Abstract
The work was done to study immunogenetic peculiarities of neuroinflammatory diseases among Korean children. A total of 13 children with neuroinflammatory diseases (8 males and 5 females; mean age 4.6±2.6 yr) were consecutively recruited. Genomic typing was performed on their HLA DRB/HLA DQB genes using PCR-SSOP/SSP techniques with gel immunoelectrophoresis. The frequencies of HLA-DR1*15 in children with acute disseminated encephalomyelitis (ADEM) (31%) and DQB1*06 in other neuroinflammatory diseases (38%) were significantly increased compared with control subjects. The frequencies of HLA-DRB3*0202 (100%), HLA-DRB1*1302 (67%), HLA-DRB3*0301 (67%), and HLA-DQB1*0301 (67%) were significantly increased in children with multiple sclerosis and the frequencies of HLA-DRB1*1501 (40%) and HLA-DRB5*0101 (40%) were significantly increased in children with ADEM. HLA-DRB1*1401, HLA-DRB3*0202, and HLA-DQB1*0502 were found in children with acute necrotizing encephalopathy. In conclusion, HLA-DR1*15 and DQB1*06 may be involved in susceptibility to inflammation in Korean children. The frequencies of HLA-DRB1*1501, HLA-DRB5*0101, HLA-DRB3*0301, and HLA-DQB1*0602 were not as high in Korean children with multiple sclerosis as in western children. However, HLA-DRB3*0202 was seen in all children with multiple sclerosis. Our data may provide further evidence that the immunogenetic background of neuroinflammatory diseases in Korean is distinctly different from the ones in western countries. Further studies are necessary to confirm this finding.
Keywords: Neuroinflammatory Diseases; HLA-DR Antigens; HLA-DQ Antigens; Encephalomyelitis, Acute Disseminated; Multiple Sclerosis; Leucoencephalitis, Acute Hemorrhagic
INTRODUCTION
Neuroinflammatory diseases are clinically heterogeneous and one of the major causes of acquired neurological disability. An underlying complex genetic susceptibility may play an important role in their etiologies; however, the role of genetic factors in determining their clinical features is still unclear. Multiple sclerosis (MS), one of the major neuroinflammatory diseases, recently was recognized and subsequently well-characterized in children, but the awareness of pediatricians and pediatric neurologists, especially in Asian countries to the occurrence of MS, is still unsatisfactory. Some data provided evidences that Asian and Western type MS are distinct regarding their immunogenetic background (1-3). Acute disseminated encephalomyelitis (ADEM) is a postinfectious encephalitis that is usually preceded by an infectious illness or vaccination. The clinical manifestation has a wide spectrum and complementary examinations are not specific, except for magnetic resonance imaging (MRI) findings which show multifocal white-matter lesions similar to those seen in MS (4-9). Acute necrotizing encephalopathy (ANE), likely postinfectious encephalomyelitis, is not uncommon in Asian countries but is very rarely seen in Western countries. The etiology is still unknown, but immunogenetic mechanism has been suggested (10-15). Some data suggest that children with postinfectious encephalomyelitis are genetically predisposed to this demyelinating disease (16). These postinfectious encephalomyelitides and multiple sclerosis have clinical, immunologic, and neuroradiographic similarities. Therefore we studied HLA determinants in thirteen children consecutively diagnosed with neuroinflammatory disease to study the immunogenetic peculiarities of neuroinflammatory diseases in Korean children.
MATERIALS AND METHODS
A total of 13 children (8 males and 5 females) with neuroinflammatory diseases were consecutively recruited at the section of Pediatric Neurology, Kyungpook National University Hospital, Daegu, Korea, between January 2000 and April 2003. The subject's ages ranged from 1-10 yr. The studied subjects included five children with ADEM, three with MS, three with acute necrotizing encephalopathy, and two with transverse myelitis. Three age-matched children with no neurological illnesses were also evaluated as controls.
The children with neuroinflammatory diseases and three age-matched children with no neurological illnesses as controls were molecularly typed for HLA class II genes. Genomic DNA was prepared from peripheral blood leukocytes by using a DNA purification kit (Promega, Madison, WI, U.S.A.) according to standard procedures. Molecular analysis was performed on HLA-DRB/HLA-DQB genes (chromosome 6p21) of 13 unrelated children with neuroinflammatory diseases using PCR-SSOP/SSP kits (INNO-LiPA HLA-DRB, Ghent, Belgium and Pel-Freez SSP UniTray with Taq polymerase, Basel, Switzerland) for low and high resolution typing, according to the manufacturer's instructions along with augmentation of gel immunoelectrophoresis for HLA-DQB. Alleles were assigned according to the nomenclature for factors of the HLA system.
