Abstract
Fragility vertebral fractures often are associated with chronic back pain controlled by analgesic compounds. Capacitive coupling electrical stimulation is a type of electrical stimulation technology approved by the US FDA to noninvasively enhance fracture repair and spinal fusion. These uses suggest it would be a possible treatment for patients with back pain attributable to vertebral fractures. We therefore randomized 51 postmenopausal women with multiple fractures and chronic pain to the use of one of two indistinguishable devices delivering either the standard capacitive coupling electrical stimulation by Osteospine™ (active group) or low intensity pulse (control group). Twenty patients of the active group and 21 of the control group (80%) completed the study for a total duration of 3 months. The mean visual analog scale values for pain and the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO) scores improved in both groups. We observed a relationship between hours of treatments and reductions in pain intensity only in the active group. Capacitive coupling electrical stimulation was not more effective than control treatment when comparing mean visual analog scale pain and QALEFFO scores in the two groups and when adjusting for the hours of treatment. However, the proportion of patients able to discontinue NSAIDs owing to elimination or reduction of pain was greater in the active group than in the control group. We interpret these findings as suggesting capacitive coupling electrical stimulation controls pain in some patients and reduces the use of NSAIDs.
Level of Evidence: Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Vertebral fractures are the most common osteoporotic fracture, affecting 25% of the elderly female population [18]. The acute pain after a new vertebral fracture traditionally is managed with rest and analgesic therapies. Prevalent vertebral deformities also increase the risk of severe chronic pain [12]. This type of pain mainly is attributable to mechanical displacement of the spine with resulting kyphosis, muscle spasm, and arthritic changes in the vertebral joints [20].
The risk of chronic pain increases with the number and severity of vertebral fractures, and it is associated with decreased physical functioning, social isolation, depression, diminished quality of life, and permanent disability [6, 16]. Functional activities are more limited when back pain symptoms (pain, limitation of movement) and deformity are present than in the sole presence of deformity [5]. In addition, the reduced mobility associated with chronic pain favors the progression of osteoporosis. Therefore it is important to ensure adequate pain control. Pain often is controlled by the continuous use of analgesic agents, which can lead to serious side effects [7]. Physical therapies such as heat, cold, ultrasound, electrical nerve stimulation, and massage therapy have been described for treatment of chronic back pain [8, 10] with substantial pain relief and improved range of motion (ROM). Electrical nerve stimulation includes transcutaneous electrical nerve stimulation [15, 19], interferential therapy [13, 14], or percutaneous electrical nerve stimulation [22]. The efficacy of all these treatments remains controversial as few studies had an acceptable methodologic design [10, 22], even if some studies indicated substantial reduction in pain intensity and pain-related disability in patients after percutaneous electrical stimulation compared with a sham group.
In two studies, a decrease in visual analog scale (VAS) pain score and an increase in Backill disability score using a new type of electric stimulation, the interferential and horizontal therapy, were reported [23, 24]. However, this therapy can be administered only in specialized centers owing to lack of available simple and portable technology and trained staff, which makes it unsuitable or inconvenient for many patients unable to attend frequent outpatient sessions.
Capacitively coupled electrical field (CCEF) stimulation is an electrical stimulation technology with the potential for increasing new bone formation by upregulating osteoblast function [3, 4, 17, 25] and can be used on an outpatient basis. CCEF has been used to prevent castration-induced osteoporosis in rat vertebrae [2]. In animal studies, it reportedly promotes fracture healing [1] and repair of nonunions [3, 21]. In patients surgically treated for degenerative disc disease, it apparently enhances spinal fusion and reduces back pain [9, 11]. It is approved by the US FDA to noninvasively enhance fracture repair and spinal fusion. These results might suggest a potential for use of CCEF stimulation to promote bone formation and decrease back pain in patients with vertebral fractures.
We therefore asked whether using CCEF stimulation in patients with previous multiple vertebral osteoporotic fractures would (1) reduce chronic back pain and enhance quality of life scores compared with control treatment, (2) reduce the need of NSAIDs to control pain, and (3) be usable at home, with adequate patient compliance.
