Abstract
The use of allograft-prosthesis composites for reconstruction after bone tumor resection at the proximal femur has generated considerable interest since the mid1980s on the basis that their use would improve function and survival, and restore bone stock. Although functional improvement has been documented, it is unknown whether these composites survive long periods and whether they restore bone stock. We therefore determined long-term allograft-prosthesis composite survival, identified major complications that led to revision, and determined whether allograft bone stock could be spared at the time of revision. We also compared the radiographic appearance of allografts sterilized by gamma radiation and fresh-frozen allografts. We retrospectively reviewed 32 patients with bone malignancy in the proximal femur who underwent reconstruction with a cemented allograft-prosthesis composite. The allograft-prosthesis composite was a primary reconstruction for 23 patients and a revision procedure for nine. The minimum followup was 2 months (median, 68 months; range, 2–232 months). The cumulative incidence of revision for any reason was 14% at 5 years (95% confidence interval, 1%–28%) and 19% at 10 years (95% confidence interval, 3%–34%). Nine patients (28%) had revision of the reconstruction during followup; four of these patients had revision surgery for infection. Allografts sterilized by gamma radiation showed worse resorption than fresh-frozen allografts. Based on reported results, allograft-composite prostheses do not appear to improve survival compared with megaprostheses.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Aggressive surgery and chemotherapy have permitted improvement in survival of patients with most bone sarcomas of the appendicular skeleton such that function and implant survival are increasingly being considered by patients and surgeons. The most frequent locations of bone sarcoma are the distal and proximal femur, the proximal tibia, and proximal humerus. The generally preferred reconstructive options in adults after bone tumor resection in the proximal femur are implantation of a megaprosthesis or an allograft-prosthesis composite [4, 12–14, 22, 24, 30, 31, 39, 42].
The use of allograft-prosthesis composites for reconstruction after bone tumor resection at the proximal femur [4, 12–14, 16–18, 24, 42], proximal humerus [9, 18, 20, 37], and proximal tibia [6, 11] has generated considerable interest since the mid1980s. The purported benefits of allograft-prosthesis composites over megaprostheses include improved function, improved longevity through the load-sharing properties of the allograft, and restoration of bone stock [10]. Comparative studies suggest minor improvement in function for patients who had reconstruction surgery with allograft-prosthesis composites compared with patients who had megaprostheses; however, to date, no evidence of improvement in survival or advantage of bone stock restoration for future revision has been reported [4, 13, 42]. Moreover, these purported benefits sometimes have been counterbalanced by risks of infection with rates ranging from 0% to 19%, risks of resorption with rates ranging from 7% to 46%, and risks of fracture with rates ranging from 12.5% to 27% [4, 6, 11, 12, 24, 30, 42].
The expected mechanical advantage of allograft-prosthesis composites over megaprostheses may be affected in different ways. The load-sharing properties of the allograft are transmitted to the host femur only if union at the allograft-host femur junction occurs. Likewise, resorption may alter the mechanical properties of the allograft and compromise the status of bone stock available at the time of revision. Nonunion at the allograft host-bone junction and graft resorption have reported rates ranging from 4% to 22% [12, 13, 24, 30, 42] and 7% to 46% [4, 12, 13], respectively, and eventually, the improvement regarding reconstruction is questionable. A deleterious effect of gamma radiation on the structural properties of the allograft have been observed in ex vivo studies [1, 2, 32], and these effects correspond to a clinically increased risk of fracture with massive structural allografts [26]. Although the effect of gamma radiation on the allograft-prosthesis composites is not known, we presumed allografts sterilized by gamma radiation would show greater resorption and lower union rates than fresh-frozen allografts.
The objectives of our study therefore were to (1) determine allograft-prosthesis composite survival with revision for any reason and for mechanical reasons as end points; (2) identify the major complications that led to revision of allograft-prosthesis composites; (3) determine whether allograft bone stock could be spared at the time of revision; and (4) compare the radiographic appearance of allografts sterilized by gamma radiation and fresh-frozen allografts with a specific rating system.
Patients and Methods
We retrospectively reviewed 32 patients with bone malignancies of the proximal femur who underwent reconstruction with allograft-prosthesis composites from 1987 to 2005. All operations were performed at a tertiary care university center by senior surgeons with expertise in musculoskeletal tumor surgery. There were 21 male and 11 female patients with a median age of 41 years (first quartile–third quartile [Q1-Q3], 27–57 years), a median body weight of 67 kg (Q1-Q3, 59–70 kg; data were missing for two patients), and a median height of 173 cm (Q1-Q3, 164–182 cm; data were missing for two patients) at the time of surgery. The right limb was affected in 12 patients (38%). According to the classification of the American Society of Anesthesiology [3], 17 patients were Grade 1 physical status (53%), 10 were Grade 2 (31%), and two were Grade 3 (6%) (data were missing for three patients). Diagnosis and classification of tumors are based on the World Health Organization Classification of Tumours [15]. The diagnosis of the index procedure was low-grade chondrosarcoma in 16 patients (50%), Ewing sarcoma in four (13%), conventional osteosarcoma in four (13%), high-grade malignant fibrous histiocytoma of bone in two (6%), bone metastasis in two (6%), and low-grade osteosarcoma, high-grade chondrosarcoma, high-grade fibrosarcoma, and high-grade leiomyosarcoma of bone in one each (3%). Five patients (16%) presented with pathologic fractures. The allograft-prosthesis composite was a primary reconstruction for 23 patients (72%) and a revision procedure for nine (28%); three patients had intralesional curettage and bone grafting and reconstruction with plate osteosynthesis for chondrosarcoma (one Grade 1, one Grade 2, and one clear-cell chondrosarcoma). Six patients had previous reconstruction with a megaprosthesis; three had revision surgery for local recurrence and three for mechanical failure. Thirteen patients had received perioperative chemotherapy and two had postoperative radiation therapy. At last followup, 21 of the 32 patients (65%) were alive, 19 with no evidence of disease and two with disease; 10 (31%) had died from disease, and one (3%) had died from unrelated causes (colorectal cancer). The minimum followup was 2 months (median, 68 months; range, 2–232 months). Six patients were followed for less than 1 year; three died from disease during the first year, two patients returned to their home country and attempts to contact them failed, and one patient with a soft tissue recurrence did not return for the 1-year followup and was considered lost to followup. We had prior approval of our local ethics committee.
