Abstract
Heptaplatin is a recently developed platinum derivative. This agent has been reported to have a response rate of 17% as a single agent, and tolerable toxicity in the treatment of advanced gastric cancer. The aim of this study was to evaluate the efficacy and toxicity of a combination of 5-fluorouracil (5-FU) and heptaplatin in patients with advanced gastric cancer. Forty-seven chemotherapy-naive patients with advanced or recurred gastric cancer were recruited. 5-FU was administered over 120 hr by continuous intravenous infusion from day 1 to 5, at a daily dose of 1,000 mg/m2 and heptaplatin was administered over 1 hr by intravenous infusion on day 1 at 400 mg/m2, and this cycle was repeated every 4 weeks. The response rate was 21%, median progression-free survival was 1.9 months (95% CI, 1.6 to 2.2 months). Median overall survival was 6.2 months (95% CI, 4 to 8.4 months) and the 1-yr survival rate was 29% for all patients. The most frequent toxicity was proteinuria. Toxicities were generally mild and reversible. This study demonstrates that the combination of 5-FU/heptaplatin combination is less active but tolerated in patients with advance gastric cancer.
Keywords: Drug Therapy, Fluorouracil, Stomach Neoplasms, Heptaplatin, Platinum Compounds
INTRODUCTION
Despite the reduction in the incidence of gastric cancer in most areas of the world, gastric cancer remains the second leading cause of cancer death worldwide (1, 2). Gastric cancer can be cured by complete surgical resection. Unfortunately, at the time of presentation patients often have advanced, unresectable disease, and therefore, become candidates for systemic chemotherapy. Based on the evidence of randomized chemotherapy trials versus best supportive care, chemotherapy confers benefits in quality of life and survival (3-5). However, there is no standard chemotherapy for gastric cancer (6).
Heptaplatin, cis-malonatol [(4R, 5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane] platinum(II) (SKI-2053R, Sunpla®, SK Chemicals, Kyungki-do, Korea) is a new drug of platinum derivatives. In vitro studies have shown that it has high antitumor activity against various cancer cell lines (7-9). A phase II study found that this agent had a response rate of 17% as a single agent and tolerable toxicity in advanced gastric cancer (10). Therefore, we conducted a study to evaluate the efficacy and toxicity of a combination of 5-fluorouracil (5-FU) and heptaplatin in patients with advanced gastric cancer.
MATERIALS AND METHODS
Forty-seven patients were enrolled between October 2001 and February 2003.
Patient selection
Patients with histologically confirmed, metastatic adenocarcinoma of the stomach were eligible. Recurred disease after gastric resection cases was also allowed but prior chemotherapy or radiotherapy was not permitted. At entry, patients were required to have measurable disease [i.e. with at least one diameter ≥2 cm, as assessed by physical or radiography examination including chest radiography or computed tomography scan] (11). The eligibility criteria were as follows: age ≤70 yr; performance status (PS) ≤3 according to the Eastern Cooperative Oncology Group (ECOG) criteria; adequate bone marrow function (absolute neutrophil count ≥1,500/µL, platelet count ≥100,000/µL), adequate hepatic function (bilirubin level <two times the upper normal limit, AST and/or ALT <three times the upper normal limit) and adequate renal function (creatinine <1.5 mg/dL). Patients with uncontrolled infections, medical instability, pregnancy, or brain metastases were excluded from the study. The protocol was approved by the institutional review board of the Ulsan University Hospital, and all patients gave written informed consent.
Treatment Schedule
5-FU was administered over 120 hr of continuous intravenous infusion from day 1 to 5, at a daily dose of 1,000 mg/m2 and heptaplatin was administered over 1 hr by intravenous infusion on day 1 at a dose of 400 mg/m2, and this cycle was repeated every 4 weeks. Prehydration with 1.5 L of normal saline mixed with 20 mEq KCl and 8 mEq MgSO4 was infused prior to the heptaplatin administration and an additional 1.5 L was given after administration. Ondansetron and metoclopropamide were given as an antiemetic prophylaxis. The dose of 5-FU was reduced by 25% for ≥grade 3 toxicities (except alopecia) and cycles were delayed until resolved to grade 2 toxicity. The dose of heptaplatin was reduced by 50% if the serum creatinine was between 1.6 and 2.5 mg/dL, and was omitted if the serum creatinine still remained greater than 2.5 mg/dL after a delay of 1 week.