RESULTS
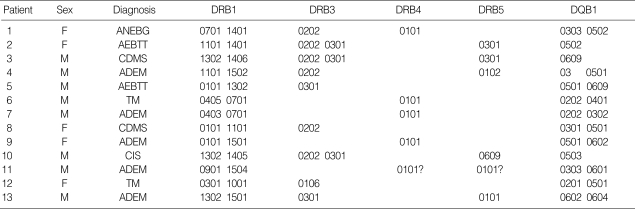
Thirteen children with neuroinflammatory diseases were typed for HLA class II genes. Their demographic and clinical characteristics are summarized in Table 1. Eight males and five females were enrolled in the study. Their ages ranged from one to ten years (mean age 4.6±2.6 yr). The three major conditions were ADEM, ANE, and MS. As shown in Table 2, the frequency of HLA-DR1*15 was significantly increased in patients with ADEM (31%) and the frequency of DQB1*06 was also increased in other neuroinflammatory diseases (38%; 4 ADEM, 1 multiple sclerosis, 1 transverse myelitis) compared with control subjects as well as known data of HLA-DRB alleles in Koreans. In Korean children with multiple sclerosis, the frequencies of HLA-DRB1*1501, HLA-DRB5*0101, HLA-DRB3*0301, and HLA-DQB1*0602 were not as high as in western children, but the frequencies of HLA-DRB3*0202 (100%), HLA-DRB1*1302 (67%), HLA-DRB3*0301 (67%), and HLA-DQB1*0301 (67%) were significantly increased instead (Table 3). The frequencies of HLA-DRB1*1501 (40%) and HLA-DRB5*0101 (40%) were significantly increased in children with ADEM compared with control subjects. In addition, three common alleles, HLA-DRB1*1401, HLA-DRB3*0202, and HLA-DQB1*0502, were found in children with acute necrotizing encephalopathy with bilateral thalamotegmental involvement (ANEBTT) (Table 4).
Table 1.
Demographic features of subjects (n=13)
IND, inflammatory neurological diseases; ADEM, acute disseminated encephalomyelitis; CDMS/CIS, clinically definite multiple sclerosis/clinically isolated syndromes suggestive of MS; AEBTT, acute encephalopathy with bilateral thalamotegmental involvement; ANEBG, acute necrotizing encephalitis with basal ganglia involvement.
Table 2.
HLA-DRB and DQB alleles in subjects (n=13)
Male (M)/Female (F).
ANEBG, acute necrotizing encephalitis with basal ganglia involvement; AEBTT, acute encephalopathy with bilateral thalamotegmental involvement; CDMS/CIS, clinically definite multiple sclerosis or clinically isolated syndromes suggestive of MS; TM, transverse myelitis; ADEM, acute disseminated encephalomyelitis.
Table 3.
Frequent alleles of HLA-DRB and DQB in patients with multiple sclerosis
Table 4.
HLA-DRB and DQB alleles of patients with ANEBTT
AEBTT, acute encephalopathy with bilateral thalamotegmental involvement.
DISCUSSION
In the absence of a biological marker, the distinction between ADEM and MS cannot be made with certainty at the time of initial manifestation and a preceding or concurrent viral illness, high lesion load on MRI, involvement of the deep gray matter, or absence of oligoclonal bands may be more indicative of ADEM (5, 17). It will be very interesting if we were able to clarify whether these conditions have their own specific biological markers or share the markers. We sought to assess a possible relationship between HLA class II genes and neuroinflammatory diseases such as MS and ADEM. This is the pilot study to evaluate the immunogenetic background of neuroinflammatory diseases in Asian children.
We analyzed HLA determinants in thirteen Korean children consecutively diagnosed with neuroinflammatory disease. As illustrated in Table 1, ADEM is still the major condition despite the fact that a lot of children with ADEM were not enrolled for many reasons. We had two cases of clinically definite multiple sclerosis (CDMS) who met the diagnostic criteria (18-20). In addition, there was a child with Clinically Isolated Syndromes (CIS) suggestive of MS. Few pediatric cases of CDMS have been reported in Korea. However, recently the number is increasing. This is probably due to rapid westernization of the country, availability of clinically qualified experts, and improvement of diagnostic tools. A few cases of ANEBTT were also included in the study.