The technique used in these patients was described previously [4, 5]. A lateral approach was used in all patients. The surgical technique involved resection of the tumor, or the previous megaprosthesis or plate osteosynthesis, and reconstruction of the joint. At the time of resection, 12 patients had the abductor mechanism continuity preserved; of these, nine had a trochanteric slide osteotomy and three had continuity preserved through the gluteus medius and vastus lateralis tendons by periosteal elevation. The abductor mechanism continuity was not preserved in 20 patients; 17 patients had soft tissue detached from the proximal femur and three had a trochanteric osteotomy. Tumor resections conformed to principles for management of malignant bone tumors; a cuff of normal tissue was left with the tumor and the biopsy track was left in continuity with the specimen with a 2-cm margin. The distal femoral cut was horizontal.
Reconstruction was performed during the same surgery. Twenty-two patients had a THA and 10 had implantation of a bipolar prosthesis. From 1998 onward, most patients had a bipolar prosthesis implanted because it was considered more stable; however, two of the six patients who had a megaprosthesis revised and one who required an extraarticular resection of the proximal femur and hip for joint contamination had a THA.
We first prepared the allograft to match the length of the skeletal defect. We then cemented the prosthesis into the allograft on a back table and performed a second trial after cement polymerization was complete. The composite prosthesis was cemented into the host bone, and care was taken so no cement was caught between the allograft and the host bone. Four patients had autograft bone added at the allograft host-bone junction.
All allografts were obtained from our institution’s bone bank. Twenty allografts were sterilized by gamma radiation (25 kGy precisely controlled by dosimeters), and 12 were fresh-frozen allografts; the institution’s policy regarding bone allograft conservation changed in 1995 in favor of fresh-frozen allografts; after 1995, we used six irradiated allografts remaining in the bone bank. The allograft tendons were not retained on the specimens. All allografts were harvested under sterile conditions, cultured before delivery and implantation, and preserved at −75°C without cryoprotective agents until preparation. For fresh-frozen allografts, when an operation was scheduled, the allograft chosen was retrieved and prepared under sterile conditions as requested by the surgeon. The allograft was immersed for 90 minutes in a solution of dimethyl sulfoxide (8 mL/100 mL), sodium chloride (0.9/1000), and rifampicin (600 mg/L). After this, the allograft was returned to −75°C until the operation. At the time of the operation, the allograft was immersed for 20 minutes in a saline solution with rifampicin (600 mg/L).
The median resection length was 175 mm (Q1-Q3, 150–211 mm), the median stem length was 335 mm (Q1-Q3, 273–350 cm), the median duration of the procedure was 180 minutes (Q1-Q3, 150–210 minutes; data missing for three cases), and the median number of red blood cell units transfused was 2 (Q1-Q3, 0–4 units; for three cases). Four patients (13%) had postoperative complications, which included two hematomas with neurologic signs of sciatic nerve palsy that required drainage; two stems were too long, as seen on the postoperative radiographs, with a supracondylar breach through the anterior cortex. These stems were shortened with a diamond saw through an anterior cortical window.
Preoperative and postoperative second-generation cephalosporins were administered for 48 hours. Patients had coaptation-suspension in the department for 2 weeks during which they had wound care. A spica cast subsequently was applied for another 6 weeks to allow scar formation around the hip. Weightbearing using two elbow crutches was allowed while wearing the spica cast after the second postoperative week. Full weightbearing with no support was allowed at the end of the eighth week, after the spica cast was removed. Low-molecular-weight heparin was administered for 8 weeks. The median hospital stay was 18 days (Quartile 1–3, 15–20 days; data missing for two patients).
Patients were followed at 8 weeks, every 6 months until the fifth year, and yearly thereafter. At each followup, a clinical evaluation was performed; an AP view of the pelvis and AP and lateral radiographs of the femur were taken at 2 months, 6 months, and yearly thereafter.
We used the International Society of Limb Salvage (ISOLS) radiographic allograft-prosthesis evaluation system [25]. The ISOLS evaluation system assesses bone remodeling, interface, anchorage, fusion, resorption, and fracture on AP and lateral radiographs and rates these items as excellent, good, fair, and poor (Table 1). Only the 21 patients, 13 who received an irradiated allograft and eight who received a fresh-frozen allograft, with radiographic followup greater than 1 year were included in this analysis. For patients who had the reconstruction revised, the radiographs were analyzed before the revision surgery. Radiographs were rated using the ISOLS evaluation system by two senior musculoskeletal radiologists (FT, EP) blinded to the allograft type; each radiologist rated the radiographs separately and disagreement was resolved by consensus.
Table 1.
