Abstract
Recently, It has been reported that the LDL receptor-related protein 5 (LRP5) regulates bone formation, and that mutations of the gene cause osteoporosis-pseudoglioma syndrome or high bone mass phenotypes. However, the mutations cannot explain a genetic trait for osteoporosis in the general population because of their rarity. From 219 Korean men aged 20-34 yr, we looked for six known polymorphisms causing amino acid changes in the LRP5 coding region, and investigated their association with bone mineral density (BMD) at the following anatomical sites: lumbar spine (L2-L4) and the left proximal femur (femoral neck, Ward's triangle, trochanter and shaft). We found that the Q89R polymorphism was significantly associated with BMD at the femoral neck and Ward's triangle (p=0.004 and <0.001, respectively). However, after adjusting for age, weight and height, a statistically significant association only occurred at the Ward's triangle (p=0.043), and a marginal association was observed at the femoral neck (p=0.098). No A400V, V667M, R1036Q and A1525V polymorphisms were found, and no statistically significant association was found between the A1330V polymorphism and BMD at any sites. Although we failed to demonstrate a clear association between the LRP5 polymorphism and peak bone mass in young men, the present study suggests that larger-scale studies on the Q89R polymorphism need to be performed.
Keywords: LDL-Receptor Related Proteins, Polymorphism (Genetic), Osteoporosis, Bone Density, Men
INTRODUCTION
Although osteoporosis is more generally considered as a disease that afflicts women, it is also prevalent in men. The incidence of osteoporotic fractures in men is about half of that in women (1). Furthermore, the mortality associated with hip fracture in elderly men is considerably higher than in women (2). However, the cellular and molecular basis underlying male osteoporosis is still poorly understood due to the emphasis on osteoporosis in women (3).
Peak bone mass, which is recognized as an important determinant in development of osteoporosis (4), is under strong genetic control, and genetic influences have been estimated to account for about 75% of the inter-individual variability in peak bone mass (5). Although many genes have been examined for their association with bone mass, most studies were carried out in either women or populations of older subjects of an age when accretion of bone mass has ceased and bone loss has begun (6). Genetic studies in men with peak bone mass are relatively rare (3).
LDL receptor-related protein 5 (LRP5) has emerged as a key regulator of osteoblast proliferation and bone formation (7, 8). Loss-of-function mutations of the LRP5 gene cause the autosomal recessive osteoporosis pseudoglioma syndrome (OPPG), a disorder causing both congenital osteoporosis and eye abnormalities (7), while autosomal dominant high bone mass (HBM) traits result from a gain-of-function mutation of the gene that causes a glycine-to-valine amino acid change (G171V) (9-11). Collectively, these studies suggest that the LRP5 gene is an important regulator of peak bone mass in vertebrates, although genetic mutations causing OPPG syndrome or HBM phenotype are very rare in the general population (7, 11).
It has been suggested that a common polymorphism in the LRP5 gene might contribute to genetic variations in bone mass in the general community (12). Torus palatinus, which was observed in subjects with the G171V mutation of the LRP5 gene, is commonly detected in the general population (13, 14), and this was reported to serve as a physical marker of high bone mineral density (BMD) in Caucasians (13) and Japanese (15). More importantly, several quantitative trait locus (QTL) analyses reported that the region 11q12-13, which contains the LRP5 gene, strongly contributes to the normal variation of BMD in the general population (16, 17). However, there is no information on whether LRP5 polymorphisms are associated with BMD in the general community.
Recently, two mutation analyses reported seven and forty-two LRP5 gene polymorphisms in Japanese (18) and Caucasians (19), respectively. In the present study, we examined LRP5 gene coding region polymorphisms in young Korean men. We determined the frequency of occurrence of six polymorphisms causing amino acid changes, and investigated whether these polymorphisms were associated with BMD.