Patients and Methods
We identified 65 postmenopausal women older than 60 years with radiographically documented multiple vertebral fractures at the thoracolumbar level (T10–L2), chronic pain for at least 6 months, and at least two weekly doses of NSAIDs to control pain. Patients with low back pain (below L3), which more likely is related to degenerative disc disease, were excluded. Patients with any malignancy, rheumatoid arthritis or spondyloarthritis (inflammatory rheumatic diseases that can affect the spine and joints, ligaments, and tendons), renal or dermatologic diseases, or previous surgery at the lumbar spine were not included in the study. The study design involved an active and a control group. Of the 65 patients matching the inclusion criteria, 51 agreed to participate in the study and were assigned randomly to treatment with either active CCEF stimulation (active group) or low stimulation (control group). For randomization of patients, a computer-generated schedule was prepared by a biostatistician. In this process, a random number seed was entered in the computer to generate a list that assigned equal numbers of devices. The number of patients for each study group (active and control) was established on the basis of a power analysis: Goodwin et al. [11] reported electrical stimulation therapies showed 80% to 90% success (the study protocol defined success as a clinical outcome rated as excellent or good and a fusion documented as solid by the investigator and the blinded independent radiologist) compared with 20% to 40% for control subjects. All patients enrolled in the study used NSAIDs for pain relief at the beginning of the study. We assumed CCEF stimulation had a successful outcome when the patient discontinued NSAID use. We assumed a 30% to 35% greater percentage of patients discontinuing NSAID use during the study compared with the control group. The power analysis indicated 20 to 21 subjects per group were needed to detect a significant difference between the active and control groups, with a two-tailed significance at 5% and a power of 80%. We then assumed a patient dropout rate of 20% during followup and concluded the minimum number of subjects per group needed was 23. The patients used one of two indistinguishable electrical stimulation devices. The active group received a 7-V peak to peak sine wave electric field. The control group received a 0.1-V peak to peak sine wave, the lowest electric field value required by the generator to detect the actual contact of the electrodes with the skin and the cable connection. This feature allowed us to record the hours of treatment and monitor patient compliance. The study protocol was approved by the local (ASL 22, Regione Veneto, Ospedale di Bussolengo) Ethical Committee. Informed consent was obtained from all patients before any enrollment procedures.
Of the 51 patients enrolled in the study, five patients in the control group interrupted treatment before the first followup: three for cutaneous reactions and two because the device limited their daily activities. Two patients in the active group discontinued the treatment immediately because they found electrode positioning difficult and two had cutaneous reactions; one patient was lost to followup. The remaining patients (20 in the active group and 21 in the control group) completed all followups. The two groups were comparable for baseline characteristics (Table 1).
Table 1.
Baseline characteristics of the two study groups
| Characteristic | Active group (n = 20) | Control group (n = 21) | p Value (t test) | CI for the difference | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 73.8 | 7.4 | 71.7 | 7.2 | 0.45 | −7.76/+3.51 |
| Weight (kg) | 63.6 | 8.8 | 64.8 | 12 | 0.73 | −5.95/+8.49 |
| Height (cm) | 154.8 | 7.7 | 155.7 | 7.8 | 0.72 | −4.39/+6.28 |
| VAS pain score (0–10) | 10.7 | 5.4 | 10.8 | 5.8 | 0.93 | −3.45/+3.76 |
| QUALEFFO (total score) | 44.9 | 14.5 | 44.2 | 14.6 | 0.88 | −9.88/+8.55 |
| QUALEFFO (pain score) | 3.3 | 0.6 | 3.4 | 0.7 | 0.60 | −0.32/+0.55 |
| Hours of treatment | 514 | 184 | 564 | 174 | 0.38 | −63.25/+162.73 |
CI = confidence interval; VAS = visual analog scale; QUALEFFO = Quality of Life Questionnaire of the European Foundation for Osteoporosis.
The device used for CCEF stimulation was the Osteospine™ (IGEA srl, Carpi, Italy), which includes a current generator able to provide a density current of 25 μA/cm2 in the region of interest. The standard electric signal used by Osteospine™ for electrical stimulation consists of electrical pulses of 12.5 Hz with a duty cycle of 50%. The active part of the electric pulse consists of a sinusoidal wave of 60 kHz. The 10 × 8 × 2 cm stimulator weighs 140 g and uses a 9 V rechargeable battery lasting 24 hours. The 10 × 5 cm electrode pads are made of a layer of highly conductive material covered with adhesive biocompatible electroconductor gel. To deliver CCEF therapy, two electrode pads are placed in contact with the skin paraspinally at the level of maximum pain. The patients were asked to wear the device for a minimum of 10 hours per day for 2 months. Per protocol, treatment outcomes were adjusted to hours of treatment.
