Abstract
Saline (0.9%, 285 mOsm) and Hartmann’s solution (255 mOsm) are two commonly used joint irrigation solutions that alter the extracellular osmolarity of in situ chondrocytes during articular surgery. We asked whether varying the osmolarity of these solutions influences in situ chondrocyte death in mechanically injured articular cartilage. We initially exposed osteochondral tissue harvested from the metacarpophalangeal joints of 3-year-old cows to solutions of 0.9% saline and Hartmann’s solution of different osmolarity (100–600 mOsm) for 2 minutes to allow in situ chondrocytes to respond to the altered osmotic environment. The full thickness of articular cartilage then was “injured” with a fresh scalpel. Using confocal laser scanning microscopy, in situ chondrocyte death at the injured cartilage edge was quantified spatially as a function of osmolarity at 2.5 hours. Increasing the osmolarity of 0.9% saline and Hartmann’s solution to 600 mOsm decreased in situ chondrocyte death in the superficial zone of injured cartilage. Compared with 0.9% saline, Hartmann’s solution was associated with greater chondrocyte death in the superficial zone of injured cartilage, but not when the osmolarity of both solutions was increased to 600 mOsm. These experiments may have implications for the design of irrigation solutions used during arthroscopic and open articular surgery.
Introduction
Arthroscopic and open interventions on articular cartilage subject the tissue to mechanical insult [9]. A mechanical injury to articular cartilage results in chondrocyte death and matrix degradation [7, 19, 22]. Articular cartilage heals poorly. Partial-thickness defects do not heal [9] and full-thickness defects repair with structurally and mechanically inferior fibrocartilage [21]. Minimizing chondrocyte death from the mechanical insult would maintain a viable chondrocyte population near the defect capable of better lateral integration and cartilage healing.
Recent work suggests the responses of in situ chondrocytes (chondrocytes embedded in their native extracellular matrix) to mechanical injury can be influenced by medium (and therefore, extracellular) osmolarity [1, 4]. In these animal models of sharp (scalpel) and blunt (impact load) mechanical injury, exposure of articular cartilage to standard culture media (Dulbecco’s Modified Eagle’s Medium [DMEM], Invitrogen, Paisley, UK) with a low osmolarity increases the extent of in situ chondrocyte death, whereas exposure to a high osmolarity decreases the extent of in situ chondrocyte death [1, 4]. The cellular mechanisms responsible for the decrease in chondrocyte death at high osmolarity have yet to be elucidated but may involve a decrease in cell volume that protects cells from the mechanical insult [3, 4]. These effects on in situ chondrocyte viability are mediated within hours with no increase in cell death from 2.5 hours to 7 days [1] suggesting (1) exposure of articular cartilage to a high medium osmolarity does not compromise in situ chondrocyte function in the long term; and (2) future investigation of decreasing chondrocyte death from mechanical injury should focus on the early (within hours) effects of high medium osmolarity. Furthermore, the majority of cell death occurs in the superficial zone of injured cartilage with relative sparing of the middle and deep zones [1]. Reasons for the increased vulnerability of cells from the superficial zone also remain to be established, although the zone-specific heterogeneity in the stress-strain relationship in cartilage that results in lower compressive strain near the articular surface (and therefore, greater cell deformation and lysis) may be important [6, 16].
Saline (0.9%) and Hartmann’s solution are commonly used joint irrigation solutions [18]. During open and arthroscopic articular surgery, synovial fluid, which normally maintains the physiologic environment in a joint, is replaced by these solutions. The mean osmolarity of human synovial fluid is approximately 400 mOsm [2]. In contrast, the mean osmolarity of routinely used 0.9% saline and Hartmann’s solution is lower (approximately 250–300 mOsm). In situ chondrocytes therefore experience a change (decrease) in extracellular osmolarity during the surgical procedure. Evidence from in vitro animal models of mechanical cartilage injury [1, 4] suggests this decrease in the extracellular osmolarity may increase in situ chondrocyte death from any subsequent surgical procedure that involves a mechanical insult on articular cartilage. However, these in vitro experiments [1, 4] have varied the extracellular osmolarity using standard culture media (DMEM), which contains various salts, amino acids, vitamins, and glucose. The composition of irrigation solutions normally used in vivo during articular surgery is different. Articular cartilage is a complex, heterogenous, viscoelastic, anisotropic tissue in which the osmotic sensitivity of in situ chondrocytes varies depending on the composition of the extracellular medium [5, 6, 10, 14, 15, 23, 24]. Therefore, to determine whether varying the osmolarity of an extracellular medium is relevant clinically, it is essential to establish that the spatial distribution of chondrocyte death and the responses of in situ chondrocytes to mechanical injury following alterations in the extracellular osmolarity previously observed in experiments using standard culture media, can be reproduced using joint irrigation solutions normally used during open and arthroscopic articular surgery.