MATERIALS AND METHODS
Subjects
The study population comprised 219 healthy young men who were medical students of University of Ulsan or residents at a university hospital (Asan Medical Center (AMC)) in Seoul, Korea. All subjects were Korean and volunteered for the study. The study was approved by the AMC ethics review committee and written informed consent was obtained from all subjects. Subjects completed a self-administered questionnaire concerning demographic characteristics, general health status, medication, weekly duration of weight-bearing physical activity, and smoking and alcohol drinking habits. Daily dietary calcium intake was assessed using a food frequency questionnaire (20, 21). All subjects were free from drugs and diseases known to affect bone metabolism.
Bone mineral densitometry
All BMD (g/cm2) measurements of the lumbar spine (L2-L4, A-P) and the left proximal femur (femoral neck, Ward's triangle, trochanter and shaft) were measured by the same trained technician using a Lunar Corp. Expert XL (Lunar, Madison, WI, U.S.A.) dual energy radiography absorptiometer (software version 1.90). The in vivo precision of the machine was 0.82% for the lumbar spine, 1.12% for the femoral neck and 2.17% for Ward's triangle.
Genotype analysis with polymerase chain reaction and restriction fragment length polymorphism analysis
Genomic DNA was extracted from peripheral blood leukocytes using a commercial kit (Wizard Genomic DNA purification kit, Promega, Madison, WI, U.S.A.). Genotyping of DNA sequence variants in the Q89R and A1330V sites was carried out by restriction enzyme analysis of polymerase chain reaction (PCR)-amplified DNA using specific pairs of oligonucleotide primers, as described previously (18). The PCR primers were sequence-specific and/or mutated for convenience. Amplification of polymorphisms was carried out using a thermocycler (Perkin-Elmer, Boston, MA, U.S.A.) using a method described previously with some modifications (18). After amplification, PCR products were digested with restriction endonucleases AvaII and DraIII (New England Biolabs Inc., Beverly, MA, U.S.A.) for the Q89R and A1330V polymorphisms, respectively. The DNA fragments were then electrophoresed on 2% and 3% agarose gels for the Q89R and A1330V polymorphisms, respectively.
Because previously reported restriction enzyme analyses of polymerase chain reaction (PCR) were not available, genotyping for A400V, V667M, R1036Q and A1525V polymorphisms was performed by SNP-IT™ assays using the SNP stream 25K™ System (Orchid Biosciences, New Jersey, U.S.A.) (22). Briefly, the genomic DNA region spanning the polymorphic site was PCR-amplified using one phosphothiolated primer and one regular PCR primer. The amplified PCR products were then digested with exonuclease, with the 5' phosphothiolates protecting one strand of the PCR product from digestion. The single-strand PCR template generated by exonuclease digestion was overlaid onto a 384-well plate that was precoated covalently with the primer extension SNP-IT™ primers. These SNP-IT™ primers were designed to hybridize immediately adjacent to the polymorphic site. The PCR primer sequences are listed in Table 1. After hybridization of template strands, SNP-IT™ primers were then extended by a single base with DNA polymerase at the polymorphic site of interest. The extension mixtures contained two labeled terminating nucleotides (one FITC, one biotin) and two unlabeled terminating nucleotides. The final single base incorporated was identified with serial colorimetric reactions with anti-FITC-AP or streptavidin-HRP. The results of blue and/or yellow color developments were analyzed using an ELISA reader, and the final genotyping (allele) calls were made using the QCReview™ program. To confirm polymorphisms identified using SNP-IT™ assays, direct DNA sequencing was performed on 20 samples using a fluorescence-based automated DNA sequencer, as previously described (19). The PCR primer sequences used for direct sequencing are listed in Table 1. The PCR products were directly sequenced on both strands using an ABI Prism 377 DNA sequencer (Perkin Elmer, CA, U.S.A.). No discrepancies were found between direct sequencing data and SNP-IT™ data (data not shown).
Table 1.