The ISOLS radiographic evaluation system for allograft-prosthesis composites [25]
| Grade | Bone remodeling | Interface | Anchorage | Fusion | Resorption | Fracture |
|---|---|---|---|---|---|---|
| Excellent | No change from discharge radiographs | No radiolucent line | No change from discharge radiographs | Fusion-osteotomy line no longer visible | No resorption or geometric change Periosteal new bone formation |
No fracture |
| Good | Hypertrophy or sclerosis or osteopenia; bone angulation < 5° | Radiolucent line < 2 mm thick and less than entire length of interface | Adequate cementation technique (no gaps or porosity) | Fusion greater than or equal to 75%; osteotomy line still visible | Resorption less than 25% and no fracture | Incomplete fracture |
| Fair | Resorption of fixation area < 50% cortical thickness and > 2 cm length | Radiolucent line > 2 mm, incomplete; or < 2 mm, complete; axial migration; stem/shaft angulation < 5° | Stem fracture; or stem deformation; or screw fracture*; or plate fracture*; or cement fracture* | Fusion 25%–75% | Resorption 25%–50% no fracture | Simple fracture without displacement |
| Poor | Resorption of fixation area > 50% cortical thickness and > 2 cm length | Radiolucent line > 2 mm, completely around stem; or > 5 mm axial migration, loosening (macromotion) | No evidence of callus; or fusion < 25% | Resorption > 50%; or type I fracture (with resorption) | Simple fracture with displacement or comminuted fracture |
* With motion of the stem; ISOLS = International Society of Limb Salvage. (Adapted from and published with permission from Springer and Dr. Bernard Tomeno from Langlais F, Tomeno B (eds). Limb Salvage: Major Reconstructions in Oncologic and Nontumoral Conditions. Heidelberg, Germany: Springer; 1991.)
The main outcome consisted of time to revision of any part of the reconstruction (stem or acetabular component) for any reason. The secondary outcome was revision of any part of the reconstruction for mechanical reasons. Survival of the allograft-prosthesis reconstruction was estimated using the cumulative incidence function to account for competing risks; this method is a better estimator than the Kaplan-Meier method in the context of limb salvage surgery [7]. Median cumulative probabilities of events with the 95% confidence intervals were determined. Factors such as chemotherapy, radiotherapy, resection length, tumor grade, and revision procedure are potentially influential regarding reconstruction survival [17, 19, 41]. Pooling patients regardless of these factors provides a summary measure that may be misleading. In the current series, the influence of these factors assessed using a Cox regression model was low (all hazard ratios between 0.5 and 2) and furthermore, no association was statistically significant (Table 2). We therefore decided to report survival for all patients as one group. We determined differences in ISOLS radiographic evaluation between irradiated and fresh-frozen allografts using a chi square test for trend. For quantitative variables (continuous variables), we report the median and first and third quartile values (Q1-Q3). For length of followup, we report the range. Categorical variables are reported as counts and proportions. All analyses were performed with R statistical software [38]. All patients were included in the analysis regardless whether they had retained the allograft-prosthesis composite. All tests are two-sided and the level of statistical significance was chosen at 0.05.
Table 2.
Hazard ratio derived from univariate Cox regression models and significance level of potentially influential factors on reconstruction survival
| Covariates | Hazard ratio (95% confidence interval) | p Value |
|---|---|---|
| Previous operation | 0.85 (0.22–3.29) | 0.82 |
| Previous megaprosthesis | 0.60 (0.12–2.94) | 0.53 |
| Chemotherapy | 0.60 (0.12–2.97) | 0.53 |
| High-grade tumor | 0.70 (0.14–3.47) | 0.66 |
Results
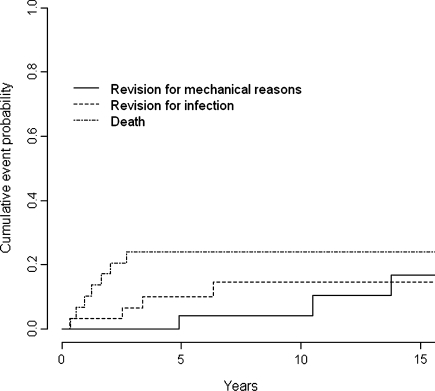
The cumulative incidence of revision for any reason was 14% at 5 years (95% confidence interval [CI], 1%–28%), 19% at 10 years (95% CI, 3%–34%), and 31% at 15 years (95% CI, 10%–53%). The cumulative incidence of revision for mechanical reasons was 4% at 5 and 10 years (95% CI, 0%–12%) and 16% at 15 years (95% CI, 0%–35%) (Fig. 1).
Fig. 1.
This graph shows the cumulative probability of revision for mechanical or infectious reasons and of death. The cumulative incidence of revision for any reason was 19% at 10 years (95% confidence interval, 3%-34%), and the cumulative incidence of revision for mechanical reasons was 4% at 10 years. Infection is an important cause for revision.