We reasoned that increasing the osmolarity of 0.9% saline and Hartmann’s solution in the model decreases in situ chondrocyte death in the superficial zone after mechanical injury (within hours). We specifically asked the following three questions: (1) What is the spatial distribution of in situ chondrocyte death in the full thickness of scalpel-injured articular cartilage exposed to solutions of 0.9% saline and Hartmann’s solution for 2.5 hours with the osmolarity varied between 100 mOsm and 600 mOsm? (2) Does varying the osmolarity of proprietary solutions of 0.9% saline (285 mOsm) and Hartmann’s (255 mOsm) influence in situ chondrocyte death in scalpel-injured articular cartilage at 2.5 hours? (3) Does the extent of in situ chondrocyte death differ between injured explants exposed to proprietary solutions of 0.9% saline and Hartmann’s and modified, high osmolarity (600 mOsm) preparations of the two solutions at 2.5 hours?
Materials and Methods
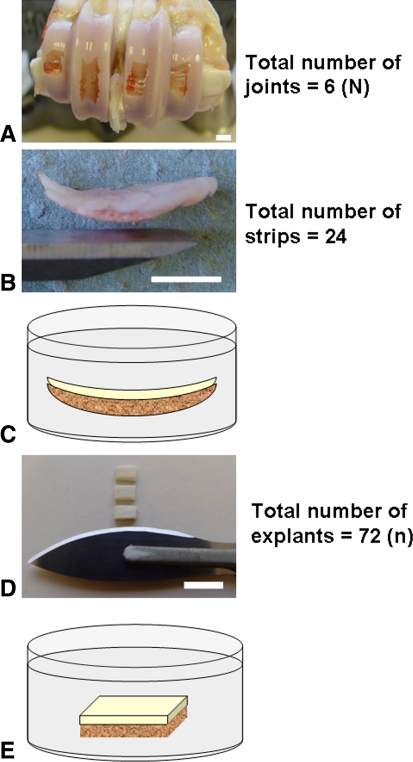
Six metacarpophalangeal joints, each from different 3-year-old cows, were skinned, rinsed in water, and opened under sterile conditions within 12 hours of slaughter. We removed four osteochondral strips from each joint with a chisel and initially exposed them to a solution (preparations of 0.9% saline: 170 mOsm, 285 mOsm [control], 500 mOsm or 600 mOsm; preparations of Hartmann’s solution: 255 mOsm [control] or 600 mOsm) for 2 minutes to allow in situ chondrocytes to experience and respond to the altered osmotic environment (Fig. 1) [3]. The articular cartilage on each osteochondral strip then was cut through its full thickness with a Number 24 scalpel to produce three rectangular osteochondral explants (approximately 5 × 3 mm) with injured (scalpel cut) edges. Therefore, 12 rectangular osteochondral explants were obtained from each joint and because these explants were obtained from six different joints, a total of 72 rectangular osteochondral explants were analyzed (N = 6, n = 72, where “N” refers to the number of different animals and “n” refers to the total number of explants obtained from “N” animals) (Fig. 1). Because the long scalpel-cut edges of each explant were of interest for the experiment, we used a strict no-touch technique with explants handled only from the short edges. A fresh Number 24 scalpel was used for every scalpel cut and discarded after one use because of concerns regarding the retention of blade sharpness [8]. We kept osteochondral strips and injured explants wet at all times with the solution of known osmolarity. All explants (N = 6, n = 72) were incubated (37°C, 5% CO2) for an additional 2.5 hours in the solution of known osmolarity (ranging between 100 and 600 mOsm). Explants were exposed to the fluorescent probes, 5-chloromethylfluorescein diacetate (CMFDA, 5 μmol/L) and propidium iodide (PI, 5 μmol/L), during the final 30 minutes of incubation to label live and dead cells, respectively. Finally, we transferred explants to 10% formalin (v/v in saline) for fixation and stored them at 4°C in phosphate-buffered saline before microscopy.
Fig. 1A–E.
Preparation of the rectangular osteochondral explants is shown. (A) The bovine metacarpophalangeal joint had four osteochondral strips removed from the flat articular surface between the condylar ridges using a chisel. (B) A sample of an osteochondral strip and the chisel used for its removal are shown. (C) The osteochondral strip initially was exposed to a solution of known osmolarity for 2 minutes to allow in situ chondrocytes to respond to the altered osmotic environment. (D) Rectangular osteochondral blocks then were cut from the osteochondral strip (three from each strip) using a Number 24 scalpel. (E) Each rectangular osteochondral explant then was incubated separately in the same solution as initially exposed to in Illustration C for an additional 2.5 hours (white bar = approximately 1 cm).
