Abstract
The aim of this study was to assess eventual differences in serum cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, LDL-C/HDL-C ratio between veterans with combat-related posttraumatic stress disorder (PTSD) only or comorbid with major depressive disorder (MDD), veterans with combat experiences with MDD, and healthy control group. PTSD and/ or MDD were diagnose according to structured clinical interview based on DSM-IV criteria. Additional criteria to diagnose PTSD were Clinician Administered PTSD Scale (CAPS), and to diagnose MDD Montgomery-Asberg Depression Rating Scale (MADRAS). Serum lipid concentrations were determined by using the enzyme-assay method. Veterans with combat-related PTSD as well as veterans with combat-related PTSD comorbid with MDD showed significantly higher concentrations of cholesterol (F=9.858, p<0.01), triglycerides (F=10.112, p<0.01), LDL-C (F=11.145, p<0.01), and LDL-C/HDL-C ratio (F=8.346, p<0.01) vs. veterans with MDD or healthy control group. Contrary healthy control group and veterans with MDD showed significantly higher concentrations of HDL-C (F=8.421, p<0.01), vs. veterans with PTSD or PTSD comorbid with MDD. In conclusion, there are no differences in serum lipid concentrations between veterans with combat-related PTSD and PTSD comorbid with MDD, but they have higher lipid concentrations than veterans with MDD or healthy control subjects.
Keywords: Croatia; Cholesterol; Lipoproteins, LDL; Lipoproteins, HDL; Triglycerides; Stress Disorder, Post-traumatic; Major Depressive Disorder; Depression, Involutional
INTRODUCTION
Serum lipid concentrations have been studied in different psychiatric disorders. Low concentrations of cholesterol and lipoproteins have been found in patients with schizophrenia (1, 2), antisocial personality (3), borderline personality disorder, suicidal (4) or aggressive behavior (5, 7), as well as in population with criminal past (8). Studies provided on patients with major depressive disorder (MDD) showed contradictory results because some of them showed low cholesterol concentrations (9, 10) while others did not find any lipid concentration changes (11, 12). In patients with manic or mixed manic episodes high concentrations of cholesterol have been found (13). In studies on patients with anxiety disorders such as panic disorder (14, 15), generalized anxiety disorder (16, 17), obsessive-compulsive disorder (18) and post-traumatic stress disorder (PTSD) (19, 20) increased concentrations of serum lipids have been found. However, epidemiological as well as clinical studies have shown that PTSD often occurs with other psychiatric disturbances (21). Those studies indicated that approximately 80% of individuals with PTSD met criteria for at least one additional psychiatric diagnosis. The MDD is one of the most frequent comorbid condition with PTSD and prevalence of MDD in patients with PTSD is expressed up to 70% (22).
Our hypothesis was that in veterans with PTSD comorbid with MDD serum lipids concentrations is lower than in veterans with PTSD only, or in other words they have similar lipids concentrations like veterans with MDD or healthy control subjects.
The aim of this study was to analyze the concentrations of serum cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, and to assess the LDL-C/HDL-C ratio in Croatian veterans with combat-related chronic PTSD opposite to war veterans with combat-related chronic PTSD comorbid with MDD, war veterans with MDD and healthy control group.