Pretreatment and follow-up studies
Before entry onto the study, all patients were required to have a physical examination, chest radiography, abdomen computed tomographic scan, complete blood counts (CBC), biochemical tests, urine analysis with microscopy and an ECG. A physical examination, CBC, biochemical tests and urine analysis with microscopy were checked before each cycle and days 14 after each cycle of therapy.
Response and Toxicity Evaluation
Patients who received at least two cycles of treatment were assessable for response unless they had definitive evidence of progression after the first cycle. Patients who had received at least one cycle of treatment were assessed for toxicity. Responses were graded according to the RECIST criteria (11). Complete remission (CR) was defined as the disappearance of all known lesions, the absence of any new lesions and the normalization of tumor markers for at least 4 weeks. Partial remission (PR) was defined as a decrease of 30% or greater in the sum of the longest diameters of the target lesions from baseline, non-progressive disease in the non-target lesion, and no new lesions. Progressive disease (PD) was defined as at least a 20% increase in the sum of the longest diameters of the target lesions or the appearance of new lesions. Stable disease (SD) was defined as an insufficient decrease in size in tumor to qualify for PR or an insufficient increase in size to qualify for PD. Toxicity grading was performed in accordance with WHO toxicity criteria.
Survival curves were constructed using the Kaplan-Meier methods and compared using the log-rank test. Analysis was performed using the SPSS 8.0 system (SPSS, Inc, Chicago, IL, U.S.A.). A p value of less than 0.05 was considered statistically significant.
RESULTS
The patient characteristics are listed in Table 1. Median patients age was 53 yr (range, 22-70). Thirty-nine patients (83%) had metastatic disease and 8 patients (13%) had recurred disease after primary curative resection. All patients received at least one cycle of chemotherapy.
Table 1.
Patient characteristics (n=47)
Treatment, response and survival
A total of 120 cycles was administered. The median number of cycles per patient was two (range, 1-9). Seventeen patients (36%) were considered non-assessable for response: 3 lost to follow up, 5 withdrawn voluntarily, 6 had chemotherapy-induced toxicity, including 5 with nausea and vomiting and 1 with renal impairment, and there were 3 early deaths due to gastric bleeding. Thus, a total of 30 patients (64%) were assessable for response.
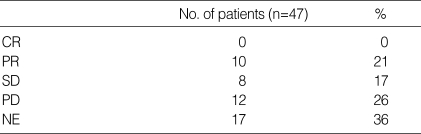
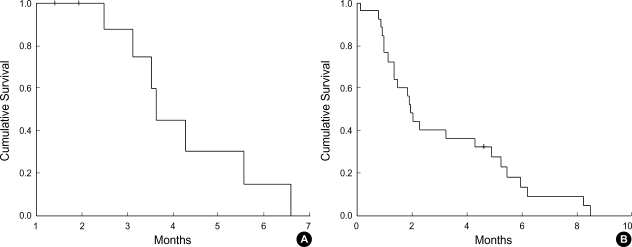
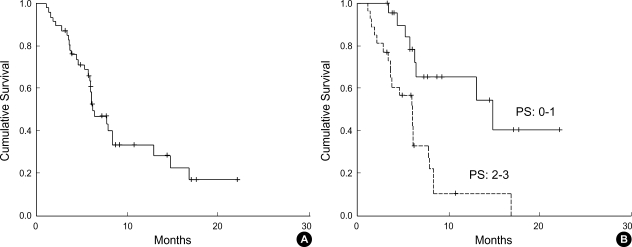
Partial responses were reported in 10 patients, resulting in an overall response rate of 21% in the intension-to-treat population (Table 2). There was no case of CR, 17% achieved SD and 26% PD. Median duration of response was 3.6 months (95% confidence interval (CI), 3.4 to 3.9 months) (Fig. 1A). Median progression-free survival was 1.9 months (95% CI, 1.6 to 2.2 months) (Fig. 1B). Median overall survival was 6.2 months (95% CI, 4 to 8.4 months), and the 1 yr survival rate for all patients was 29% (Fig. 2A). Patients with a PS of 0 or 1 showed significantly better survival than those with a PS of 2 or 3 (1-yr survival rate: 54% vs. 0%, p=0.001) (Fig. 2B). However, ages, sex, and metastatic sites did not affect overall survival.
Table 2.
Objective response*
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable.
*Intention-to-treat analysis.
Fig. 1.
(A) Duration of response, (B) Progression free survival.