Primer sequences for SNP-IT™ assays and for direct sequencing within the LDL receptor-related protein 5 gene
Statistical analysis
Descriptive characteristics are expressed as mean and standard deviation. The independent contribution of the clinical characteristics to BMD was determined using multiple linear regression analysis. Linkage disequilibrium between genotypes and significance of deviation from the Hardy-Weinberg equilibrium were analyzed by the χ2 test. Haplotypes were constructed using the E-M algorithm (Arlequin, http://anthro.unige.ch/arlequin/). The effect of single or combined genotypes on BMD was evaluated using either unpaired t-tests or one-way analyses of variance. Multiple linear regression analyses were performed to adjust for risk factors, with BMD as a dependent variable, and genotypes, age, weight and height each being independent variables. Unless indicated otherwise, p values less than 0.05 were considered significant. The SPSS 10.0 program (SPSS Inc., Chicago, IL, U.S.A.) was used for statistical procedures.
RESULTS
Subject characteristics
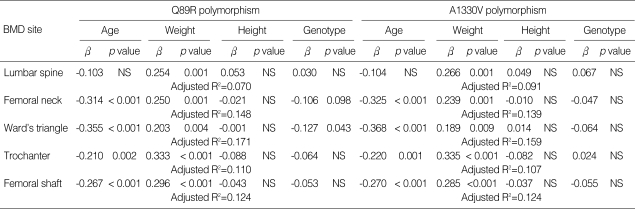
The mean age of participants was 25.6±3.7 yr (range 20-34 yr). Body mass index was 22.9±2.8 kg/m2 (range 17.2-32.1 kg/m2). Multiple linear regression analysis showed a positive association between body weight and BMD at all anatomical sites: β=0.275, p=0.001 at the lumbar spine; β=0.253, p=0.001 at the femoral neck; β=0.216, p=0.004 at the Ward's triangle; β=0.329, p<0.001 at the trochanter; β=0.299, p<0.001 at the femoral shaft. Age was negatively correlated with BMD at the proximal femur, with regression analysis showing β=-0.312, p<0.001 at the femoral neck; β=-0.349, p<0.001 at the Ward's triangle; β=-0.192, p=0.010 at the trochanter; and β=-0.223, p=0.003 at the femoral shaft. There was no association between BMD and height, calcium intake, smoking, alcohol consumption or exercise (data not shown).
Genotype frequencies
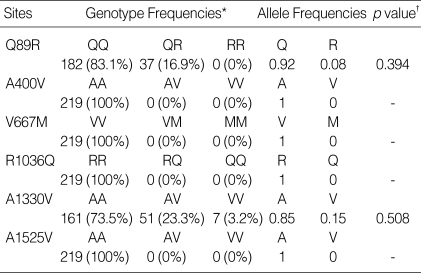
The genotype distributions and allele frequencies for LRP5 gene polymorphisms are presented in Table 2. No polymorphisms of the A400V, V667M, R1036Q or A1525V type were observed. These data differ from those obtained from Caucasian subjects (19). We detected Q89R and A1330V polymorphisms, and these occurred at allelic frequencies comparable to those previously reported in Japanese studies (18). The Q89R and A1330V polymorphisms were in Hardy-Weinberg equilibrium. Because the number of subjects bearing the VV genotype of the A1330V polymorphism was so small (n=7), we compared those carrying the V allele (AV or VV) with those that did not (AA). Contradictory to the previous result suggesting that the two polymorphisms were in another linkage disequilibrium blocks in European (23), the distribution of combination genotypes indicated that the two polymorphisms were in linkage disequilibrium in our subjects (χ2=19.526, p<0.001). The haplotype frequencies were: QA, 357 (81.5%); QV, 44 (10.0%); RA, 16 (3.7%); RV, 21 (4.8%). Combined genotypes were constructed from the haplotypes and their frequencies were: QA-QA, 145 (66.2%); QA-QV, 32 (14.6%); QA-RA, 16 (7.3%); QA-RV, 19 (8.7%); QV-QV, 5 (2.3%); QV-RV, 2 (0.9%).
Table 2.
Genotype distribution and allele frequencies of polymorphisms of the LDL receptor-related protein 5 gene
*Values represent number of times the allele was detected (% of all subjects). †Deviation from the Hardy-Weinberg equilibrium as determined by χ2 test. A p value >0.05 indicates the genotypes are in Hardy-Weinberg equilibrium.