At the time of the recruitment visit, the following general data were recorded: age, height, weight, and clinical and pharmacologic history. The patients’ physical condition also was evaluated. Pain intensity was determined by a VAS on a scale of 0 to 10 (worst pain). Quality of life was determined using the QUALEFFO [16] with the two domains: total score and pain score. Forty-five of the 51 patients (89%) were taking either diclofenac or nimesulide and the remaining patients were taking other NSAIDs. These assessments were made at baseline; after 2, 4, and 8 weeks of treatment; and 4 weeks after the end of the treatment (Week 12). The patients were asked to record on a weekly basis the use of NSAIDs, including the name of the drug, to control pain. Use of NSAIDs was kept free among patients: the criterion for NSAID reduction was no pain or tolerable pain. Clinical information was collected by one of the authors (OV) blinded to type and duration of exposure to the intervention, which was disclosed only after completion of the study.
Means and standard deviations were obtained for continuous variables (VAS and QUALEFFO at baseline and at each followup, percentage of patients using NSAIDs during each week of the followup). We used paired Student’s t test to compare within the same group at followup versus baseline and unpaired Student’s t test for comparisons between the two groups after ANOVA. Data analyses were adjusted for exposure to treatment by covariance analysis. We explored the association between duration of treatment and VAS and QUALEFFO by linear regression analysis and Spearman correlation coefficient. Comparisons between groups for the use of NSAIDs were performed using a contingency table and the chi square test with a one-tailed level of significance. Statistical analyses were performed using SPSS® 13.0 software (SPSS Inc, Chicago, IL).
Results
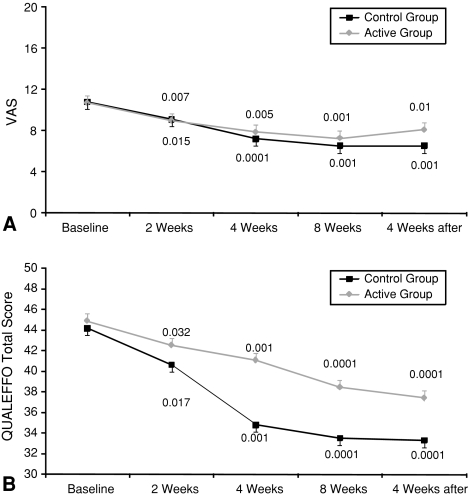
We observed an improvement (decrease) in mean VAS pain scores and QUALEFFO scores in both groups by the first followup (Fig. 1). The improvements were similar when data were adjusted for the hours of treatment.
Fig. 1A–B.
The mean (A) VAS values for pain and (B) QUALEFFO scores at baseline and at all followups indicate pain reduction and QUALEFFO changes in both groups. P values are reported for differences between each followup versus baseline (paired t test). The error bars are represented only in one direction for clarity of representation. The reductions in VAS and QUALEFFO were similar in the two groups.
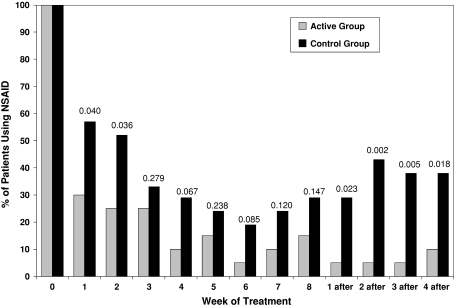
The percentage of patients who required the use of NSAIDs declined in both groups (Fig. 2). However, by the end of treatment, the number of patients taking NSAIDs was less (p < 0.001) in the active group (two to three of 20) than in the control group (five to six of 21) and remained less (p < 0.001) in the active group (two of 20) than in the control group (eight of 21) at the last posttreatment followup.
Fig. 2.
A graph shows the percentage of patients continuing the use of NSAIDs to control pain during followup and after completion of treatment in the two groups. P values are reported for differences between groups at each time (chi square test).