We obtained saline (0.9% [Na+] = 154 mmol/L, [Cl−] = 154 mmol/L) from Baxter Healthcare Ltd (Norfolk, UK). Hartmann’s solution ([Na+] = 131 mmol/L, [Cl−] = 111 mmol/L, [HCO−3] = 29 mmol/L, [K+] = 5 mmol/L, [Ca2+] = 2 mmol/L) was obtained from Fresenius Kabi Ltd (Cheshire, UK). We obtained the fluorescent probes, CMFDA and PI, from Invitrogen Ltd (Paisley, UK) and prepared them as 1 mmol/L stock solutions in dimethyl sulfoxide and water, respectively. Formaldehyde solution (10% v/v in normal saline) was obtained from Fisher Scientific (Leicestershire, UK). The osmolarity of all solutions was measured using a freezing point osmometer (Advanced Micro Osmometer, Model 3300; Vitech Scientific Ltd, West Sussex, UK). The osmolarities of the proprietary (control) solutions of 0.9% saline and Hartmann’s solution were measured as 285 mOsm and 255 mOsm, respectively. The osmolarity of the solutions was varied between 100 and 600 mOsm by the addition of distilled water or sucrose.
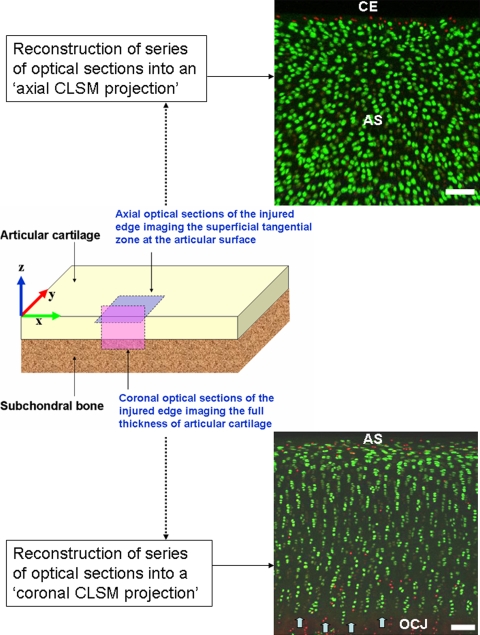
Using established techniques with CLSM [1, 4, 7], we acquired 921 × 921-μm2 optical sections at 10-μm intervals in the coronal and axial planes of mechanically injured articular cartilage (Fig. 2). Coronal optical sections imaged the full thickness of cartilage and were acquired at the scalpel-cut surface to a depth of approximately 80 μm into the tissue (y-axis). We acquired axial optical sections near the cut edge of the articular surface to a depth of approximately 60 μm into the tissue (z-axis), exclusively imaging the superficial zone in this region. By imaging the scalpel-cut edges of articular cartilage in coronal and axial planes, in situ chondrocyte death was evaluated in the entire region of injured cartilage (ie, full thickness and articular surface perspectives). Three-dimensional reconstructions of the imaged volume of articular cartilage, referred to as CLSM projections, were created from the consecutive series of optical sections using Volocity 4 (Improvision, Coventry, UK) imaging software (Fig. 2).
Fig. 2.
CLSM of the coronal and axial planes at the cartilage edge is shown. The x, y, and z axes are labeled on a diagram of a rectangular osteochondral explant. The pink-shaded area on the diagram represents the plane in which coronal optical sections were obtained imaging the full thickness (cut surface) of injured articular cartilage. The reconstructed coronal CLSM projection (bottom image) represents the series of optical sections combined into a single three-dimensional rendition of the imaged volume of cartilage. The articular surface (AS) is oriented toward the top of the image, the osteochondral junction (OCJ) at the bottom of the image, and the tidemark is indicated by the block arrows. The blue-shaded area on the diagram represents the plane in which axial optical sections were obtained, imaging the superficial zone near the cut edge of cartilage. In the corresponding axial CLSM projection (top image), in situ chondrocyte death from the scalpel injury is seen as a band of red-stained nuclei at the cut edge (CE) of cartilage. Farther from the CE, the viable superficial zone chondrocytes in the AS are seen as bright green-stained cells (PI stains the nuclei of dead cells red; CMFDA stains the cytoplasm of live cells green, white bar = 100 μm). CLSM = confocal laser scanning microscopy PI = propidium iodide; CMFDA = 5-chloromethylfluorescein diacetate.
We determined the percentage cell death (100× number of dead cells/number of dead and live cells) in three-dimensional regions of interest (ROI) positioned on coronal and axial CLSM projections. For coronal CLSM projections (imaging all zones in the full thickness of cartilage), percentage cell death (PCDFT, where FT refers to full thickness) was quantified at 100-μm intervals from the articular surface downward in a ROI measuring 921 × 80 × 100 μm3 (x-y-z axes, respectively). The limit for PCDFT measurements was taken as 400 μm from the articular surface because the ROIs overlapped with the tidemark at greater depths into cartilage. For axial CLSM projections (imaging only the superficial zone), we determined percentage of cell death (PCDSZ, where SZ refers to the superficial zone) in a ROI measuring 921 × 200 × 60 μm3 (x-y-z axes, respectively) positioned at the injured cartilage edge. This ROI included the band of cell death at the scalpel-cut edge and the adjacent uninjured region of the articular surface. Objects (individual cells) in the green (live cells) and red (dead cells) channels in each ROI were identified by thresholding voxel (volumetric pixel) intensity, a validated and reproducible technique of automated computer-generated cell counting [1, 12, 13]. For this study, percentage thresholds of voxel intensity were set using a histogram of measured values for all objects identified in each channel. The upper limit was always 100% with minor adjustments of the lower limit (minimum 5%) to account for variations in cell dye loading, detector sensitivity, and noise between images. All cells touching the ROI were included in the counts and combined objects (neighboring cells in close proximity incorrectly identified as single objects) in the ROI separated. This protocol returned a list of measured objects in the green and red channels. When ordered by volume, objects in the green channel less than 500 μm3 and in the red channel less than 200 μm3 were attributed to background noise and excluded from the cell counts. Finally, the entire ROI was observed in three dimensions to adjust for any remaining background noise before the software program generated automated live and dead cell counts.
