condyle fresh osteochondral allografts for OCD. Standard surgical technique for a fresh osteochondral allograft and postoperative protocols were utilized identically in both cases.
Seven years after surgery, T1 reported a Musculoskeletal Tumor Society (MSTS) score [8] of 100%, with bony healing of the allograft to host bone visible on radiograph (Fig. 5). T2 also reported a MSTS score of 100% 5 years postoperatively and also showed radiographic incorporation of graft to host healing (Fig. 6).
Fig. 5.
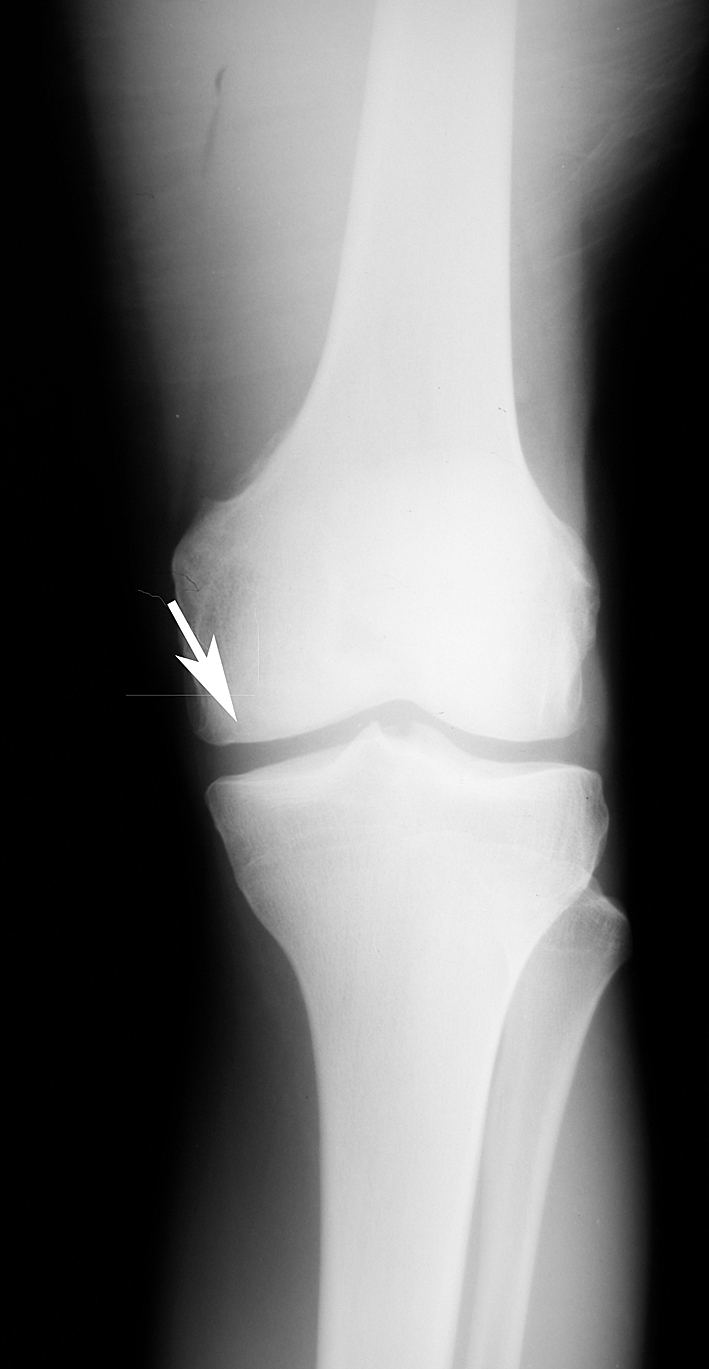
An arrow points to the area of healing of the allograft in the first twin 18 months after surgery.
Fig. 6.
An arrow points to the area of healing of the allograft in the second twin 5 months after surgery.
Discussion
OCD can be a debilitating problem leading to premature arthritis and substantial pain. Although the etiology is not entirely understood, genetic factors, along with mechanical, traumatic, and ischemic etiologies, have been suggested to play a role in the disease.
We encountered a unique situation with identical twins who both presented with OCD of the same anatomic location, thus suggesting a genetic influence in the etiology of OCD. The incidence of bilateral OCD of the femoral condyles has been reported at 30% [2]. In our situation, both twins presented with OCD of the left side and one twin presented with a bilateral occurrence. Our findings support those of Petrie [17], who also suggested genetics play a role in the formation of OCD. However, in that paper and our study, genetic causation could not be established. We suggest identical twins offer an excellent opportunity to examine the possibility of a genetic predisposition for OCD and more genetically related patients should be investigated to determine what the role of genetics is in OCD.
There are several treatment options depending on the stage of the disease and surgeon preference. These vary from nonsurgical treatments, such as protected weightbearing, to subchondral drilling of the defect, fragment fixation, fragment excision, arthroplasty, or the use of an osteochondral autograft or allograft [1, 5, 6, 13].
Osteochondral allografts have been transplanted since the early 1900s. Lexer [15] first described harvesting osteochondral grafts with immediate implantation. A study by Williams et al. [22] suggested the optimal time of implant was between 17 and 28 days after harvest if the grafts are in a cell culture medium or simulated environment. This time period allows for examination of bacterial cultures and serum immunoassays to reduce the risk of transmission of disease while keeping the allograft chondrocytes viable. The use of a fresh osteochondral allograft for treating a lesion of the femoral condyle has been previously reported [21] although we describe its use in a unique situation involving identical twins with the same lesion in the same location. Unlike frozen allografts, the fresh allografts in this study had never been frozen and were obtained from the donor and implanted within 14 to 28 days. The use of a fresh allograft for the procedure is relatively new and is recommended as chondrocyte viability is higher (95%–99%) than the use of a frozen graft (50%–70%) in this procedure [10, 22]; the higher chondrocyte viability helps with healing of the defect and merging with the host bone [5, 10, 22]. The technique is limited in that the time required to find a matching allograft can be several months, but allograft transplantation is a one-stage procedure, unlike autologous chondrocyte implantation, and fresh allografts have been used with great success by the surgeons at our clinic [19, 20]. A similar procedure, osteochondral autograft transplantation, uses bone from the contralateral knee or a nonweightbearing area of the same knee, but the amount of material that can be used is restricted and chondrocyte viability is limited compared to the method used in this procedure [10]. We describe an unusual situation of OCD in twins and present a possible surgical method for treating the condition.