Association between LRP5 gene polymorphisms and BMD
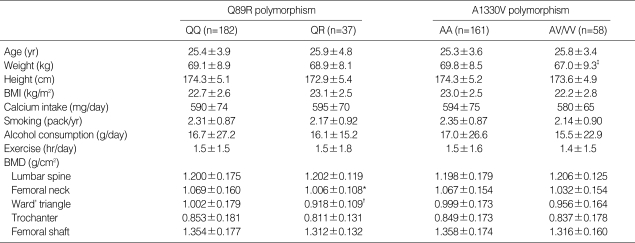
The study population data were separated into two categories on the basis of Q89R or A1330V polymorphisms in the LRP5 gene. No significant differences in any clinical parameters were observed between the two groups (Table 3). However, subjects with the QQ genotype had significantly higher BMD at the femoral neck and Ward's triangle, compared with those with the QR genotype (p=0.004 and p<0.001, respectively). Lumbar BMD was not different between the two groups. Multiple linear regression analyses were undertaken in order to adjust confounding variables such as age, weight and height. The Q89R polymorphism was significantly associated with BMD at the Ward's triangle (p=0.043), and marginally associated at the femoral neck (p=0.098) (Table 4).
Table 3.
Comparison of clinical characteristics and BMD (g/cm2). The study population was separated into two groups on the basis of having either the Q89R or A1330V polymorphism in the LDL receptor-related protein 5 gene
All values are mean±SD. Differences in clinical characteristics between the two groups were tested using unpaired t-tests, where: *p<0.005 vs. QQ; †p<0.001 vs. QQ; ‡p<0.05 vs. AA.
Table 4.
Multiple linear regression analysis with BMD as the dependent variable, and age, weight, height and genotypes as the independent variables
*NS denotes not significant.
When the study subjects were divided into two groups according to the presence of the V allele of the A1330V polymorphism, those with the V allele (AV or VV genotype) had lower body weight than those without (AA genotype) (p=0.035, Table 3). Other clinical parameters were similar between the two groups (data not shown). No differences in BMD values at any sites were noted between these two groups both before (Table 3) and after (Table 4) adjusting for confounding variables.
We also examined effects of the combined genotypes on BMD. The subjects were divided into three groups according to the number of QA haplotypes: (QA haplotype=2)=(1, 1), (QA haplotype=1)=(1, 0), and (QA haplotype=0)=(0, 0). No associations between the combined genotypes and BMD were noted at any sites before or after adjusting for confounding variables (data not shown).
DISCUSSION
To our knowledge, this is the first study examining the association between LRP5 gene polymorphisms and BMD in the general population. We observed a statistically significant association between the Q89R polymorphism and BMD at Ward's triangle after adjusting for confounding variables. This association may have to be viewed with circumspection. The precision error for Ward's triangle measurement is considerably higher than for other regions of interest (24, 25). Further, the association was not noted at the lumbar spine, which has similar compositions of cortical and trabecular bone to the Ward's triangle. These suggest that the significant association may result from false positive one. However, the association was suggested at the femoral neck, albeit marginal (p=0.098). Therefore, we cannot exclude a possibility that this somewhat weak association is due to the small sample size providing insufficient statistical power to show modest but statistically significant associations.
The association of the Q89R polymorphism with BMD appears to be further supported by the position at which this polymorphism is situated in the LRP5 gene. The LRP5 gene contains 23 exons and spans >100 kb (26). The LRP5 protein consists of a distinct ensemble of five structural motifs-four structures resembling a propeller with six blades containing YWTD spacer repeat domains, epidermal growth factor-like repeats, an LDL receptor-like ligand-binding domain, a single transmembrane domain, and a short cytoplasmic tail (27, 28). The Q89R polymorphism is located in exon 2 encoding the first of four propellers, while the A1330V polymorphism is in exon 18 encoding the LDL receptor-like domain. Although the precise function of each region is uncertain, the four propellers are of structural importance. It has been suggested that mutation in the first propeller region can alter the local hydrophobic environment, thus possibly affecting the interaction of LRP5 with other proteins (11). In addition, a number of mutations in this region in the LDL receptor propeller module can cause familial hypercholesterolemia (28), confirming that this domain is important for protein function. These suggest a possibility that the Q89R or related linked polymorphisms coding the region might alter LRP5 protein function and might be associated with BMD, suggesting need for further studies on functional differences of these polymorphisms in relation to bone metabolism.