Nine patients (28%) had revision of the reconstruction during followup, including four for infection. One patient had a two-stage revision procedure and reconstruction with a long stem THA 4 months after the index procedure. She subsequently had infection develop again and a resection arthroplasty was performed. One patient had a two-stage revision procedure to a long stem THA at another center 30 months after the index operation. One patient had a two-stage revision procedure to another allograft-prosthesis composite 41 months after the index operation. The reconstruction eventually failed 134 months after the index procedure and revision surgery using another allograft-prosthesis composite was performed. Finally, one patient had a one-stage revision procedure to a long stem THA 76 months after the index operation. The allograft was never retained when revision was performed to treat infection. Five patients had revision surgery to treat mechanical complications. One patient sustained a stem fracture 59 months after the reconstruction and had a standard long cemented stem implanted. Extraction of the proximal stem resulted in some allograft being extracted as well, and only 3 cm of the allograft could be retained on the host femur. One patient had a megaprosthesis implanted 126 months after the index procedure to treat aseptic loosening of the femoral stem. The allograft showed severe resorption (Fig. 2) and no allograft bone was retained. One patient had revision surgery for a worn acetabular component 165 months after the index procedure. She had an all-polyethylene acetabular component cemented and reinforced with an acetabular ring. One patient had a local recurrence located against the anterior acetabular horn excised 204 months after the index procedure. The acetabular component was exchanged during the same procedure owing to macroscopic wear. The patient had an all-polyethylene acetabular component cemented and reinforced with an acetabular ring. Finally, one patient had another allograft-prosthesis composite 212 months after the index procedure to treat acetabular aseptic loosening and allograft resorption. During revision surgery, the proximal part of the allograft was split longitudinally to preserve soft tissue attachment and access the acetabulum. During extraction of the stem, only the last 2 cm of the allograft remained attached to the host femur and it was decided to implant another allograft-composite prosthesis.
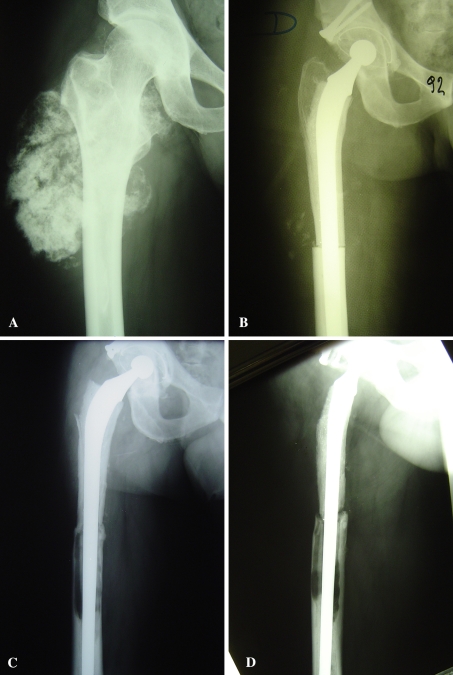
Fig. 2A–D.
(A) This radiograph shows an allograft-composite prosthesis after resection of a chondrosarcoma of the right proximal femur. (B) The immediate postoperative and (C) 16-year postoperative AP and (D) lateral radiographs show allograft resorption, allograft-host bone nonunion, and stem loosening with time. Some mismatch between the allograft and host femur can be seen on the postoperative radiograph.
Little if any allograft bone stock was retained in the seven patients whose stems were revised. The allograft was removed entirely for all infected reconstructions (four patients) and only the more distal few centimeters could be preserved for two of the three patients who had mechanical complications.
Allograft sterilized by gamma radiation showed worse resorption (p = 0.041) than fresh-frozen allografts according to the ISOLS radiographic allograft-prosthesis evaluation system (Table 3; Figs. 2 and 3) We observed no difference in bone remodeling (p = 0.87), fusion at the allograft-host bone junction (p = 0.75), interface (p = 0.13), anchorage (p = 1), and fracture (p = 0.42).
Table 3.
Radiographic evaluation of proximal femoral allograft composites using ISOLS rating system
| ISOLS rating | Resorption | Fusion | Interface | Bone remodeling | Anchorage | Fracture | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Irradiated | Fresh | Irradiated | Fresh | Irradiated | Fresh | Irradiated | Fresh | Irradiated | Fresh | Irradiated | Fresh | |
| Excellent | 0 | 4 | 4 | 0 | 4 | 3 | 1 | 1 | 13 | 8 | 12 | 8 |
| Good | 4 | 2 | 3 | 6 | 4 | 5 | 9 | 5 | 0 | 0 | 1 | 0 |
| Fair | 5 | 0 | 3 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Poor | 4 | 2 | 3 | 0 | 3 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
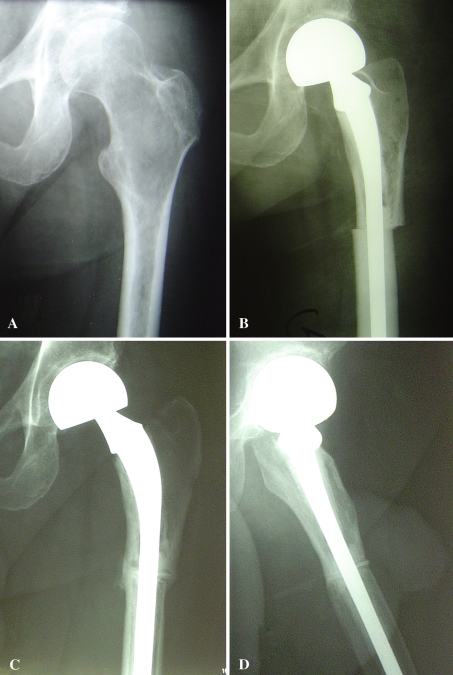
Fig. 3A–D.
(A) This radiograph shows an allograft-composite prosthesis after resection of a malignant fibrous histiocytoma of the left proximal femur. (B) The immediate postoperative and (C) 7-year postoperative AP and (D) lateral radiographs show allograft-host bone union and absence of allograft resorption with time.