Footnotes
Dr. Wilkins is the medical director for Allosource, Centennial, CO, a company from which the allografts were obtained. This author certifies that he has or may receive payments or benefits from a commercial entity related to this work.
Each author certifies that his or her institution approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that both patients were informed the case report is being published.
References
- 1.Bakay A, Csonge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft: clinical analysis of 33 cases. Int Orthop. 1998;22:277–281. doi: 10.1007/s002640050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bednarz PA, Paletta GA, Stanitski CL. Bilateral osteochondritis dissecans of the knee and elbow. Orthopedics. 1998;21:716–719. doi: 10.3928/0147-7447-19980601-17. [DOI] [PubMed] [Google Scholar]
- 3.Bradley J, Dandy DJ. Osteochondritis dissecans and other lesions of the femoral condyles. J Bone Joint Surg Br. 1989;71:518–522. doi: 10.1302/0301-620X.71B3.2722949. [DOI] [PubMed] [Google Scholar]
- 4.Bramer JA, Maas M, Dallinga RJ, te Slaa RL, Vergroesen DA. Increased external fixation torsion and osteochondritis dissecans of the knee. Clin Orthop Relat Res. 2004;442:187–189. doi: 10.1097/01.blo.0000126310.02631.f2. [DOI] [PubMed] [Google Scholar]
- 5.Bugbee WD. Fresh osteochondral allografts. J Knee Surg. 2002;15:191–195. [PubMed] [Google Scholar]
- 6.Cepero S, Ullot R, Sastre S. Osteochondritis of the femoral condyles in children and adolescents: our experience over the last 28 years. J Pediatr Orthop B. 2005;14:24–29. doi: 10.1097/01202412-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 7.DiPaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101–104. doi: 10.1016/0749-8063(91)90087-e. [DOI] [PubMed] [Google Scholar]
- 8.Enneking W. Modification of the system for functional evaluation in the surgical management of musculoskeletal tumors. In: Enneking W, editor. Limb Salvage in Musculoskeletal Oncology. New York, NY: Churchill Livingstone; 1987. pp. 626–639. [Google Scholar]
- 9.Federico DJ, Lynch JK, Jokl P. Osteochondritis dissecans of the knee: a historical review of etiology and treatment. Arthroscopy. 1990;6:190–197. doi: 10.1016/0749-8063(90)90074-n. [DOI] [PubMed] [Google Scholar]
- 10.Gole MD, Poulsen D, Marzo JM, Ko SH, Ziv I. Chondrocyte viability in press-fit cryopreserved osteochondral allografts. J Orthop Res. 2004;22:781–787. doi: 10.1016/j.orthres.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg Am. 1984;66:1340–1348. [PubMed] [Google Scholar]
- 12.Jaberi FM. Osteochondritis dissecans of the weight-bearing surface of the medial femoral condyle in adults. Knee. 2002;9:201–207. doi: 10.1016/S0968-0160(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 13.Karataglis D, Green MA, Learmonth DJ. Autologous osteochondral transplantation for the treatment of chondral defects of the knee. Knee. 2005;13:32–35. doi: 10.1016/j.knee.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Kenniston JA, Beredjiklian PK, Bozentka DJ. Osteochondritis dissecans of the capitellum in fraternal twins: case report. J Hand Surg Am. 2008;33:1380–1383. doi: 10.1016/j.jhsa.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Lexer E. Substitution of whole or half joints from freshly amputated extremities by free-plastic operation. Surg Gynecol Obstet. 1908;6:601–607. [Google Scholar]
- 16.Obedian RS, Grelsamer RP. Osteochondritis dissecans of the distal femur and patella. Clin Sports Med. 1997;16:157–174. doi: 10.1016/S0278-5919(05)70012-0. [DOI] [PubMed] [Google Scholar]
- 17.Petrie PW. Aetiology of osteochondritis dissecans: failure to establish a familial background. J Bone Joint Surg. 1977;59:366–367. doi: 10.1302/0301-620X.59B3.893517. [DOI] [PubMed] [Google Scholar]
- 18.Pill SG, Ganley TJ, Flynn JM, Milam RA, King PJ, Gregg JR. Osteochondritis dissecans of the knee: experiences at The Children’s Hospital of Philadelphia and a review of literature. UPOJ. 2001;14:25–33. [Google Scholar]
- 19.Wilkins RM. Complications of allograft reconstruction. In: Simon MA, Springfield D, editors. Surgery for Bone and Soft-Tissue Tumors. Philadelphia, PA: Lippincott-Raven; 1998. pp. 487–496. [Google Scholar]
- 20.Wilkins RM, Kelly CM. Revision of the failed distal femoral replacement to allograft prosthetic composite. Clin Orthop Relat Res. 2002;397:114–118. doi: 10.1097/00003086-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Williams RJ, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- 22.Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, Sah RL, Bugbee WD. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Woods K, Harris I. Osteochondritis dissecans of the talus in identical twins. J Bone Joint Surg Br. 1995;77:331. [PubMed] [Google Scholar]