MATERIALS AND METHODS
Subjects
The first study group consisted of 43 male veterans with chronic combat-related PTSD, aged between 26 and 47 yr. The duration of their combat activity ranged from 1 to 4 yr. The number of their combat traumas ranged from 2-5. Time elapsed since they had experienced combat traumas ranged from 6 to 10 yr. The second study group consisted of 37 male veterans with chronic combat-related PTSD comorbid with MDD, aged between 25 and 45 yr. The duration of their combat activity ranged from 1 to 4 yr. The number of their combat traumas ranged from 1-5. Time elapsed since they had experienced combat traumas ranged 6 to 10 yr. The third study group consisted of 38 male veterans with MDD, aged between 25 and 46 yr. The duration of their combat activity ranged from 1 to 4 yr. The number of their combat traumas ranged from 2-4. Time elapsed since they had experienced combat traumas ranged from 6 to 10 yr. Subjects from all study groups, when they had been drafted for active military service, they went through rigorous entrance tests, which ensured that they had not had any previous major psychiatric or somatic disorders. All investigated patients did not have any other psychiatric comorbid disorders or medical problems, and took different medications in medical history prior to this study, mostly antidepressants (fluoxetine 8.9%, fluvoxamine 5.2%, paroxetine 25.4%, sertraline 27%, clomipramine 18.7%, trazadone 1.4%, and maprotiline 13.4%) and anxiolytics (alprazolam 18.7%, oxazepam 8.6%, diazepam 57.6%, clonazepam 15.1%). 75% of the patients sometimes took some hypnotics during therapy (nitrazepam, funitrazepam or zolpidem). None of the above mentioned drugs influence serum lipids concentrations (23). The control group consisted of 39 healthy men, mostly our hospital workers, aged 28-47 yr. They had negative anamnesis data of alcohol or other drugs of dependence abuse. Informed consent was obtained from patients and controls after complete and extensive description of the study profile.
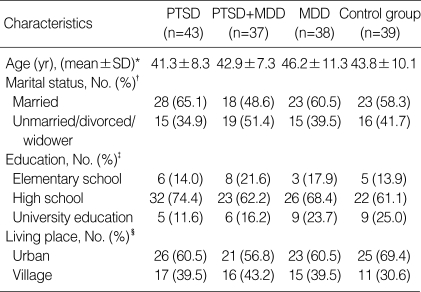
Because lipid concentration are sensitive to even relatively subtle psychosocial influences, our experience has indicated that it is important to describe these factors in some details, which characterize PTSD patients as well as the control group. Descriptive parameters of all included subjects are presented in Table 1. Almost half of the subjects were married (65.1% in PTSD, 48.6% in PTSD comorbid with MDD, 60.5% in MDD and 58.3% in the control group). Most of the subjects had secondary schooling (74.4% in PTSD, 62.2% in PTSD comorbid with MDD, 68.4% in MDD, and 71.8% in control group). Most of them lived in urban areas (60.5% in PTSD, 56.8% in PTSD comorbid with MDD, 60.5% in MDD, and 69.4% in control group). Subjects with PTSD, PTSD comorbid with MDD, or MDD were in inpatient hospital treatment. During hospitalization, they undertook an intensive schedule of 30 hr of individual and group therapy per week, as well as other socio-therapeutic activities. Treatment program was based on the therapeutic community (24).
Table 1.
Sociodemographic characteristic of the veterans with post-traumatic stress disorder (PTSD), PTSD comorbid with major depressive disorder (MDD), MDD, and control healthy group
*F=1.939, p=0.126; †χ2=2.318, p=0.509; ‡χ2=5.691, p=0.459; §χ2=1.346, p=0.718.
Medical examination and study design
From the pool of all male patients (n=891) who were receiving treatment in the University Department of Psychiatry in "Sestre milosrdnice" University Hospital in 2001 and 2002, we elected three study groups; patients with combat-related PTSD only (n=43) or comorbid with MDD (n=37), and patients with combat-experiences with MDD only (n=38). Patients with other psychiatric disorders (n=673), and comorbid psychiatric disorders in PTSD group (n=63), (except MDD in PTSD group) or somatic conditions (n=28) were excluded from the study in all study groups. Also because the patients did not give informed consent we excluded them from the study (2 in PTSD group, 3 in PTSD comorbid with MDD group, and 4 in MDD group).