Numerous additional operations were performed during followup. Three patients had revision of a nonunion of the greater trochanter; one patient had a supracondylar fracture below the stem that was opened and internally fixed; three patients had washout procedures (two patients cited previously; one patient died from metastatic dissemination 7 months after surgery with no evidence of infection); one patient had a negative biopsy; one patient had corticocancellous bone grafting at the allograft host-bone junction; and three patients had excision of a local recurrence.
Discussion
Development of aggressive treatment has permitted remarkable improvement in survival of patients with most bone sarcoma of the appendicular skeleton and patients want surgeons to provide reconstructions that restore function and last for decades. Although allograft-prosthesis composites have generated substantial interest since the mid1980s, their purported benefits over megaprostheses have not been confirmed. We therefore (1) evaluated allograft-prosthesis composite survival with revision for any reason and for mechanical reasons as end points; (2) identified the major complications that led to revision of allograft-prosthesis composites; (3) determined whether allograft bone stock could be spared at the time of revision; and (4) compared the radiographic appearance of allografts sterilized by gamma radiation and fresh-frozen allografts with a specific rating system.
Our study has some limitations regarding to the internal and external validity of the results. First, we lost some patients to followup (three of 32 [9%]) and had a limited number of patients with radiographic followup more than 1 year (21 of 32 [66%]). However, these cases are relatively infrequent and it is difficult to accumulate a large number. Further, analysis of time to event data, either using the Kaplan-Meier estimator or cumulative incidence estimator, takes account of censored observations in such a way that patients with short followup do not bias the results because their observations are censored early. Except for graft resorption, the small number of patients with radiographic followup more than 1 year implies the study has limited power to detect meaningful effects in radiographic differences between both groups. Therefore, the absence of differences between irradiated allografts and fresh-frozen allografts for items other than resorption should be interpreted in light of other in vitro and vivo studies. Second, our data are most likely only applicable to centers and surgeons that have developed a specific expertise in the treatment of malignant bone tumors. For instance, access to a bone bank is a prerequisite to perform allograft-prosthesis composite reconstructions. Third, the Kaplan-Meier estimator used in some studies to assess the survival of the reconstruction overestimates the risk of the event to occur [7]; comparisons with survival estimated with the cumulative incidence estimator need to account for this bias. Fourth, factors such as chemotherapy, radiotherapy, resection length, tumor grade, and revision procedure potentially influence reconstruction survival. Although we did not find these factors had an effect on reconstruction survival in this limited series, we do not believe their potential influence should be disregarded for future studies.
We found the cumulative incidence of revision for any reason was 14% at 5 years and 19% at 10 years. These results are comparable to those in other studies [4, 11, 13, 14, 22, 31, 42]. In series ranging from 16 to 27 patients, the allograft-prosthesis composite survival rates ranged from 76% to 100% at 5 years and 76% to 86% at 10 years [11, 13, 42]. In series ranging from 17 to 96 patients, the megaprosthesis survival rates ranged from 65% to 86% at 5 years and from 0% to 86% at 10 years [4, 13, 14, 22, 31, 42]. To date, however, comparative studies of allograft-prosthesis composites versus megaprostheses have failed to show improved longevity of the former [4, 13, 42] (Table 4). Junction union is paramount to the theoretical advantage of allograft-prosthesis composites over megaprostheses. When union is not obtained, the purported load-sharing properties of the allograft are negligible [19]. Four reconstructions of 21 (19%) showed union less than 25% on the AP or lateral radiographs (union rated as poor). Nonunion at the allograft host-bone junction is reported between 4% and 22% in series ranging from 18 to 27 patients [12, 13, 24, 30, 42] (Table 5). Factors affecting bone union are bone grafting at the junction, postoperative chemotherapy, and radiotherapy [17, 19]. Although use of intramedullary cement reportedly offers stability without adverse effects on healing [40], it is important that no cement be placed at the host-bone allograft junction during fixation of the stem and that cancellous bone be added at the periosteal surface [17]. McGoveran et al. proposed using an uncemented stem to improve the chances of host-graft bone union through compression loading [30] (Table 5). To increase stability at the host-graft junction and enhance union, some authors advise performing a step-cut osteotomy instead of a transverse osteotomy [24, 30]. Although this osteotomy provides a mechanical advantage, it is technically difficult and may lead to inadequate contact between the allograft and the host femur or rotational malunion. The treatment of nonunion at the allograft host-bone junction is corticocancellous bone autograft.
Table 4.