Most patients could perform the treatment at home as instructed although there was a 20% dropout. Patient compliance was good and similar (p = 0.43) in both groups: 9.2 ± 3.3 hours per day in the active group and 10.0 ± 3.1 hours per day in the control group. We observed a correlation between treatment time and VAS and QUALEFFO scores only in the active group at most times (Table 2).
Table 2.
Correlation coefficients between global number of hours of treatment and changes from baseline
| Followup | VAS | QUALEFFO | ||||||
|---|---|---|---|---|---|---|---|---|
| Active group | p Value | Control group | p Value | Active group | p Value | Control group | p Value | |
| 2 weeks of treatment | −0.46 | 0.024 | −0.36 | 0.109 | −0.44 | 0.028 | −0.33 | 0.070 |
| 4 weeks of treatment | −0.47 | 0.021 | −0.31 | 0.172 | −0.43 | 0.028 | −0.30 | 0.142 |
| 8 weeks of treatment | −0.48 | 0.018 | −0.31 | 0.166 | −0.34 | 0.080 | −0.30 | 0.142 |
| 4 weeks after treatment termination | −0.34 | 0.080 | −0.31 | 0.164 | −0.27 | 0.123 | −0.38 | 0.041 |
VAS = visual analog scale; QUALEFFO = Quality of Life Questionnaire of the European Foundation for Osteoporosis.
Discussion
Chronic low back pain is a severe complication of multiple vertebral osteoporotic fractures that limits a patient’s mobility, reduces the quality of life, and requires prolonged use of NSAIDs with associated negative side effects. Attempts have been made to control pain in these patients by means of locally delivered physical treatments. CCEF stimulation has proven effective in favoring the healing of spinal fusions, further showing a positive effect on pain that was substantially less in actively treated patients. Most important, no negative side effects have been associated with CCEF stimulation even when used for long periods, which could make the treatment feasible for management of chronic diseases. We hypothesized CCEF could be used efficaciously to alleviate spine pain and improve quality of life in patients with osteoporosis who have multiple vertebral deformities and severe chronic back pain. We designed a prospective, randomized, placebo-controlled, double-blind study to investigate the effect of CCEF stimulation on pain, quality of life, use of NSAIDs, and treatment feasibility.
Our study has several limitations. First, although CCEF stimulation has been used to prevent castration-induced osteoporosis in rats [3], in this study, we did not investigate the effect of CCEF stimulation on bone mineral density for the following reasons: (1) the presence of fractures creates artifacts in DEXA measurement; and (2) to observe evidence of bone mineral density changes would require a larger cohort of patients and a longer treatment and followup than was available for our patients. Second, our study was limited to 3 months’ followup, and we cannot estimate how long the effect of CCEF stimulation may last and if and to what extent repeated cycles of stimulation could improve or maintain the clinical results we described. Finally, the prior power analysis provided us with an indicative number of patients required to obtain significant results, however, a significant difference is not necessarily equivalent to a clinically important difference, but it can be considered a starting point.
If we do not take into account the discontinuation of NSAIDs among patients (more in the CCEF stimulation group), we found CCEF stimulation no more effective than control treatment when comparing mean VAS and QUALEFFO scores in the two groups during the study. In the active and control groups, pain and QUALEFFO scores decreased progressively and the effect persisted after discontinuation of treatment (Fig. 1). These results likely are explained by an important placebo effect characterizing any type of physical therapy [24]. However, the changes for VAS and QUALEFFO scores were correlated with duration of exposure in the active group but not in the control group. CCEF currents are not perceived by the patients and this excludes any additional placebo effect of the CCEF stimulation.
Among actively treated patients, a larger proportion of patients could discontinue NSAID use for controlling pain as compared with the control group, especially at the followup after the end of treatment. The proportion of patients using NSAIDs to control pain decreased markedly in both groups within the first week of treatment. However, although in the control group, the proportion of patients taking NSAIDs tended to return to initial values within a few weeks, most patients in the active group were not taking rescue NSAIDs even after discontinuation of the CCEF stimulation. These results apparently are inconsistent only with the results observed for pain and QUALEFFO scores: in the control group, NSAID use was required to obtain the same improvements observed in the active group. This, together with the correlation between duration of active CCEF stimulation and reported symptomatic improvements, supports the superiority of CCEF stimulation as compared with control treatment.