The rationale for using scalpel-injured, rectangular osteochondral explants to study the effects of medium osmolarity on chondrocyte death was reported previously [1]. CLSM allowed tissue imaging without physical sectioning (that is often necessary for conventional histologic analyses) and closely reflects the extent of in situ chondrocyte death in three dimensions at the mechanically injured cartilage edge. Quantification of in situ chondrocyte death from full-thickness (coronal imaging) and articular surface (axial imaging) perspectives, using intensity thresholding techniques for automated cell counting and large ROIs containing more than 1000 cells, provides objective, spatial definitions of the heterogeneous, zone-specific responses of in situ chondrocytes to mechanical injury.
All data are presented as means ± standard error and represent observations from at least three different animals for each statistical analysis. The data were normally distributed and parametric tests were used to compare observations between groups. Percentage cell death in the full thickness of articular cartilage (PCDFT) was quantified for injured explants as a function of increasing depth from the articular surface to determine the spatial distribution of in situ chondrocyte death after mechanical injury. Because chondrocyte death was localized to the superficial zone of articular cartilage, we quantified percentage cell death in the zone (PCDSZ) in axial CLSM projections, which exclusively imaged the superficial zone in an en face view, observing the band of cell death at the injured cartilage edge and the adjacent uninjured region of the articular surface. We determined the difference in percentage cell death in the superficial zone (PCDSZ) between explants exposed to increasing osmolarity (100 mOsm to 600 mOsm) of the 0.9% saline solution using an analysis of variance (ANOVA). Because this ANOVA included replicates of explants from the same animal, a two-way analysis was used with the treatment being a fixed factor and the animal from which the explant came being regarded as a random factor. We determined differences in PCDSZ between explants exposed to solutions of Hartmann’s and 0.9% saline using paired Student’s t-tests. For paired comparisons, values of replicates of an experiment from the same animal were averaged to obtain a single data point for each animal. We performed all statistical analyses using SPSS Version 13.0 (SPSS Inc, Chicago, IL).
Results
PCDFT indicated cell death mainly occurred in the superficial zone (approximately the first 100 μm from the articular surface) of injured cartilage for explants exposed to 100, 285, and 500 mOsm 0.9% saline solutions and the 255 mOsm Hartmann’s solution with relative sparing of the middle and deep zones (Figs. 3, 4). For explants exposed to the modified, high osmolarity (600 mOsm) preparations of 0.9% saline and Hartmann’s, PCDFT in the first 100 μm of the articular surface was similar to the PCDFT at depths greater than 100 μm (Figs. 3, 4).
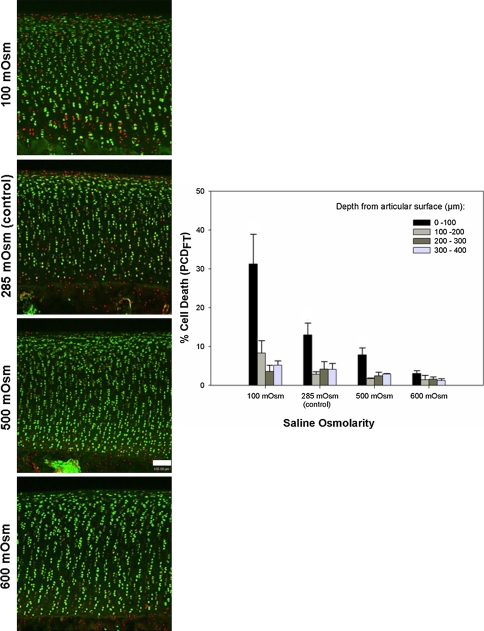
Fig. 3.