A psychiatrist performed the structured clinical interview and made diagnosis based on DSM-IV criteria for PTSD or/and MDD (25). Clinical psychologist applied Clinician Administered PTSD Scale (CAPS) PTSD interview based on DSM-IV criteria to measure post-traumatic stress reaction (26). Final diagnosis of PTSD was reached only in cases where both sets of criteria were fulfilled. Diagnostic agreement between psychiatric and psychological criteria was high κ=0.96. The alpha coefficient for the CAPS questionnaire in our study was 0.91. We accepted the diagnosis of MDD if the patients fulfilled two criteria: indicative result on the Montgomery-Asberg Depression Rating Scale (MADRAS) (27) and sufficient number of symptoms shown in the structural clinical interview based on DSM-IV criteria for MDD (25). Diagnostic balance between the MADRAS and clinical interview based on DSM-IV criteria for MDD was κ=0.98. MADRAS questionnaire was used because of its simplicity and reliability, in our study alpha coefficient was 0.93. Two psychiatrists performed this part of the evaluation, each of them examining independently all the subjects. The agreement between two psychiatrists was high, κ=0.97.
We also compared PTSD group and MDD group according to DSM IV criteria for nicotine dependence (25) because smoking has a strong effect on elevation of serum lipids (28). And last, body mass index (BMI) was calculated for PTSP and MDD group because BMI correlated well with serum lipids level (28). BMI is ratio representing each patients weight in kilograms divided by square of height in meters (BMI=kg/m2). Since diet and activity can influence the results of examinations, laboratory measures and psychiatric examinations were carried out between days 14 and 16 of inpatient stay. Blood lipids measurements at this time may be preferable to values on admission to ensure that all patients had equivalent diets and activity levels.
Biochemical measurements
Blood samples were collected from forearm vein, in glass red-topped, vacuum tubes without any anticoagulant, in the morning between 8-9 a.m., after overnight fast of 12 hr and 30 min resting immediately prior to blood collection. Serum concentrations for cholesterol, HDL, and triglyceride were measured by enzyme assay (EA), immediately after taking the samples with commercial kits (Olympus Diagnostic, GmbH, Hamburg, Germany) on Olympus AU 600 automatic analyzator. The interassay coefficient of variation in our laboratory was 3.2% for cholesterol, 2.5% for triglycerides, and 3.0% for HDL-C. Serum LDL-C concentrations were calculated by following formula: LDL-C=cholesterol-(HDL-C -tryglicerides/5)] (29). Our laboratory's referent intervals for lipids are parameters measured as follow: cholesterol 147-220 mg/dL, LDL lower than 150.0 mg/dL, HDL higher than 42.0 mg/dL, LDL-C/HDL-C ratio lower than 130.0, and triglyceride 53-177 mg/dL.
Statistical analyses
We use chi-quadrate test to analyze difference in nicotine dependence or sociodemographic variables between four groups. The normal distribution was assessed for all measures and for each group by Kolmogorov-Smirnov test. Subjects with PTSD, subjects with PTSD comorbid with MDD, subjects with MDD, and control group (except in war variables for control group) were compared in age, blood glucose concentration, systolic or diastolic blood pressure, duration of combat activity, duration of PTSD symptoms, and number of combat traumas by analysis of variance (ANOVA) and Scheffe's post-hoc analysis for pair-wise comparisons. Analysis of covariance (ANCOVA) with age, BMI, blood glucose concentrations, diastolic or systolic blood pressure as a covariates, was used to analyze differences in serum cholesterol, triglycerides, LDL-C, HDL-C, and LDL-C/HDL-C ratio in subjects with PTSD, subjects with PTSD comorbid with MDD, subjects with MDD, and control group. Age, BMI, blood glucose concentrations, diastolic or systolic blood pressure was used as covariates because that variable can influence serum lipid concentrations (28).
A p value of <0.01 was considered to denote the presence of a statistically significant difference. Statistics was done with SPSS software (SPSS for Windows 8.0, SPSS, Chicago, IL, U.S.A.).