Fig. 2.
(A) Overall survival, (B) Effect of performance status on overall survival.
Toxicities
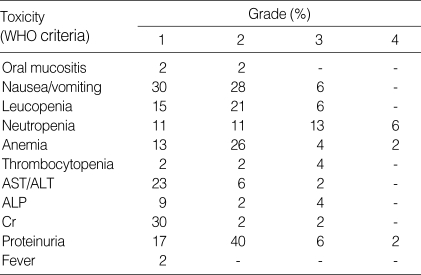
The hematologic and nonhematologic toxicities were evaluated in 44 patients, and are shown in Table 3. Hematologic toxicities were mild. Neutropenia (grade 3-4) and anemia (grade 3-4) appeared in 19% and 6% of the patients, respectively. 4% of the patients experienced grade 3 thrombocytopenia. Nonhematologic toxicities were also mild. However, nausea and vomiting occurred frequently (64%) and grade 3 toxicity occurred in 6% of patients. Azotemia was observed in 34% of the patients with 2% (grade 3). Proteinuria appeared frequently (65%) and 8% of the patients showed grade 3-4 toxicity. Observed renal insufficiency was reversible.
Table 3.
Frequency of toxicity
DISCUSSION
This study based on a continuous infusion of 5-FU with heptaplatin (FH) demonstrated a response rate of 21% in all patients with advanced gastric cancer who had not previously received chemotherapy. Median progression-free survival and overall survival were 1.9 months and 6.2 months, respectively. Even though no standard regimens have yet been established, infusional 5-FU-based regimens have shown promise in combinations in the treatment of gastric cancer (12-14).
Despite the activity of cisplatin as a single agent in first-line chemotherapy for gastric cancer has not been well defined. Nevertheless, cisplatin is one of the most common agents used for the treatment of gastric cancer (12-17). In particular, the regimen of a continuous 5-day infusion of 5-FU with cisplatin (FP) produced response rates of 41% and 43%, with a median survival of 10.6 and 9 months in nonrandomized phase II studies (12, 13). Heptaplatin is a recently developed platinum derivative. This agent had shown a response rate of 17% as a single agent and tolerable toxicity in the treatment of advanced gastric cancer (10).
In the present study, the FH regimen showed inferior results in terms of response rate and survival when compared with the two studies. Otherwise, the FH regimen showed favorable profiles in terms of toxicities. Firstly, the hematologic toxicities of the FH regimen were similar to those found in the two trials with the FP regimen. The most common hematologic toxicity was anemia. Grade 3-4 anemia and grade 3-4 neutropenia occurred in 6% and 19% of patients, respectively. Secondly, gastrointestinal toxicities of the FH regimen were mild. Grade 3 nausea and vomiting only occurred in 6% of patients. Otherwise, 22% and 36.5% of patients had grade 3-4 nausea and vomiting in the two trials with FP regimen. Azotemia was also mild with 4% of patients having grade 2-3 toxicities, but proteinuria was frequent. Proteinuria was already found to be the most remarkable toxicity of the FH regimen (10, 18), 65% of patients had proteinuria with 8% of patients having grade 3-4 toxicities. A phase III study, which compared the toxicity of heptaplain and cisplatin found that nephrotoxicity was more severe in patients treated with heptaplatin than in those treated with cisplatin (18). Further efforts to detect and minimize the nephrotoxicity of heptaplatin seem to be warranted.
One of the limitations in this study is that 36% of patients were non-assessable for response. So, the result of this study do not allow a conclusion to be drawn on difference between the FH and the FP regimens in terms of efficacy or toxicity for the treatment of advanced gastric cancer. The Korean Cancer Study Group (KCSG) has initiated a randomized phase III study in patients with advanced gastric cancer, which incorporates a comparison between FP and FH.
In conclusion, our study shows that the 5-FU/heptaplatin combination is less active but tolerable by patients with advanced gastric cancer.
ACKNOWLEDGEMENT
Authors thank Geum Suk Kim, a research nurse, for her assistance in data collection.