Published studies of reconstruction with an allograft-prosthesis composite or a megaprostheses
| Study | Date of publication | Number of patients (number of procedures) | Diagnosis | Age (years) | Reconstruction | Followup | Infection | Local recurrence | Reconstruction survival |
|---|---|---|---|---|---|---|---|---|---|
| Zehr et al. [42] | 1996 | 16 (18) | Primary malignant tumors (88%); aggressive benign bone tumors (12%) | 38 (13–72) | Allograft-prosthesis composite | 5 (0.8–10) | 3 of 16 (19%) | 3 of 16 (19%) | 76% at 5 and 10 years |
| 17 (18) | Primary malignant tumors (88%); aggressive benign bone tumors (12%) | 49 (23–77) | Prosthesis; cemented | 9.5 (0.3–23) | 1 of 17 (6%) | 2 of 17 (12%) | 65% at 5 years; 58% at 10 years | ||
| Kabukcuoglu et al. [22] | 1999 | 54 | Primary malignant tumors (96%); aggressive benign bone tumors (4%) | 40 (15–76) | Prosthesis; cemented | 9 (5–24) | 3 of 54 (6%) | 15 of 54 (28%) | 67% at 10 years |
| McGoveran et al. [30] | 1999 | 16 | Primary malignant tumors (100%) | 51 (25–83) | Allograft-prosthesis composite | 4 (2–8) | 3 of 16 (19%) | Not reported | 4 failures of 16 (25%) |
| Anract et al.* [4] | 2000 | 21 | Primary malignant tumors (81%); metastases (19%) | 40 (15–67) | Allograft-prosthesis composite | 4 (0.5–10) | 2 of 21 (10%) | 0 | 77% at 5 years and 10 years |
| 20 | Primary malignant tumors (75%); aggressive benign bone tumors (10%); metastases (15%) | 43 (20–90) | Prosthesis; cemented | 6 (0.3–10) | 2 of 20 (10%) | 2 of 20 (10%) | 73% at 5 years; 0% at 10 years | ||
| Donati et al. [12] | 2002 | 27 | Primary malignant tumors (67%); aggressive benign bone tumors (30%); metastases (4%) | 32 (11–64) | Allograft-prosthesis composite | 5 (0.9–10.5) | 1 of 27 (4%) | 1 of 27 (4%) | 3 of 27 (11%) |
| Langlais et al. [24] | 2003 | 21 | Primary malignant tumors (100%) | 38 (14–77) | Allograft-prosthesis composite | 5.7 (0.25–15) | 0 | 6 of 21 (29%) | 4 failures of 21 (19%) |
| Menendez et al. [31] | 2006 | 96 | Primary malignant tumors (25%); metastases (75%) | 59 (14–86) | Prosthesis; cemented | 1.5 (0.1–11) | 6 of 96 (6%) | 3 of 96 (3%) | 82% at 5 and 10 years |
| Farid et al. [13] | 2006 | 20 | Primary malignant tumors (100%) | 44 (17–64) | Allograft-prosthesis composite | 7* (2–28) | 1 of 20 (5%) | Not reported | 100% at 5 years; 86% at 10 years |
| 52 | Primary malignant tumors (60%); aggressive benign bone tumors (6%); metastases (35%) | 39 (16–87) | Prosthesis; cemented | 8/18* (2–28) | 2 of 52 (4%) | Not reported | 86% at 5 and 10 years | ||
| Finstein et al. [14] | 2007 | 62 | Primary malignant tumors (40%); aggressive benign bone tumors (8%); metastases (52%) | 49 (10–83) | Prosthesis; cemented | 5 (0.1–22) | 3 of 62 (5%) | 6 of 62 (7%) | 79% at 5 years; 57% at 10 years |
| Selek et al. | 2008 | 44 (45) | Metastases (100%) | 55 (29–84) | Prosthesis; cemented | Not reported | 2 of 44 (5%) | Not reported | Not reported |
Mean (range) are presented for age and followup; *some patients included in this series also are included in the current series.
Table 5.
Techniques for tumoral reconstruction with an allograft-prosthesis composite
| Study | Number of patients (number of procedures) | Allograft type | Fixation of stem into the allograft | Fixation into host femur | Junction procedure | Allograft resorption | Allograft host-bone nonunion | Allograft fracture |
|---|---|---|---|---|---|---|---|---|
| Zehr et al. [42] | 16 (18) | Fresh-frozen | Cemented (n = 14); uncemented (n = 2) | Cemented stem (n = 14); uncemented stem (n = 2) | Osteotomy interface milled; autogenous bone graft (n = 11) | Not reported | 4 nonunion | 0 |
| McGoveran et al. [30] | 16 | Irradiated | Cemented | Cemetend stem (n = 5); uncemented stem (n = 9); plate fixation (n = 2) | Step-cut osteotomy (n = 15); autogenous bone graft (n = 15) | Not reported | 2 nonunion; 1 delayed union | 2 |
| Anract et al.* [4] | 21 | Irradiated | Cemented | Cemented stem | Not reported | 10 partial or complete resorption | 1 nonunion | 0 |
| Donati et al. [12] | 27 | Fresh-frozen | Cemented | Cemented stem (n = 4); uncemented stem (n = 23) | Transverse osteotomy; no bone grafting | 2 marked resorption | 1 nonunion; 4 delayed union | (17 trochanter fracture) |
| Langlais et al. [24] | 21 | Fresh-frozen | Cemented | Cemented stem | Step-cut osteotomy; autogenous bone graft unless chemotherapy scheduled | 5 mild resorption; 1 severe | 2 nonunion | 0 |
| Farid et al. [13] | 20 | Not reported | Cemented | Cemented stem (n = 18); uncemented stem (n = 2); supplemental plate fixation (n = 10) | Autogenous bone graft (n = 3); allogenous bone graft (n = 4) | Not reported | 2 nonunion | Not reported |
* Some patients included in this series also are included in the current series.
Infection was an important mode of early failure in the current series with five of 32 patients (16%) having the component removed for this reason. Reported rates of infection vary from 0% to 19% in series ranging from 16 to 96 patients (Table 4). Numerous risk factors have been incriminated in the development of postoperative infection such as patients’ comorbidities [28, 33, 34], previous surgery [36], complexity and duration of the operation [34, 36], prophylactic antibiotic protocol [23, 35, 36], blood transfusion [28, 33, 35], radiation, and chemotherapy [8, 21, 23, 35]. Our series shows possible increased susceptibility for allograft-prosthesis composites toward infection when compared with megaprostheses. Superior infection rates have been reported for allograft-prosthesis composite reconstructions than for megaprostheses (Table 4) [13, 42]. This could be attributable to the greater complexity of allograft-prosthesis composite reconstructions and to the transmission of pathogen agents through the allograft [27, 29]. The use of fresh-frozen instead of irradiated-sterilized allografts may increase the risk of infection-related complications. In the absence of an unbiased comparative study, we believe it is inappropriate to draw any conclusion and this finding should merely serve as the basis for future research. Retention of bone stock was never possible when revision for treating infection was performed. Bone stock could not be preserved because of allograft resorption or technical difficulties either when revision was performed for treating aseptic stem loosening or stem fracture or when exchange of the stem was necessary to facilitate exposure.