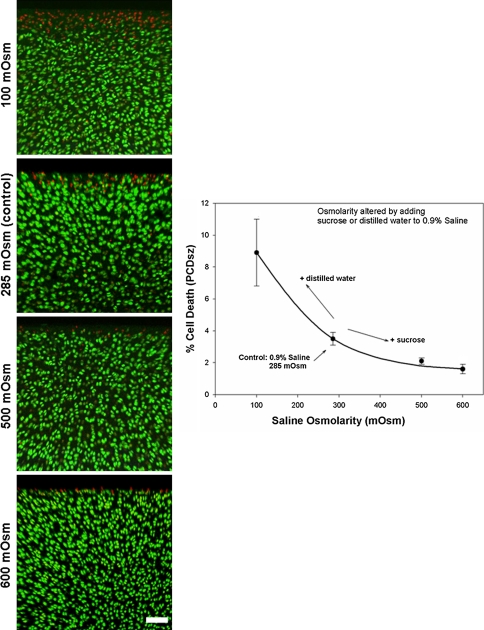
Coronal CLSM projections of the injured cartilage edge and PCDFT as a function of increasing saline osmolarity (100–600 mOsm) are shown. The panels show coronal CLSM projections of the full thickness of articular cartilage. The bar chart shows the corresponding pooled data for PCDFT as a function of increasing depth from the articular surface. In situ chondrocyte death is localized mainly near the articular surface (ie, superficial zone, 0- to 100-μm depth interval) for explants exposed to 100, 285 (control), and 500 mOsm saline solutions with relative sparing of the middle and deep zones. At 600 mOsm, there is a decrease in PCDFT in the 0- to 100-μm depth interval of injured articular cartilage. Cell death has been quantified only in hyaline cartilage tissue; the PI staining in the calcified layer of cartilage below the tidemark has not been included. (N = 3, n = 12, white bar = 100 μm). PCD = percentage cell death.
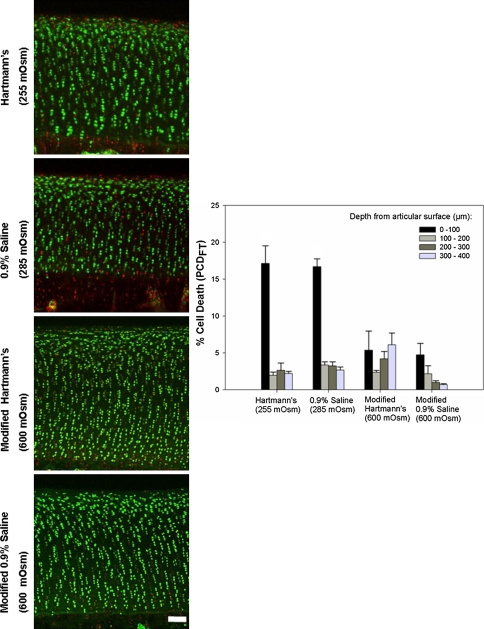
Fig. 4.
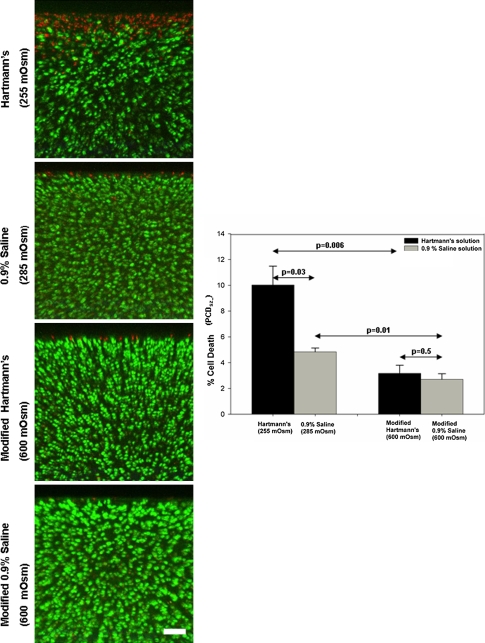
Coronal CLSM projections of the injured cartilage edge and PCDFT compare the extent of cell death between 0.9% saline and Hartmann’s solution. The panels show coronal CLSM projections of the full thickness of articular cartilage. The bar chart shows the corresponding pooled data for PCDFT as a function of increasing depth from the articular surface. In situ chondrocyte death is localized mainly near the articular surface (ie, superficial zone, 0- to 100-μm depth interval) for explants exposed to control solutions of Hartmann’s solution (255 mOsm) and 0.9% saline (285 mOsm) with relative sparing of the middle and deep zones. For explants exposed to the modified, high osmolarity (600 mOsm) of Hartmann’s solution and 0.9% saline, there is a decrease in PCDFT in the 0- to 100-μm depth interval of injured articular cartilage (N = 3, n = 12, white bar = 100 μm). CLSM = confocal laser scanning microscopy; PCD = percentage cell death.
PCDSZ decreased (p = 0.048) in explants exposed to increasing osmolarity (from 100 mOsm to 600 mOsm) of the 0.9% saline solution (N = 3, n = 36) (Fig. 5). Compared with the control explants exposed to Hartmann’s solution (255 mOsm) and 0.9% saline (285 mOsm), PCDSZ decreased (p = 0.006 for the Hartmann’s solutions; p = 0.01 for the saline solutions) for explants exposed to the modified, high osmolarity (600 mOsm) preparations of Hartmann’s and 0.9% saline (N = 3, n = 36) (Fig. 6).
Fig. 5.