The mechanism whereby CCEF stimulation reduced the use of NSAIDs, presumably as a result of elimination or reduction of pain remains unclear. In two randomized, double-blind, placebo-controlled studies, it was observed two sorts of electrical nerve stimulation (interferential therapy and horizontal therapy) may alleviate pain and disability in patients with chronic back pain attributable to multiple vertebral deformities [23, 24]. However, CCEF stimulation is not expected to have any effect on peripheral nerves or local pain perception.
Three types of electrical stimulation devices have received US FDA approval for treating spinal fusions: direct current electrical stimulation, inductive coupling such as pulsed electromagnetic fields and combined magnetic fields, and CCEF stimulation. Preclinical investigations [4, 17, 25] suggest CCEF stimulation interacts with the calcium signal transduction pathway leading to TGF-β1 synthesis increase via the calcium-calmodulin pathway and to downregulation of proinflammatory cytokines (IL-1β). We hypothesize the symptomatic improvements observed in our patients may stem from an antiinflammatory effect mediated by proinflammatory cytokine downregulation.
Additional studies are needed to investigate if and to what extent the treatment regimen, daily hours, number of days, and times per year can be improved. Nevertheless, we believe our data are important because they show it is possible to control pain in some patients, reducing the use of analgesics and their side effects.
Footnotes
Francesca de Terlizzi is an IGEA employee. This author certifies that she has or may receive payments or benefits from a commercial entity related to this work.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at University of Verona.
References
- 1.Brighton CT, Hozack WJ, Brager MD, Windsor RE, Pollack SR, Vreslovic EJ, Kotwick JE. Fracture healing in the rabbit fibula when subjected to various capacitively coupled electrical fields. J Orthop Res. 1985;3:331–340. doi: 10.1002/jor.1100030310. [DOI] [PubMed] [Google Scholar]
- 2.Brighton CT, Luessenhop CP, Pollack SR, Steinberg DR, Petrik ME, Kaplan FS. Treatment of castration-induced osteoporosis by a capacitively coupled electrical signal in rat vertebrae. J Bone Joint Surg Am. 1989;71:228–236. [PubMed] [Google Scholar]
- 3.Brighton CT, Pollack SR. Treatment of recalcitrant non-union with a capacitively coupled electrical field: a preliminary report. J Bone Joint Surg Am. 1985;67:577–585. [PubMed] [Google Scholar]
- 4.Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR. Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am. 2001;83:1514–1523. doi: 10.2106/00004623-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Edmond SL, Kiel DP, Samelson EJ, Kelly-Hayes M, Felson DT. Vertebral deformity, back symptoms, and functional limitations among older women: the Framingham Study. Osteoporos Int. 2005;16:1086–1095. doi: 10.1007/s00198-004-1815-y. [DOI] [PubMed] [Google Scholar]
- 6.Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int. 2008;19:895–903. doi: 10.1007/s00198-007-0530-x. [DOI] [PubMed] [Google Scholar]
- 7.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 8.Gadsby JG, Flowerdew MW. Transcutaneous electrical nerve stimulation and acupuncture-like transcutaneous electrical nerve stimulation for chronic low back pain. Cochrane Database Syst Rev. 2000;2:CD000210. doi: 10.1002/14651858.CD000210. [DOI] [PubMed] [Google Scholar]
- 9.Gan JC, Glazer PA. Electrical stimulation therapies for spinal fusions: current concepts. Eur Spine J. 2006;15:1301–1311. doi: 10.1007/s00586-006-0087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoname EA, Craig WF, White PF, Ahmed HE, Hamza MA, Henderson BN, Gajraj NM, Huber PJ, Gatchel RJ. Percutaneous electrical nerve stimulation for low back pain: a randomized crossover study. JAMA. 1999;281:818–823. doi: 10.1001/jama.281.9.818. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA. A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine (Phila Pa 1976) 1999;24:1349–1356. doi: 10.1097/00007632-199907010-00013. [DOI] [PubMed] [Google Scholar]