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 3.Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995;71:587–591. doi: 10.1038/bjc.1995.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Rausch M. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 5.Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H, Heuman R. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–168. doi: 10.1023/a:1008243606668. [DOI] [PubMed] [Google Scholar]
- 6.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14(Suppl 2):ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 7.Kim DK, Kim G, Gam J, Cho YB, Kim HT, Tai JH, Kim KH, Hong WS, Park JG. Synthesis and antitumor activity of a series of [2-substituted-4,5-bis(aminomethyl)-1,3-dioxolane]platinum(II) complexes. J Med Chem. 1994;37:1471–1485. doi: 10.1021/jm00036a013. [DOI] [PubMed] [Google Scholar]
- 8.Kim DK, Kim HT, Cho YB, Tai JH, Ahn JS, Kim TS, Kim KH, Hong WS. Antitumor activity of cis-malonato[(4R, 5R)-4,5 bis(aminomethyl)-2-isopropyl-1,3-dioxolane]platinum(II), a new platinum analogue, as an anticancer agent. Cancer Chemother Pharmacol. 1995;35:441–445. doi: 10.1007/s002800050260. [DOI] [PubMed] [Google Scholar]
- 9.Kim HT, Kim DK, Cho YB, Kim TS, Jung I, Kim KH, Heo DS, Bang YJ, Shin SG, Kim NK. Influence of exposure and infusion times on the cytotoxicity and pharmacokinetics of cis-malonato [(4R, 5R)-4,5-bis(aminomethyl)-2-isopropyl-1,3-dioxolane]platinum(II) Cancer Chemother Pharmacol. 1998;41:109–116. doi: 10.1007/s002800050716. [DOI] [PubMed] [Google Scholar]
- 10.Kim NK, Im SA, Kim DW, Lee MH, Jung CW, Cho EK, Lee JT, Ahn JS, Heo DS, Bang YJ. Phase II clinical trial of SKI-2053R, a new platinum analog, in the treatment of patients with advanced gastric adenocarcinoma. Cancer. 1999;86:1109–1115. doi: 10.1002/(sici)1097-0142(19991001)86:7<1109::aid-cncr3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. J Nat Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Lacave AJ, Barón FJ, Antón LM, Estrada E, De Sande LM, Palacio I, Esteban E, Gracia JM, Buesa JM, Fernández OA, Barón MG. Combination chemotherapy with cisplatin and 5-fluorouracil 5-day infusion in the therapy of advanced gastric cancer: a phase II trial. Ann Oncol. 1991;2:751–754. doi: 10.1093/oxfordjournals.annonc.a057858. [DOI] [PubMed] [Google Scholar]
- 13.Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J, Bognel C, Lasser P, Ychou M, Elias D, Cvitkovic E, Armand JP, Droz JP. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas: a phase II trial with prognostic factor analysis. Eur J Cancer. 1994;30:1263–1269. doi: 10.1016/0959-8049(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 14.Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, Hughes M, Mansi J, Findlay M, Hill A, Oates J, Nicolson M, Hickish T, O'Brien M, Iveson T, Watson M, Underhill C, Wardley A, Meehan M. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 15.Preusser P, Wilke H, Achterrath W, Fink U, Lenaz L, Heinicke A, Meyer J, Meyer HJ, Buente H. Phase II study with the combination etoposide, doxorubicin, and cisplatin in advanced measurable gastric cancer. J Clin Oncol. 1989;7:1310–1317. doi: 10.1200/JCO.1989.7.9.1310. [DOI] [PubMed] [Google Scholar]
- 16.Cascinu S, Labianca R, Alessandroni P, Marcellini M, Silva RR, Pancera G, Testa E, Martignoni G, Barni S, Frontini L, Zaniboni A, Luporini G, Cellerino R, Catalano G. Intensive weekly chemotherapy for advanced gastric cancer using fluorouracil, cisplatin, epi-doxorubicin, 6S-leucovorin, glutathione, and filgrastim: a report from the Italian group for the study of digestive tract cancer. J Clin Oncol. 1997;15:3313–3319. doi: 10.1200/JCO.1997.15.11.3313. [DOI] [PubMed] [Google Scholar]
- 17.Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish T, Nicolson V, Nash A, Sacks N, Ford H, Carter R, Hill A. A phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF) Ann Oncol. 1994;5:609–616. doi: 10.1093/oxfordjournals.annonc.a058932. [DOI] [PubMed] [Google Scholar]
- 18.Ahn JH, Kang YK, Kim TW, Bahng H, Chang HM, Kang WC, Kim WK, Lee JS, Park JS. Nephrotoxicity of heptaplatin: a randomized comparison with cisplatin in advanced gastric cancer. Cancer Chemother Pharmacol. 2002;50:104–110. doi: 10.1007/s00280-002-0483-x. [DOI] [PubMed] [Google Scholar]