Axial CLSM projections are shown of the injured cartilage edge and PCDSZ as a function of increasing saline osmolarity (100–600 mOsm). The panels show axial CLSM projections of the articular surface. The graph shows the corresponding pooled data for PCDSZ as a function of increasing saline osmolarity. The band of superficial zone chondrocyte death at the cut cartilage edge (PI-stained red nuclei) decreases for explants exposed to increasing osmolarity of the saline solution (N = 3, n = 36, white bar = 100 μm). CLSM = confocal laser scanning microscopy; PCD = percentage cell death; PI = propidium iodide.
Fig. 6.
Axial CLSM projections of the injured cartilage edge and PCDSZ compare the extent of cell death between 0.9% saline and Hartmann’s solution. The panels show axial CLSM projections of the articular surface. The bar chart shows the corresponding pooled data for PCDSZ as a function of the osmolarity of Hartmann’s solution and 0.9% saline. The band of superficial zone chondrocyte death at the cut cartilage edge decreases after increasing the osmolarity of Hartmann’s solution (255 mOsm) and 0.9% saline (285 mOsm) to 600 mOsm (N = 3, n = 36, white bar = 100 μm). CLSM = confocal laser scanning microscopy; PCD = percentage cell death.
PCDSZ was greater (p = 0.03) for explants exposed to the control solution of Hartmann’s solution (255 mOsm) compared with the control solution of 0.9% saline (285 mOsm), but there was no difference (p = 0.5) in PCDSZ between explants exposed to the modified, high osmolarity (600 mOsm) preparations of Hartmann’s solution and 0.9% saline (Fig. 6).
Discussion
Two commonly used joint solutions during arthroscopy, saline (0.9%, 285 mOsm) and Hartmann’s solution (255 mOsm), alter the extracellular osmolarity of in situ chondrocytes during articular surgery and ostensibly are associated with increased superficial zone chondrocyte death in the face of injury [1, 4]. We therefore asked the following questions: (1) What is the spatial distribution of in situ chondrocyte death in the full thickness of scalpel-injured articular cartilage exposed to solutions of 0.9% saline and Hartmann’s solution for 2.5 hours with the osmolarity varied between 100 mOsm and 600 mOsm? (2) Does varying the osmolarity of proprietary solutions of 0.9% saline (285 mOsm) and Hartmann’s (255 mOsm) influence in situ chondrocyte death in scalpel-injured articular cartilage at 2.5 hours? (3) Does the extent of in situ chondrocyte death differ between injured explants exposed to proprietary solutions of 0.9% saline and Hartmann’s and modified, high osmolarity (600 mOsm) preparations of the two solutions at 2.5 hours?
There are certain limitations to this study. First, caution must be exercised in extrapolating conclusions from an in vitro bovine cartilage system to the human clinical scenario, because the responses to mechanical injury and the osmotic sensitivity of in situ chondrocytes may differ [20]. In this model we used a single scalpel cut to represent a sharp mechanical injury to articular cartilage to (1) orientate cut edges in the same plane, avoiding variation in matrix damage and chondrocyte death that may be attributed to anisotropic properties and (2) allow a single scalpel cut to be made in push-through mode [7] minimizing variation in the applied force that potentially could influence the extent of cell death. Therefore, any variation in chondrocyte death from the mechanical injury was minimized between explants, allowing the effects of altering medium osmolarity on cell death to be studied with greater sensitivity. However, during open and arthroscopic surgery the magnitude and direction of the applied force during a surgical procedure on articular cartilage is likely to be more variable and consequently, the magnitude of any decrease in chondrocyte death from increasing medium osmolarity in vivo also may be different from that observed in vitro. Additionally, the effects of high osmolarity solutions on other tissue in synovial joints such as synovial lining and menisci are not known at present. However, the osmolarity of human synovial fluid ranges between approximately 350 and 450 mOsm [2], greater than the osmolarity of routinely used joint irrigation solutions (250–300 mOsm). Therefore, it is unlikely that high osmolarity solutions would have a negative effect on the soft tissues in synovial joints, although such investigation would be important before their use in vivo. Notwithstanding these limitations, such animal models form the basis for future in vivo research, and we believe the data may have implications for the design of irrigation solutions used during arthroscopic and open articular surgery—the decrease in chondrocyte death by increasing the osmolarity of irrigation solutions merits future investigation in human articular cartilage. Second, the extracellular osmolarity experienced by in situ chondrocytes has not been measured directly. Cartilage (with high fixed negative charges) contains a high concentration of free cations (mainly Na+) and a low concentration of free anions (mainly Cl−) compared with surrounding synovial fluid, and its interstitial osmolarity is greater with precise values determined by the local proteoglycan concentrations and the Gibbs-Donnan equilibrium conditions [23, 24]. As medium osmolarity increases, there is a corresponding increase in extracellular osmolarity in cartilage. This is evident from the reciprocal changes in chondrocyte volume that occur with alterations in medium osmolarity [3]. A mathematical calculation for estimating the extracellular osmolarity for in situ bovine articular chondrocytes as a function of medium osmolarity has been described [3]. Third, although we used a validated and reproducible computer-generated cell counting technique to quantify cell death [12, 13], there is always the potential for error in such an automated system. However, with automated analyses, the error is consistent between different images [12] and, therefore unlikely to affect eventual conclusions. In our experience of using automated cell counts, measured values of cell density [1] have been comparable to zone-specific measurements in bovine cartilage derived using histologic and stereologic quantitative techniques [26].