The subjects were genotyped using PCR-SSOP/SSP techniques for HLA DRB/HLA DQB genes with augmentation and gel immunoelectrophoresis. As shown in Table 2, the frequencies of HLA-DR1*15 and DQB1*06 were significantly increased in neuroinflammatory diseases compared with control subjects as well as known data of HLA-DRB alleles in Korean (21, 22). The results are similar to previous reports from Western countries and partly supports our hypothesis (23). The five most common alleles in these children were DRB3*0202 (46%), DRB1*0101 (23%), DRB1*1302 (23%), DQB1*0301 (23%), and DQB1*0501 (23%). Like previous Japanese studies (24, 25), the frequencies of HLA-DRB1*1501, HLA-DRB5*0101, HLA-DRB3*0301, and HLA-DQB1*0602 were not as high in Korean children with MS as in western children (1-3, 26-29). A very strong correlation of the condition with HLA-DRB3*0202, HLA-DRB1*1302, HLA-DRB3*0301, and HLA-DQB1*0301 alleles was verified instead (Table 3). These findings may provide the evidence for correlation of these alleles and susceptibility of Korean children to MS. The frequencies of HLA-DRB1*1501 (40%) and HLA-DRB5*0101 (40%) were significantly increased in children with ADEM compared with control subjects. Interestingly, there was one child with a clinically strong feature of ADEM, but he had the western genotype of MS, HLA-DRB1*1501 and HLA-DQB1*0602 (Fig. 1). He is still visiting the clinic and has not had any relapse yet. Although the same HLA determinants were found in this patient as in those with MS, further studies on a larger number of patients with postinfectious encephalomyelitis are needed before we can conclude with certainty that the two diseases share a common genetic propensity. Finally, it is noteworthy that three common alleles, HLA-DRB1*1401, HLA-DRB3*0202, and HLA-DQB1*0502 were found in children with ANEBTT (Table 4). With our limited knowledge, ANEBTT is not a common illness worldwide and has never been molecularly evaluated, so this result might be valuable. However, further studies are required to elucidate the condition.
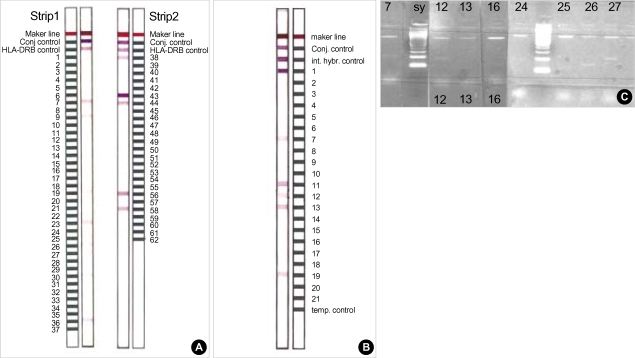
Fig. 1.
Molecular analysis of HLA-DRB/HLA-DQB genes in a case of ADEM with the western genotype of multiple sclerosis. PCR-SSOP (A), PCR-SSP (B) with gel Immunoelectrophoresis for HLA-DQB (C) illustrates HLA-DRB1*1501 and HLA-D QB1*0602/0604.
In conclusion, HLA-DR1*15 and DQB1*06 may be involved in susceptibility to inflammation in Korean children. The frequencies of HLA-DRB1*1501, HLA-DRB5*0101, HLA-DRB3*0301, and HLA-DQB1*0602 were not as high in Korean children with MS as in western children. HLA-DRB3*0202 was seen in all children with MS. Our data may provide further evidence that the immunogenetic background of neuroinflammatory diseases in Asians is distinctly different from that in western countries. Further studies are necessary to confirm this finding.
Footnotes
The study was financially supported by Medical Research Institute, Kyungpook National University Hospital, Daegu, Korea.