We observed a difference in radiographic evaluation between irradiated and fresh-frozen allografts only for graft resorption. Radiation affects the structural properties of allografts [1, 2, 32] and increases the risk for fracture for massive allografts [26]. For these reasons, we presumed irradiation could have played a role in allograft resorption. Our observations with fresh-frozen allografts are consistent with those in several previous studies where marked or severe allograft resorption occurred in 5% to 7% of fresh-frozen allografts [12, 24]. Based on the deleterious effect of gamma radiation on allograft-prosthesis composites observed in our series, and the findings of other in vivo and ex vivo studies [1, 2, 26, 32], we recommend radiation not be used routinely for sterilization.
Allograft-prosthesis composite reconstruction survival in our series showed comparable survival to reported rates of megaprosthesis reconstruction survival. However, allograft bone stock could never be retained when stem revision was performed. Allografts sterilized by gamma radiation were at increased risk for allograft resorption compared to fresh-frozen allografts.
Acknowledgments
We thank Drs. L. Vastel and B. Bourely (Hôpital Cochin, Bone Bank) for help with preparation of the manuscript, and Dr. E. Pluot for evaluating the postoperative radiographs as the second senior radiologist. Unidentified patient level data are available by request to promote and facilitate research on rare diseases.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This study was performed at Hôpital Cochin.
References
- 1.Akkus O, Belaney RM, Das P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res. 2005;23:838–845. doi: 10.1016/j.orthres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Akkus O, Rimnac CM. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927–934. doi: 10.1016/S0736-0266(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 3.American Society of Anesthesiologists New classification of physical status. Newsletter of the American Society of Anesthesiologists. Anesthesiology. 1963;24:111. [Google Scholar]
- 4.Anract P, Coste J, Vastel L, Jeanrot C, Mascard E, Tomeno B. [Proximal femoral reconstruction with megaprosthesis versus allograft prosthesis composite: a comparative study of functional results, complications and longevity in 41 cases] [in French] Rev Chir Orthop Reparatrice Appar Mot. 2000;86:278–288. [PubMed] [Google Scholar]
- 5.Biau DJ, Davis A, Vastel L, Tomeno B, Anract P. Function, disability, and health-related quality of life after allograft-prosthesis composite reconstructions of the proximal femur. J Surg Oncol. 2008;97:210–215. doi: 10.1002/jso.20936. [DOI] [PubMed] [Google Scholar]
- 6.Biau DJ, Dumaine V, Babinet A, Tomeno B, Anract P. Allograft-prosthesis composites after bone tumor resection at the proximal tibia. Clin Orthop Relat Res. 2007;456:211–217. doi: 10.1097/BLO.0b013e31802ba478. [DOI] [PubMed] [Google Scholar]
- 7.Biau DJ, Latouche A, Porcher R. Competing events influence estimated survival probability: when is Kaplan-Meier analysis appropriate? Clin Orthop Relat Res. 2007;462:229–233. doi: 10.1097/BLO.0b013e3180986753. [DOI] [PubMed] [Google Scholar]
- 8.Bow EJ. Infection risk and cancer chemotherapy: the impact of the chemotherapeutic regimen in patients with lymphoma and solid tissue malignancies. J Antimicrob Chemother. 1998;41(suppl D):1–5. doi: 10.1093/jac/41.suppl_4.1. [DOI] [PubMed] [Google Scholar]
- 9.Chen WM, Chen TH, Huang CK, Chiang CC, Lo WH. Treatment of malignant bone tumours by extracorporeally irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg Br. 2002;84:1156–1161. doi: 10.1302/0301-620X.84B8.13508. [DOI] [PubMed] [Google Scholar]
- 10.Clatworthy MG, Gross AE. The allograft prosthetic composite: when and how. Orthopedics. 2001;24:897–898. doi: 10.3928/0147-7447-20010901-34. [DOI] [PubMed] [Google Scholar]
- 11.Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res. 2008;466:459–465. doi: 10.1007/s11999-007-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donati D, Giacomini S, Gozzi E, Mercuri M. Proximal femur reconstruction by an allograft prosthesis composite. Clin Orthop Relat Res. 2002;394:192–200. doi: 10.1097/00003086-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 13.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–229. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 14.Finstein JL, King JJ, Fox EJ, Ogilvie CM, Lackman RD. Bipolar proximal femoral replacement prostheses for musculoskeletal neoplasms. Clin Orthop Relat Res. 2007;459:66–75. doi: 10.1097/BLO.0b013e31804f5474. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher CDM, Unni KK, Merten SF, editors. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 16.Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;397:106–113. doi: 10.1097/00003086-200204000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hanson PD, Warner C, Kofroth R, Osmond C, Bogdanske JJ, Kalscheur VL, Frassica FJ, Markel MD. Effect of intramedullary polymethylmethacrylate and autogenous cancellous bone on healing of frozen segmental allografts. J Orthop Res. 1998;16:285–292. doi: 10.1002/jor.1100160303. [DOI] [PubMed] [Google Scholar]