We found in situ chondrocytes in the superficial zone were most susceptible to the full-thickness cartilage injury. These findings confirm those in previous experiments with scalpel-injured cartilage [1] and after blunt injuries from impact and cyclical trauma [4, 5, 11, 17]. Furthermore, exposure of articular cartilage to modified, high osmolarity (600 mOsm) preparations of 0.9% saline and Hartmann’s solution decreased the extent of cell death in the superficial zone (Figs. 3, 4). These data suggest cells in the superficial zone are not only most susceptible to mechanical injury, but also most sensitive to the effects exerted by high osmolarity solutions. However, the precise mechanism responsible for the decrease in superficial zone chondrocyte death remains to be elucidated. The superficial zone has the highest permeability to solute and fluid [14, 15], and it is possible that by virtue of their location close to the articular surface, superficial zone chondrocytes may be most sensitive to the osmotic effects of a bathing solution. It follows that these cells also may derive the most benefit from any potential chondroprotective effect exerted by the osmolarity of the solution.
Our data suggest increasing the osmolarity of 0.9% saline and Hartmann’s solution decreases in situ chondrocyte death (in the superficial zone) after mechanical injury. We used sucrose for increasing the osmolarity of the solutions because the disaccharide is impermeable across animal cell membranes and is not metabolized by articular chondrocytes. An alternative would be to use sodium chloride to increase the osmolarity of the solutions because it has similar, but not identical, effects on matrix synthesis rates compared with sucrose when used at identical osmolarities [24]. The small difference is the result of differential effects of sucrose and sodium on intracellular composition [24]. Sucrose addition would decrease chondrocyte volume, thereby raising intracellular ion concentrations. When sodium chloride is used as an osmotic replacement, it is likely that in addition to a decrease in chondrocyte volume, there will be an additional increase in the intracellular sodium ion concentration potentially leading to altered activity of sodium-dependent membrane transporters, particularly those involved in intracellular pH regulation [25]. Thus, interpretation of the results using sucrose is easier because only chondrocyte volume is altered with minimal changes to cell sodium levels. These in vitro experiments also confirm that the decrease in chondrocyte death by increasing the osmolarity of standard culture media (DMEM) noted in previous studies [1, 4] can be reproduced using joint irrigation solutions in common use during articular surgery. These data strengthen the rationale for increasing the osmolarity of joint irrigation solutions to decrease chondrocyte death from mechanical trauma, for instance during débridement of cartilage defects, placement of intraarticular screws for articular fractures, or harvest of osteochondral plugs for transplantation using circular osteotomes.
Hartmann’s solution (255 mOsm) was associated with greater superficial zone chondrocyte death at the cut edge compared with 0.9% saline (285 mOsm) (Fig. 6). However, the difference in the extent of cell death between these two solutions was not completely accounted for by the lower osmolarity of the Hartmann’s solution. In addition to sodium chloride, Hartmann’s solution also contains 2 mmol/L of calcium as a chloride salt. The presence of calcium in Hartmann’s solution is believed to support chondrocyte metabolism better than solutions without added calcium such as 0.9% saline, which have been considered unphysiologic [18]. However, recent work suggests a reduction in calcium in the extracellular medium also decreases chondrocyte death after mechanical injury, possibly through the prevention of an increase in cytoplasmic calcium [10]. We suggest the greater chondrocyte death associated with Hartmann’s solution compared with 0.9% saline is not only the result of the lower osmolarity of the Hartmann’s solution, but also the result of the calcium present in its preparations. We observed no difference in the extent of cell death between Hartmann’s solution (255 mOsm) and 0.9% saline (285 mOsm) if the osmolarity of either solutions was increased to 600 mOsm, suggesting a higher osmolarity not only elicits a relatively greater beneficial effect for the Hartmann’s solution, but also negates the additional cell death that may be attributed to the elevated calcium.
Increasing the osmolarity of 0.9% saline (285 mOsm) and Hartmann’s solution (255 mOsm) to 600 mOsm decreases in situ chondrocyte death in a bovine model of mechanical cartilage injury. These experiments may have implications for the design of irrigation solutions used during articular surgery and merit future investigation in human articular cartilage.
Acknowledgments
We thank Dr. Martin Simmen for help with the medical statistics for the study.
Footnotes
One or more of the authors received funding from the Royal College of Surgeons of Edinburgh/Lorna Smith Charitable Trust (AKA) and the Wellcome Trust (075753/Z/04/Z) (ACH).
This study was performed at the Centre for Integrative Physiology, School of Biomedical Sciences, University of Edinburgh, UK.