- 12.Huang C, Ross PD, Wasnich RD. Vertebral fracture and other predictors of physical impairment and health care utilization. Arch Intern Med. 1996;156:2469–2475. doi: 10.1001/archinte.156.21.2469. [DOI] [PubMed] [Google Scholar]
- 13.Hurley DA, Minder PM, McDonough SM, Walsh DM, Moore AP, Baxter DG. Interferential therapy electrode placement technique in acute low back pain: a preliminary investigation. Arch Phys Med Rehabil. 2001;82:485–493. doi: 10.1053/apmr.2001.21934. [DOI] [PubMed] [Google Scholar]
- 14.Johnson MI, Tabasam G. A single-blind placebo-controlled investigation into the analgesic effects of interferential currents on experimentally induced ischaemic pain in healthy subjects. Clin Physiol Funct Imaging. 2002;22:187–196. doi: 10.1046/j.1475-097X.2002.00416.x. [DOI] [PubMed] [Google Scholar]
- 15.Köke AJ, Schouten JS, Lamerichs-Geelen MJ, Lipsch JS, Waltje EM, Kleef M, Patijn J. Pain reducing effect of three types of transcutaneous electrical nerve stimulation in patients with chronic pain: a randomized crossover trial. Pain. 2004;108:36–42. doi: 10.1016/j.pain.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Lips P, Cooper C, Agnusdei D, Caulin F, Egger P, Johnell O, Kanis JA, Kellingray S, Leplege A, Liberman UA, McCloskey E, Minne H, Reeve J, Reginster JY, Scholz M, Todd C, Vernejoul MC, Wiklund I. Quality of life in patients with vertebral fractures: validation of the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO). Working Party for Quality of Life of the European Foundation for Osteoporosis. Osteoporos Int. 1999;10:150–160. doi: 10.1007/s001980050210. [DOI] [PubMed] [Google Scholar]
- 17.Lorich DG, Brighton CT, Gupta R, Corsetti JR, Levine SE, Gelb ID, Seldes R, Pollack SR. Biochemical pathway mediating the response of bone cells to capacitive coupling. Clin Orthop Relat Res. 1998;350:246–256. doi: 10.1097/00003086-199805000-00033. [DOI] [PubMed] [Google Scholar]
- 18.Melton LJ, III, Lane AW, Cooper C, Eastell R, O’Fallon WM, Riggs BL. Prevalence and incidence of vertebral deformities. Osteoporos Int. 1993;3:113–119. doi: 10.1007/BF01623271. [DOI] [PubMed] [Google Scholar]
- 19.Melzack R, Vetere P, Finch L. Transcutaneous electrical nerve stimulation for low back pain: a comparison of TENS and massage for pain and range of motion. Phys Ther. 1983;63:489–493. doi: 10.1093/ptj/63.4.489. [DOI] [PubMed] [Google Scholar]
- 20.Pluijm SM, Tromp AM, Smit JH, Deeg DJ, Lips P. Consequences of vertebral deformities in older men and women. J Bone Miner Res. 2000;15:1564–1572. doi: 10.1359/jbmr.2000.15.8.1564. [DOI] [PubMed] [Google Scholar]
- 21.Scott G, King JB. A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones. J Bone Joint Surg Am. 1994;76:820–826. doi: 10.2106/00004623-199406000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Weiner DK, Rudy TE, Glick RM, Boston JR, Lieber SJ, Morrow LA, Taylor S. Efficacy of percutaneous electrical nerve stimulation for the treatment of chronic low back pain in older adults. J Am Geriatr Soc. 2003;51:599–608. doi: 10.1034/j.1600-0579.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 23.Zambito A, Bianchini D, Gatti D, Rossini M, Adami S, Viapiana O. Interferential and horizontal therapies in chronic low back pain due to multiple vertebral fractures: a randomized, double blind, clinical study. Osteoporos Int. 2007;18:1541–1545. doi: 10.1007/s00198-007-0391-3. [DOI] [PubMed] [Google Scholar]
- 24.Zambito A, Bianchini D, Gatti D, Viapiana O, Rossini M, Adami S. Interferential and horizontal therapies in chronic back pain: a randomized, double blind, clinical study. Clin Exp Rheumatol. 2006;24:534–539. [PubMed] [Google Scholar]
- 25.Zhuang H, Wang W, Seldes RM, Tahernia AD, Fan H, Brighton CT. Electrical stimulation induces the level of TGF-beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun. 1997;237:225–229. doi: 10.1006/bbrc.1997.7118. [DOI] [PubMed] [Google Scholar]