- 18.Hejna MJ, Gitelis S. Allograft prosthetic composite replacement for bone tumors. Semin Surg Oncol. 1997;13:18–24. doi: 10.1002/(SICI)1098-2388(199701/02)13:1<18::AID-SSU4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. doi: 10.1097/00003086-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Jensen KL, Johnston JO. Proximal humeral reconstruction after excision of a primary sarcoma. Clin Orthop Relat Res. 1995;311:164–175. [PubMed] [Google Scholar]
- 21.Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87:842–849. doi: 10.2106/JBJS.C.01222. [DOI] [PubMed] [Google Scholar]
- 22.Kabukcuoglu Y, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement for primary malignant tumors of the proximal femur. Clin Orthop Relat Res. 1999;358:8–14. doi: 10.1097/00003086-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Konishi T, Watanabe T, Kishimoto J, Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg. 2006;244:758–763. doi: 10.1097/01.sla.0000219017.78611.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langlais F, Lambotte JC, Collin P, Thomazeau H. Long-term results of allograft composite total hip prostheses for tumors. Clin Orthop Relat Res. 2003;414:197–211. doi: 10.1097/01.blo.0000079270.91782.23. [DOI] [PubMed] [Google Scholar]
- 25.Langlais F, Tomeno B, editors. Limb Salvage: Major Reconstructions in Oncologic and Nontumoral Conditions. Heidelberg, Germany: Springer; 1991. [Google Scholar]
- 26.Lietman SA, Tomford WW, Gebhardt MC, Springfield DS, Mankin HJ. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res. 2000;375:214–217. doi: 10.1097/00003086-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Liu JW, Chao LH, Su LH, Wang JW, Wang CJ. Experience with a bone bank operation and allograft bone infection in recipients at a medical centre in southern Taiwan. J Hosp Infect. 2002;50:293–297. doi: 10.1053/jhin.2002.1192. [DOI] [PubMed] [Google Scholar]
- 28.Liu SA, Wong YK, Poon CK, Wang CC, Wang CP, Tung KC. Risk factors for wound infection after surgery in primary oral cavity cancer patients. Laryngoscope. 2007;117:166–171. doi: 10.1097/01.mlg.0000249737.05840.29. [DOI] [PubMed] [Google Scholar]
- 29.Mankin HJ, Hornicek FJ, Raskin KA. Infection in massive bone allografts. Clin Orthop Relat Res. 2005;432:210–216. doi: 10.1097/01.blo.0000150371.77314.52. [DOI] [PubMed] [Google Scholar]
- 30.McGoveran BM, Davis AM, Gross AE, Bell RS. Evaluation of the allograft-prosthesis composite technique for proximal femoral reconstruction after resection of a primary bone tumour. Can J Surg. 1999;42:37–45. [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez LR, Ahlmann ER, Kermani C, Gotha H. Endoprosthetic reconstruction for neoplasms of the proximal femur. Clin Orthop Relat Res. 2006;450:46–51. doi: 10.1097/01.blo.0000229332.91158.05. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell EJ, Stawarz AM, Kayacan R, Rimnac CM. The effect of gamma radiation sterilization on the fatigue crack propagation resistance of human cortical bone. J Bone Joint Surg Am. 2004;86:2648–2657. doi: 10.2106/00004623-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Morris CD, Sepkowitz K, Fonshell C, Margetson N, Eagan J, Miransky J, Boland PJ, Healey J. Prospective identification of risk factors for wound infection after lower extremity oncologic surgery. Ann Surg Oncol. 2003;10:778–782. doi: 10.1245/ASO.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Neumayer L, Hosokawa P, Itani K, El-Tamer M, Henderson WG, Khuri SF. Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1178–1187. doi: 10.1016/j.jamcollsurg.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Olsen MA, Lefta M, Dietz JR, Brandt KE, Aft R, Matthews R, Mayfield J, Fraser VJ. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207:326–335. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Penel N, Yazdanpanah Y, Chauvet MP, Clisant S, Giard S, Neu JC, Lefebvre D, Fournier C, Bonneterre J. Prevention of surgical site infection after breast cancer surgery by targeted prophylaxis antibiotic in patients at high risk of surgical site infection. J Surg Oncol. 2007;96:124–129. doi: 10.1002/jso.20796. [DOI] [PubMed] [Google Scholar]
- 37.Potter BK, Adams SC, Pitcher JD, Jr, Malinin TI, Temple HT. Proximal humerus reconstructions for tumors. Clin Orthop Relat Res. 2009;467:1035–1041. doi: 10.1007/s11999-008-0531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. Available at: www.R-project.org. Accessed May 13, 2009.
- 39.Selek H, Basarir K, Yildiz Y, Saglik Y. Cemented endoprosthetic replacement for metastatic bone disease in the proximal femur. J Arthroplasty. 2008;23:112–117. doi: 10.1016/j.arth.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Straw RC, Powers BE, Withrow SJ, Cooper MF, Turner AS. The effect of intramedullary polymethylmethacrylate on healing of intercalary cortical allografts in a canine model. J Orthop Res. 1992;10:434–439. doi: 10.1002/jor.1100100316. [DOI] [PubMed] [Google Scholar]
- 41.Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78:5–13. [PubMed] [Google Scholar]
- 42.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop Relat Res. 1996;322:207–223. doi: 10.1097/00003086-199601000-00026. [DOI] [PubMed] [Google Scholar]