References
- 1.Ma JJ, Nishimura M, Mine H, Saji H, Ohta M, Saida K, Ozawa K, Kawakami H, Saida T, Uchiyama T. HLA-DRB1 and tumor necrosis factor gene polymorphisms in Japanese patients with multiple sclerosis. J Neuroimmunol. 1998;92:109–112. doi: 10.1016/s0165-5728(98)00189-1. [DOI] [PubMed] [Google Scholar]
- 2.Ono T, Zambenedetti MR, Yamasaki K, Kawano Y, Kamikawaji N, Ito H, Sakurai M, Nishimura Y, Kira J, Kanazawa I, Sasazuki T. Molecular analysis of HLA class I (HLA-A and -B) and HLA class II (HLA-DRB1) genes in Japanese patients with multiple sclerosis (Western type and Asian type) Tissue Antigens. 1998;52:539–542. doi: 10.1111/j.1399-0039.1998.tb03084.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamasaki K, Horiuchi I, Minohara M, Kawano Y, Ohyagi Y, Yamada T, Mihara F, Ito H, Nishimura Y, Kira J. HLA-DPB*10501-associated opticospinal multiple sclerosis: clinical, neuroimaging and immunogenetic studies. Brain. 1999;122:1689–1696. doi: 10.1093/brain/122.9.1689. [DOI] [PubMed] [Google Scholar]
- 4.Dale RC, de Sousa C, Chong WK, Cox TC, Harding B, Neville BG. Acute disseminated encephalomyelitis, multiphasic disseminated encephalomyelitis and multiple sclerosis in children. Brain. 2000;123:2407–2422. doi: 10.1093/brain/123.12.2407. [DOI] [PubMed] [Google Scholar]
- 5.Hynson JL, Kornberg AJ, Coleman LT, Shield L, Harvey AS, Kean MJ. Clinical and neuroradiologic features of acute disseminated encephalomyelitis in children. Neurology. 2001;56:1308–1312. doi: 10.1212/wnl.56.10.1308. [DOI] [PubMed] [Google Scholar]
- 6.Khong PL, Ho HK, Cheng PW, Wong VC, Goh W, Chan FL. Childhood acute disseminated encephalomyelitis: the role of brain and spinal cord MRI. Pediatr Radiol. 2002;32:59–66. doi: 10.1007/s00247-001-0582-6. [DOI] [PubMed] [Google Scholar]
- 7.Murthy SN, Faden HS, Cohen ME, Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110(2 Pt 1):e21. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Prabhakar S, Korah IP, Warade SS, Alexander M. Acute disseminated encephalomyelitis and multiple sclerosis: magnetic resonance imaging differentiation. Australas Radiol. 2000;44:404–411. doi: 10.1046/j.1440-1673.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 9.Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–1231. doi: 10.1212/wnl.59.8.1224. [DOI] [PubMed] [Google Scholar]
- 10.Ashtekar CS, Jaspan T, Thomas D, Weston V, Gayatri NA, Whitehouse WP. Acute bilateral thalamic necrosis in a child with Mycoplasma pneumoniae. Dev Med Child Neurol. 2003;45:634–637. doi: 10.1017/s0012162203001154. [DOI] [PubMed] [Google Scholar]
- 11.Goo HW, Choi CG, Yoon CH, Ko TS. Acute necrotizing encephalopathy: diffusion MR imaging localized proton MR spectroscopic findings in two infants. Korean J Radiol. 2003;4:61–65. doi: 10.3348/kjr.2003.4.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mizuguchi M, Hayashi M, Nakano I, Kuwashima M, Yoshida K, Nakai Y, Itoh M, Takashima S. Concentric structure of thalamic lesions in acute necrotizing encephalopathy. Neuroradiology. 2002;44:489–493. doi: 10.1007/s00234-002-0773-3. [DOI] [PubMed] [Google Scholar]
- 13.Ravid S, Topper L, Eviatar L. Acute necrotizing encephalopathy presenting as a basal ganglia syndrome. J Child Neurol. 2001;16:461–462. doi: 10.1177/088307380101600618. [DOI] [PubMed] [Google Scholar]
- 14.Yoshikawa H, Watanabe T, Abe T, Oda Y. Clinical diversity in acute necrotizing encephalopathy. J Child Neurol. 1999;14:249–255. doi: 10.1177/088307389901400407. [DOI] [PubMed] [Google Scholar]