RESULTS
There was no statistically significant difference in age between veterans with combat-related chronic PTSD, combatrelated chronic PTSD comorbid with MDD, MDD, and control group, as well as in marital status, education, and living place (Table 1). Regarding combat activity duration, number of combat traumas as well as duration of PTSD symptoms and/or MDD, there was no statistically significant difference between veterans with combat-related PTSD, veterans with combat-related PTSD comorbid with MDD, veterans with MDD, and healthy control group (Table 2).
Table 2.
Duration of combat activity, duration of symptoms, and number of combat traumas in veterans with post-traumatic stress disorder (PTSD), PTSD with comorbid major depressive disorder (MDD), and MDD
*mean±SD.
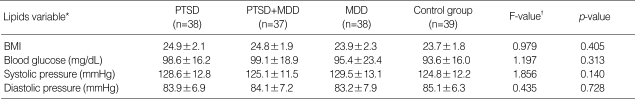
DSM-IV criteria for nicotine dependence were met by 74.4% of veterans with combat-related PTSD and 83.8% of veterans with PTSD comorbid with MDD, 65.8% of veterans with MDD, and 72.2% of healthy control subjects (χ2=3.237, p=0.356). There was no statistically significant difference in BMI, blood glucose concentrations, systolic or diastolic blood pressure between veterans with combat-related chronic PTSD, combat-related chronic PTSD comorbid with MDD, MDD, and control group (Table 3).
Table 3.
Body mass index (BMI), blood glucose concentrations, systolic blood pressure, and diastolic blood pressure in veterans with post-traumatic stress disorder (PTSD), PTSD comorbid with major depressive disorder (MDD), MDD, and in healthy control group
*(mean±SD), †ANOVA.
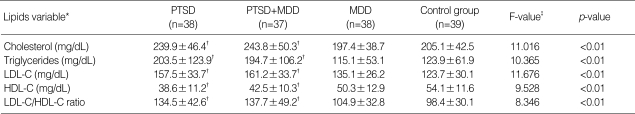
Veterans with combat-related PTSD as well as veterans with PTSD comorbid with MDD showed significantly higher cholesterol, LDL-C, LDL-C/HDL-C ratio, and triglyceride concentrations compared to veterans with combat-experiences with MDD and healthy control group (Table 4). HDL-C was statistically significantly lower in veterans with combat-related PTSD and veterans with PTSD comorbid with MDD opposite to veterans with MDD or healthy control group (Table 4).
Table 4.
Concentrations of serum cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides, in veterans with post-traumatic stress disorder (PTSD), PTSD comorbid with major depressive disorder (MDD), MDD, and in healthy control group
*(mean±SD), †significantly different from the MDD and control group (post-hoc Scheffe testing, <0.01), ‡ANCOVA (after adjusted for BMI, ages, blood glucose concentrations, systolic and diastolic blood pressure as covariates).
DISCUSSION
Our results showed higher concentrations of cholesterol and triglycerides in veterans with combat-related chronic PTSD as well as in veterans with combat-related PTSD comorbid with MDD opposite to veterans with only MDD or healthy control group. Also our results show a very high risk of developing arteriosclerosis in veterans with combat-related PTSD only or comorbid with MDD contrary to veterans with MDD or healthy control group. The reason is the low level of HDL-C, high level of LDL-C, and high LDL-C/HDL-C ratio.
These findings are in accordance with the results of the investigation on Vietnam or Croatian veterans with chronic PTSD (19, 20). However, the research on Vietnam veterans did not include the analysis of the LDL-C and HDL-C. Also this study contrary to prior study provided on Croatian war veterans in which control group was formed of patients with MDD, included, veterans with combat-experiences with MDD as well as healthy control subjects. Our opinion is that it was necessary because some researchers pointed out that MDD is associated with low serum lipids concentrations (9, 10). Because of those findings our hypothesis was that in veterans with PTSD comorbid with MDD serum lipids concentrations is lower than in veterans with PTSD only, or in other words they have similar lipids concentrations like veterans with MDD or healthy control subjects. However, our results are opposite to our expectations, and further more our results are contrary to the results of the studies which find low serum lipids concentrations in patients with MDD. The explanation for those differences maybe lies in cross-cultural differences in diet habit, sample size, or in rigorous diagnostic criteria that were used for subject inclusion in our study contrary than others.