References
- 1.Amin AK, Huntley JS, Bush PG, Simpson AH, Hall AC. Osmolarity influences chondrocyte death in wounded articular cartilage. J Bone Joint Surg Am. 2008;90:1531–1542. doi: 10.2106/JBJS.G.00857. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarten M, Bloebaum RD, Ross SD, Campbell P, Sarmiento A. Normal human synovial fluid: osmolarity and exercise-induced changes. J Bone Joint Surg Am. 1985;67:1336–1339. [PubMed] [Google Scholar]
- 3.Bush PG, Hall AC. The osmotic sensitivity of isolated and in situ bovine articular chondrocytes. J Orthop Res. 2001;19:768–778. doi: 10.1016/S0736-0266(01)00013-4. [DOI] [PubMed] [Google Scholar]
- 4.Bush PG, Hodkinson PD, Hamilton GL, Hall AC. Viability and volume of in situ bovine articular chondrocytes: changes following a single impact and effects of medium osmolarity. Osteoarthritis Cartilage. 2005;13:54–65. doi: 10.1016/j.joca.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J Orthop Res. 2003;21:888–898. doi: 10.1016/S0736-0266(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 6.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 7.Huntley JS, Bush PG, McBirnie JM, Simpson AH, Hall AC. Chondrocyte death associated with human femoral osteochondral harvest as performed for mosaicplasty. J Bone Joint Surg Am. 2005;87:351–360. doi: 10.2106/JBJS.D.02086. [DOI] [PubMed] [Google Scholar]
- 8.Huntley JS, McBirnie JM, Simpson AH, Hall AC. Cutting-edge design to improve cell viability in osteochondral grafts. Osteoarthritis Cartilage. 2005;13:665–671. doi: 10.1016/j.joca.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 10.Huser CA, Davies ME. Calcium signaling leads to mitochondrial depolarization in impact-induced chondrocyte death in equine articular cartilage explants. Arthritis Rheum. 2007;56:2322–2334. doi: 10.1002/art.22717. [DOI] [PubMed] [Google Scholar]
- 11.Jeffrey JE, Gregory DW, Aspden RM. Matrix damage and chondrocyte viability following a single impact load on articular cartilage. Arch Biochem Biophys. 1995;322:87–96. doi: 10.1006/abbi.1995.1439. [DOI] [PubMed] [Google Scholar]
- 12.Jomha NM, Anoop PC, Elliott JA, Bagnall K, McGann LE. Validation and reproducibility of computerised cell viability analysis of tissue slices. BMC Musculoskelet Disord. 2003;4:5. doi: 10.1186/1471-2474-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin G, Bjornsson CS, Smith KL, Abdul-Karim MA, Turner JN, Shain W, Roysam B. Automated image analysis methods for 3-D quantification of the neurovascular unit from multichannel confocal microscope images. Cytometry A. 2005;66:9–23. doi: 10.1002/cyto.a.20149. [DOI] [PubMed] [Google Scholar]
- 14.Maroudas A. Physiochemical properties of articular cartilage. In: Freeman MAR, ed. Adult Articular Cartilage. London, England: Pitman Medical; 1973:131–170.
- 15.Maroudas A, Bullough P, Swanson SA, Freeman MA. The permeability of articular cartilage. J Bone Joint Surg Br. 1968;50:166–177. [PubMed] [Google Scholar]
- 16.Milentijevic D, Helfet DL, Torzilli PA. Influence of stress magnitude on water loss and chondrocyte viability in impacted articular cartilage. J Biomech Eng. 2003;125:594–601. doi: 10.1115/1.1610021. [DOI] [PubMed] [Google Scholar]
- 17.Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: effects of strain rate and peak stress. J Orthop Res. 2001;19:242–249. doi: 10.1016/S0736-0266(00)00025-5. [DOI] [PubMed] [Google Scholar]
- 18.Reagan BF, McInerny VK, Treadwell BV, Zarins B, Mankin HJ. Irrigating solutions for arthroscopy: a metabolic study. J Bone Joint Surg Am. 1983;65:629–631. [PubMed] [Google Scholar]
- 19.Redman SN, Dowthwaite GP, Thomson BM, Archer CW. The cellular responses of articular cartilage to sharp and blunt trauma. Osteoarthritis Cartilage. 2004;12:106–116. doi: 10.1016/j.joca.2002.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Roach HI, Shearer JR, Archer C. The choice of an experimental model: a guide for research workers. J Bone Joint Surg Br. 1989;71:549–553. doi: 10.1302/0301-620X.71B4.2768295. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum. 2000;43:215–225. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Urban JP. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33:901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- 24.Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 25.Wilkins RJ, Hall AC. Measurement of intracellular pH in isolated bovine articular chondrocytes. Exp Physiol. 1992;77:521–524. [DOI] [PubMed]
- 26.Wong M, Wuethrich P, Eggli P, Hunziker E. Zone-specific cell biosynthetic activity in mature bovine articular cartilage: a new method using confocal microscopic stereology and quantitative autoradiography. J Orthop Res. 1996;14:424–432. doi: 10.1002/jor.1100140313. [DOI] [PubMed] [Google Scholar]