In the present study we observed different genetic backgrounds between Koreans and Caucasians with respect to allele frequencies of LRP5 gene polymorphisms. The allele frequencies of the A400V, V667M, R1036Q and A1525V polymorphisms in Caucasians were 2-8% (19), whereas no such polymorphisms were found in our subjects. More importantly, the Q89R polymorphism was very rare in Caucasians (19, 29), whereas the polymorphism was prevalent in Japanese (18) and our Korean subjects. These data suggest that the Q89R polymorphism would not be a useful marker for bone mass in Caucasians.
In conclusion, we failed to demonstrate a clear association between the LRP5 polymorphism and peak bone mass in young men. However, the present study suggests that larger-scale studies on the Q89R polymorphism are needed.
Footnotes
This work was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health & Welfare, Republic of Korea (Project No. 01-PJ3-PG6-01GN11-0002).
References
- 1.Cooper C, Atkinson EJ, O'Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 2.Myers AH, Robinson EG, Van Natta ML, Michelson JD, Collins K, Baker SP. Hip fractures among the elderly: factors associated with in-hospital mortality. Am J Epidemiol. 1991;134:1128–1137. doi: 10.1093/oxfordjournals.aje.a116016. [DOI] [PubMed] [Google Scholar]
- 3.Byers RJ, Hoyland JA, Braidman IP. Osteoporosis in men: a cellular endocrine perspective of an increasingly common clinical problem. J Endocrinol. 2001;168:353–362. doi: 10.1677/joe.0.1680353. [DOI] [PubMed] [Google Scholar]
- 4.Newton-John HF, Morgan DB. The loss of bone with age, osteoporosis, and fractures. Clin Orthop. 1970;71:229–252. [PubMed] [Google Scholar]
- 5.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 6.Ralston SH. Genetic control of susceptibility to osteoporosis. J Clin Endocrinol Metab. 2002;87:2460–2466. doi: 10.1210/jcem.87.6.8621. [DOI] [PubMed] [Google Scholar]
- 7.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, Van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML Osteoporosis-Pseudoglioma Syndrome Collaborative Group. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 8.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- 10.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 11.Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel MS, Karsenty G. Regulation of bone formation and vision by LRP5. N Engl J Med. 2002;346:1572–1574. doi: 10.1056/NEJM200205163462011. [DOI] [PubMed] [Google Scholar]
- 13.Belsky JL, Hamer JS, Hubert JE, Insogna K, Johns W. Torus palatinus: a new anatomical correlation with bone density in postmenopausal women. J Clin Endocrinol Metab. 2003;88:2081–2086. doi: 10.1210/jc.2002-021726. [DOI] [PubMed] [Google Scholar]
- 14.Kolas S, Halperin V, Jerreris K, Huddleston S, Robinson HB. The occurrence of torus palatinus and torus mandibularis in 2,478 dental patients. Oral Surg Oral Med Oral Pathol. 1953;6:1134–1141. doi: 10.1016/0030-4220(53)90225-4. [DOI] [PubMed] [Google Scholar]
- 15.Hosoi T, Yoda T, Yamaguchi M, Amano H, Orimo H. Elderly women with oral exostoses had higher bone mineral density. J Bone Miner Metab. 2003;21:120–122. doi: 10.1007/s007740300020. [DOI] [PubMed] [Google Scholar]
- 16.Koller DL, Rodriguez LA, Christian JC, Slemenda CW, Econs MJ, Hui SL, Morin P, Conneally PM, Joslyn G, Curran ME, Peacock M, Johnston CC, Foroud T. Linkage of a QTL contributing to normal variation in bone mineral density to chromosome 11q12-13. J Bone Miner Res. 1998;13:1903–1908. doi: 10.1359/jbmr.1998.13.12.1903. [DOI] [PubMed] [Google Scholar]