Our study has several limitations, also. For example we do not compare our subjects in diet anamnesis data. Also we compared only war veterans with PTSD only or comorbid with MDD with veterans with MDD and healthy control subjects. In future research it will be interesting to explore serum lipid concentrations in civil subjects with PTSD. And the third limitation is in male gender of our subjects in all four groups because male gender is connected with higher cholesterol, triglycerides, and LDL-C as well as low HDL-C concentrations than female.
However, former research on the patients with PTSD showed many biological alterations such as hypersensitivity of steroid receptors (30), and alterations in hypothalamic-pituitary-adrenal/thyroid axis (31). In interpretation of our results, it is important to note the findings that focused on high concentration of catecholamines (32), and an increase of sympathetic nerve system activity, which manifested itself through an intensified heartbeat and blood pressure (33, 34). Therefore, the drugs that increase the activity of noradrenergic system can induce symptomatology of PTSD (35). On the other hand, in large investigation of patients with arterial hypertension and other risk factors for developing arteriosclerosis, researchers found increased activity of noradrenergic system, and a correlation between the increased levels of cholesterol and catecholamines (36, 37). In these studies researchers pointed out that norepinephrine increase the activity of lipoprotein lipase, and the result of increased activity of lipoprotein lipase is increase of free fatty acids in the serum, which in turn may be converted by the liver into cholesterol. Furthermore, in one study provided on patients with panic disorder authors was found correlation between increased activity of lipoprotein lipase, cholesterol, and catecholamines concentrations (14).
In conclusion, we can say that high levels of serum lipids in PTSD are probably the consequence of the increased activity of the noradrenergic system, because there is strong correlation between noradrenalin and lipids level (36, 37). Therefore, the population with PTSD is at risk of numerous somatic complications, especially cerebro/cardio-vascular disorders, because, the increase of serum lipids is directly related to the higher risk of arteriosclerosis and vascular incidents (38). The new research should investigate the correlation between the PTSD, the level of the serum lipids, and concentration of catecholamines. Also, in the future routine serum lipids screening in of patients with PTSD is necessary as well as undertaking preventive actions for decrease of atherosclerosis with all consequences in patients with PTSD.
References
- 1.Boston PF, Dursun SM, Reveley MA. Cholesterol and mental disorder. Br J Psychiatry. 1996;169:682–689. doi: 10.1192/bjp.169.6.682. [DOI] [PubMed] [Google Scholar]
- 2.Steinert T, Woelfle M, Gebhardt RP. No correlation of serum cholesterol levels with measures of violence in patients with schizophrenia and non-psychotic disorders. Eur Psychiatry. 1999;14:346–348. doi: 10.1016/s0924-9338(99)00157-1. [DOI] [PubMed] [Google Scholar]
- 3.Vikkunen MD. Serum cholesterol in anti-social personality. Neuropsychobiology. 1994;5:27–30. doi: 10.1159/000117660. [DOI] [PubMed] [Google Scholar]
- 4.Alverez JC, Cremniter D, Gluck N, Quintin P, Leboyer M, Berlin I, Therond P, Spreux-Varoquaux O. Low serum cholesterol in violent but not in non-violent suicide attempters. Psychiatry Res. 2000;95:103–108. doi: 10.1016/s0165-1781(00)00171-2. [DOI] [PubMed] [Google Scholar]