- 15.Campistol J, Gassio R, Pineda M, Fernandez-Alvarez E. Acute necrotizing encephalopathy of childhood (infantile bilateral thalamic necrosis): two non-Japanese cases. Dev Med Child Neurol. 1998;40:771–774. doi: 10.1111/j.1469-8749.1998.tb12346.x. [DOI] [PubMed] [Google Scholar]
- 16.Woody RC, Steele RW, Charlton RK, Smith V. Histocompatibility determinants in childhood postinfectious encephalomyelitis. J Child Neurol. 1989;4:204–207. doi: 10.1177/088307388900400311. [DOI] [PubMed] [Google Scholar]
- 17.Brass SD, Caramanos Z, Santos C, Dilenge ME, Lapierre Y, Rosenblatt B. Multiple sclerosis vs acute disseminated encephalomyelitis in childhood. Pediatric Neurology. 2003;29:227–231. doi: 10.1016/s0887-8994(03)00235-2. [DOI] [PubMed] [Google Scholar]
- 18.McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lubin FD, MacFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, van den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 19.Barkhof F, Rocca M, Francis G, Van Waesberghe JH, Uitdehaag BM, Hommes OR, Hartung HP, Durelli L, Edan G, Fernandez O, Seeldrayers P, Sorensen P, Margrie S, Rovaris M, Corni G, Filippi M. Validation of diagnostic magnetic resonance imaging criteria for multiple sclerosis and response to interferon beta-1a. Ann Neurol. 2003;53:718–724. doi: 10.1002/ana.10551. [DOI] [PubMed] [Google Scholar]
- 20.Poser CM, Paty PW, Scheinberg L, McDonald WI, Davis FA, Johnson KP, Sibley WA, Silberberg DH, Tourtellotte WW. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 21.Park MH, Kim HS, Kang SJ. HLA-A, B, DRB1 allele and haplotype frequencies in 510 Koreans. Tissue Antigens. 1999;53:386–390. doi: 10.1034/j.1399-0039.1999.530412.x. [DOI] [PubMed] [Google Scholar]
- 22.Lim YA, Kwak YS. HLA-DQB1 allele frequencies and haplotypic associations with DRB1 genes in Koreans. Korean J Clin Pathol. 1999;19:535–541. [Google Scholar]
- 23.Fogdell-Hahn A, Ligers A, Gronning M, Hillert J, Olerup O. Multiple sclerosis: a modifying influence of HLA class I genes in a HLA class II associated autoimmune disease. Tissue Antigens. 2000;55:140–148. doi: 10.1034/j.1399-0039.2000.550205.x. [DOI] [PubMed] [Google Scholar]
- 24.Ito H, Yamasaki K, Kawano Y, Horiuchi I, Yun C, Nishimura Y, Kira J. HLA-DP-associated susceptibility to the optico-spinal form of multiple sclerosis in the Japanese. Tissue Antigens. 1998;52:179–182. doi: 10.1111/j.1399-0039.1998.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 25.Kira J, Kanai T, Nishimura Y, Yamasaki K, Matsushita S, Kawano Y, Hasuo K, Tobimatsu S, Kobayashi T. Western versus Asian types of multiple sclerosis: immunogenetically and clinically distinct disorders. Ann Neurol. 1996;40:569–574. doi: 10.1002/ana.410400405. [DOI] [PubMed] [Google Scholar]
- 26.Kwon OJ, Karni A, Israel S, Brautbar C, Amar A, Meiner Z, Abramsky O, Karussis D. HLA class II susceptibility to multiple sclerosis among Ashkenazi and non-Ashkenazi Jews. Arch Neurol. 1999;56:555–560. doi: 10.1001/archneur.56.5.555. [DOI] [PubMed] [Google Scholar]
- 27.Barcellos LF, Oksenberg JR, Green AJ, Bucher P, Rimmler JB, Schmidt S, Garcia ME, Lincoln RR, Pericak-Vance MA, Haines JL, Hauser SL Multiple Sclerosis Genetics Group. Genetic basis for clinical expression in multiple sclerosis. Brain. 2002;125(Pt 1):150–158. doi: 10.1093/brain/awf009. [DOI] [PubMed] [Google Scholar]
- 28.Rasmussen HB, Kelly MA, Clausen J. Additive effect of the HLA-DR 15 haplotype on susceptibility to multiple sclerosis. Mult Scler. 2001;7:91–93. doi: 10.1177/135245850100700203. [DOI] [PubMed] [Google Scholar]
- 29.Liblau R, Gautam AM. HLA, molecular mimicry and multiple sclerosis. Rev Immunogenet. 2000;2:95–104. [PubMed] [Google Scholar]