- 17.Koller DL, Econs MJ, Morin PA, Christian JC, Hui SL, Parry P, Curran ME, Rodriguez LA, Conneally PM, Joslyn G, Peacock M, Johnston CC, Foroud T. Genome screen for QTLs contributing to normal variation in bone mineral density and osteoporosis. J Clin Endocrinol Metab. 2000;85:3116–3120. doi: 10.1210/jcem.85.9.6778. [DOI] [PubMed] [Google Scholar]
- 18.Okubo M, Horinishi A, Kim DH, Yamamoto TT, Murase T. Seven novel sequence variants in the human low density lipoprotein receptor related protein 5 (LRP5) gene. Hum Mutat. 2002;19:186–188. doi: 10.1002/humu.9012. [DOI] [PubMed] [Google Scholar]
- 19.Allen KM, Anisowicz A, FitzGerald MG, Van Eerdewegh P, Little RD, Keith T, Recker RR, Johnson ML. Polymorphism analysis of LDL receptor-related protein 5 (LRP5) J Bone Miner Res. 2002;17:S424. [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Won HS, Kim WY. Development and validation of a semi-quantitative food frequency questionnaire to evaluate nutritional status of Korean elderly. Korean J Nutr. 2000;33:314–323. (in Korean) [Google Scholar]
- 22.Lee JK, Kim HT, Cho SM, Kim KH, Jin HJ, Ryu GM, Oh B, Park C, Kimm K, Jo SA, Jung SC, Kim S, In SM, Lee JE, Jo I. Characterization of 458 single nucleotide polymorphisms of disease candidate genes in the Korean population. J Hum Genet. 2003;48:213–216. doi: 10.1007/s10038-003-0011-9. [DOI] [PubMed] [Google Scholar]
- 23.Twells RC, Mein CA, Phillips MS, Hess JF, Veijola R, Gilbey M, Bright M, Metzker M, Lie BA, Kingsnorth A, Gregory E, Nakagawa Y, Snook H, Wang WY, Masters J, Johnson G, Eaves I, Howson JM, Clayton D, Cordell HJ, Nutland S, Rance H, Carr P, Todd JA. Haplotype structure, LD blocks, and uneven recombination within the LRP5 gene. Genome Res. 2003;13:845–855. doi: 10.1101/gr.563703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazess RB, Barden H, Ettinger M, Schultz E. Bone density of the radius, spine, and proximal femur in osteoporosis. J Bone Miner Res. 1988;3:13–18. doi: 10.1002/jbmr.5650030104. [DOI] [PubMed] [Google Scholar]
- 25.Pouilles JM, Tremollieres F, Todorovsky N, Ribot C. Precision and sensitivity of dual-energy x-ray absorptiometry in spinal osteoporosis. J Bone Miner Res. 1991;6:997–1002. doi: 10.1002/jbmr.5650060914. [DOI] [PubMed] [Google Scholar]
- 26.Hey PJ, Twells RC, Phillips MS, Nakagawa Y, Brown SD, Kawaguchi Y, Cox R, Xie G, Dugan V, Hammond H, Metzker ML, Todd JA, Hess JF. Cloning of a novel member of the low-density lipoprotein receptor family. Gene. 1998;216:103–111. doi: 10.1016/s0378-1119(98)00311-4. [DOI] [PubMed] [Google Scholar]
- 27.Springer TA. An extracellular beta-propeller module predicted in lipoprotein and scavenger receptors, tyrosine kinases, epidermal growth factor precursor, and extracellular matrix components. J Mol Biol. 1998;283:837–862. doi: 10.1006/jmbi.1998.2115. [DOI] [PubMed] [Google Scholar]
- 28.Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC. Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol. 2001;8:499–504. doi: 10.1038/88556. [DOI] [PubMed] [Google Scholar]
- 29.Van Wesenbeeck L, Cleiren E, Gram J, Beals RK, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman ML, De Vernejoul MC, Bollerslev J, Van Hul W. Six novel missense mutations in the LDL receptor-related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet. 2003;72:763–771. doi: 10.1086/368277. [DOI] [PMC free article] [PubMed] [Google Scholar]