- 5.Bond AJ. Serum cholesterol, triglycerides, and aggression in the general population. Br J Psychiatry. 1993;163:666–668. doi: 10.1192/bjp.163.5.666. [DOI] [PubMed] [Google Scholar]
- 6.Tripodianakis J, Markianos M, Sarantidis D, Agouridaki M. Biogenic amine turnover and serum cholesterol in suicide attempt. Eur Arch Psychiatry Clin Neurosci. 2002;252:38–43. doi: 10.1007/s004060200007. [DOI] [PubMed] [Google Scholar]
- 7.Buydens-Branchey L, Branchey M, Hudson J, Fergeson P. Low HDL cholesterol, aggression and altered central serotonergic activity. Psychiatry Res. 2000;93:93–102. doi: 10.1016/s0165-1781(99)00126-2. [DOI] [PubMed] [Google Scholar]
- 8.Repo-Tiihonen E, Halonen P, Tiihonen J, Virkkunen M. Total serum cholesterol level, violent criminal offences, suicidal behavior, mortality and the appearance of conduct disorder in Finnish male criminal offenders with antisocial personality disorder. Eur Arch Psychiatry Clin Neurosci. 2002;252:8–11. doi: 10.1007/s004060200001. [DOI] [PubMed] [Google Scholar]
- 9.Yates WR, Wallace R. Cardiovascular risk factors in affective disorder. J Affect Disord. 1987;12:129–134. doi: 10.1016/0165-0327(87)90004-8. [DOI] [PubMed] [Google Scholar]
- 10.Oxenkrug GF, Branconnier RJ, Harto-Truax N, Cole JO. Is serum cholesterol a biological marker for major depressive disoder? Am J Psychiatry. 1983;140:920–921. doi: 10.1176/ajp.140.7.920. [DOI] [PubMed] [Google Scholar]
- 11.Dealberto MJ, Ducimetiere P, Mainard F, Alperovitch A. Serum lipids and depression. Lancet. 1993;341:435. [PubMed] [Google Scholar]
- 12.Morgan RE, Palinkas LA, Barrett-Connor EL, Wingard DL. Plasma cholesterol and depressive symptoms in older men. Lancet. 1993;341:75–79. doi: 10.1016/0140-6736(93)92556-9. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy F, Carroll BJ. Hypocholesterolemia during mixed manic episodes. Eur Arch Psychiatry Clin Neurosci. 2002;252:110–114. doi: 10.1007/s00406-002-0367-4. [DOI] [PubMed] [Google Scholar]
- 14.Bajwa WK, Asnis GM, Sanderson WC, Irfan A, van Praag HM. High cholesterol levels in patients with panic disorder. Am J Psychiatry. 1992;149:376–378. doi: 10.1176/ajp.149.3.376. [DOI] [PubMed] [Google Scholar]
- 15.Shioiri T, Fujii K, Someya T, Takahashi S. Serum cholesterol levels and panic symptoms in patients with panic disorder: a preliminary study. J Affect Disorders. 2000;58:167–170. doi: 10.1016/s0165-0327(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 16.Kuczmierczyk AR, Barbee JG, Bologna NA, Townsend MH. Serum cholesterol levels in patients with generalized anxiety disorder (GAD) and with GAD and comorbid major depression. Can J Psychiatry. 1996;41:465–468. doi: 10.1177/070674379604100712. [DOI] [PubMed] [Google Scholar]
- 17.Sevincok L, Buyukozturk A, Dereboy F. Serum lipid concentrations in patients with comorbid generalized anxiety disorder and major depressive disorder. Can J Psychiatry. 2001;46:68–71. doi: 10.1177/070674370104600110. [DOI] [PubMed] [Google Scholar]
- 18.Peter H, Hand I, Hohagen F, Koenig A, Mindermann O, Oeder F, Wittich M. Serum cholesterol level comparison: control subjects, anxiety disorder patients, and obsessive-compulsive disorder patients. Can J Psychiatry. 2002;47:557–561. doi: 10.1177/070674370204700608. [DOI] [PubMed] [Google Scholar]
- 19.Kagan BL, Leskin G, Haas B, Wilkins J, Foy D. Elevated lipid levels in Vietnam veterans with chronic posttraumatic stress disorder. Biol Psychiatry. 1999;45:374–377. doi: 10.1016/s0006-3223(98)00059-6. [DOI] [PubMed] [Google Scholar]
- 20.Solter V, Thaller V, Karlović D, Crnković D. Elevated serum lipids in veterans with combat-related chronic posttraumatic stress disorder. Croat Med J. 2002;43:685–689. [PubMed] [Google Scholar]
- 21.Zoričić Z, Karlović D, Buljan D, Marušić S. Comorbid alcohol addiction increases aggression level in soldiers with combat-related posttraumatic stress disorder. Nord J Psychiatry. 2003;57:199–202. doi: 10.1080/08039480310001337. [DOI] [PubMed] [Google Scholar]
- 22.Kozarić-Kovačić D, Hercigonja DK, Grubišić-Ilić M. Posttraumatic stress disorder and depression in soldiers with combat experiences. Croat Med J. 2001;42:165–170. [PubMed] [Google Scholar]
- 23.Stahl S. Neuroscientific basis and practical applications. Cambridge: Cambridge University Press; 2002. Essential psychopharmacology; pp. 199–295. [Google Scholar]
- 24.Thaller V, Breitenfeld D, Buljan D. Alcohol consumption among Croatian combat veterans. Eur J Psychiat. 1997;11:43–50. [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and statistic manual of mental disorders. IV. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 26.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery SA, Asperg M. A new depression scale designed to be sensitive to change. Brit J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 28.Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298:784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eastham RD. Biochemical values in clinical medicine. London: John wright & Sons; 1978. pp. 103–109. [Google Scholar]
- 30.Zoričić Z, Buljan D, Thaller V, Karlović D. Aggression in posttraumatic stress disorder comorbid with alcohol dependence. Eur J Psychiat. 2003;17:243–247. [Google Scholar]
- 31.Karlović D, Kozarić-Kovačić D, Kocijan-Hercigonja D. Elevation of serum total triiodothironine and free triiodothironine in Croatian veterans with combat-related post-traumatic stress disorder. Mil Med. 2002;167:846–849. [PubMed] [Google Scholar]
- 32.Thaller V, Marušić S, Katinić K, Buljan D, Golik-Gruber V, Potkonjak J. Biological factors in patients with post-traumatic stress disorder and alcoholism. Eur J Psychiat. 2003;17:87–98. [Google Scholar]
- 33.McCann BS, Magee MS, Broyles FC, Vaughan M, Albers JJ, Knopp RH. Acute psychological stress and epinephrine infusion in normolipidemic and hyperlipidemic men: effects on plasma lipid and apoprotein concentrations. Psychosom Med. 1995;57:165–176. doi: 10.1097/00006842-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Kjeldsen SE, Rostrup M, Moan A, Mundal HH, Gjesdal K, Eide IK. The sympathetic nervous system may modulate the metabolic cardiovascular syndrome in essential hypertension. J Cardiovasc Pharmacol. 1992;20:S32–S39. [PubMed] [Google Scholar]
- 35.Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 36.Kjeldsen SE, Rostrup M, Moan A, Murdal HH, Gjesdal K, Eide IK. The sympathetic nervous system may modulate the metabolic cardiovascular syndrome in essential hypertension. J Cardiovasc Pharmacol. 1992;20(Suppl 8):S32–S39. [PubMed] [Google Scholar]
- 37.McCann BS, Magee S, Broyles FC, Vaughan M, Albers JJ, Knopp RH. Acute psychological stress and epinephrine infusion in normolipidemic and hyperlipidemic men: effects on plasma lipid and apoprotein concentrations. Psychosom Med. 1995;57:165–176. doi: 10.1097/00006842-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Quiziblash N, Duffy SW, Warlow C, Mann JI. Lipids are risk factors for ischemic stroke: overview and review. Cerebrovasc Dis. 1992;2:127–136. [Google Scholar]