Abstract
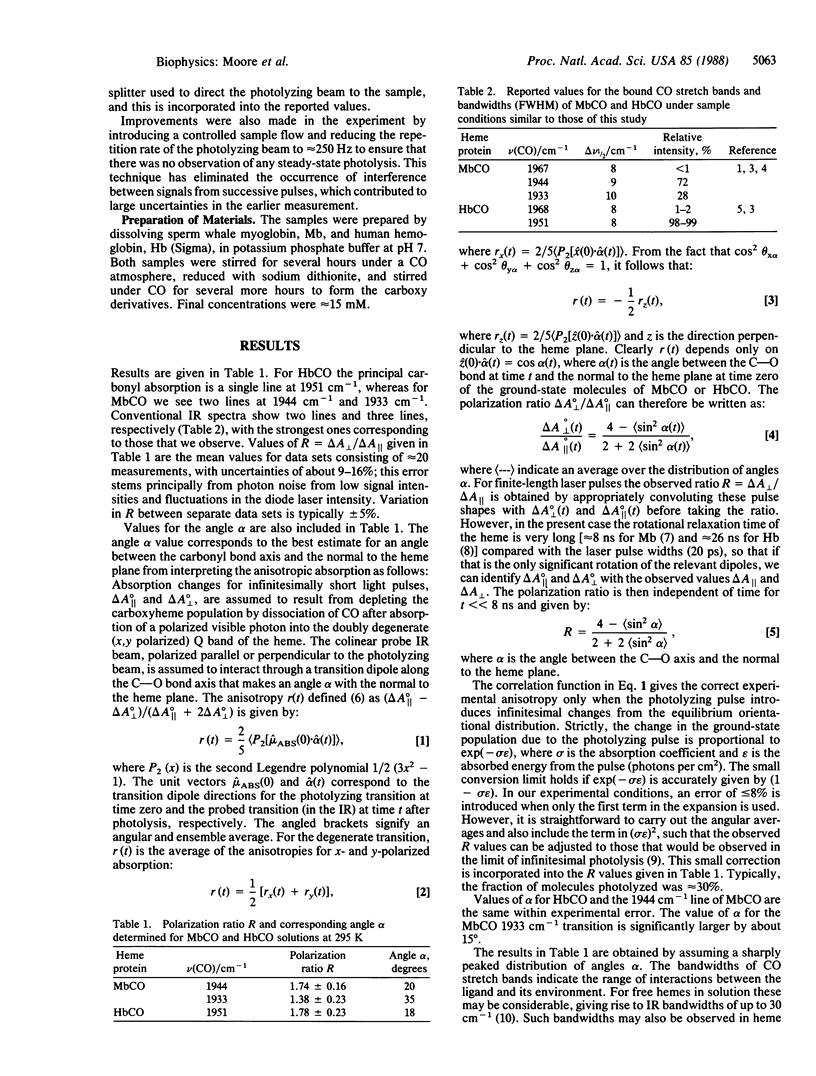
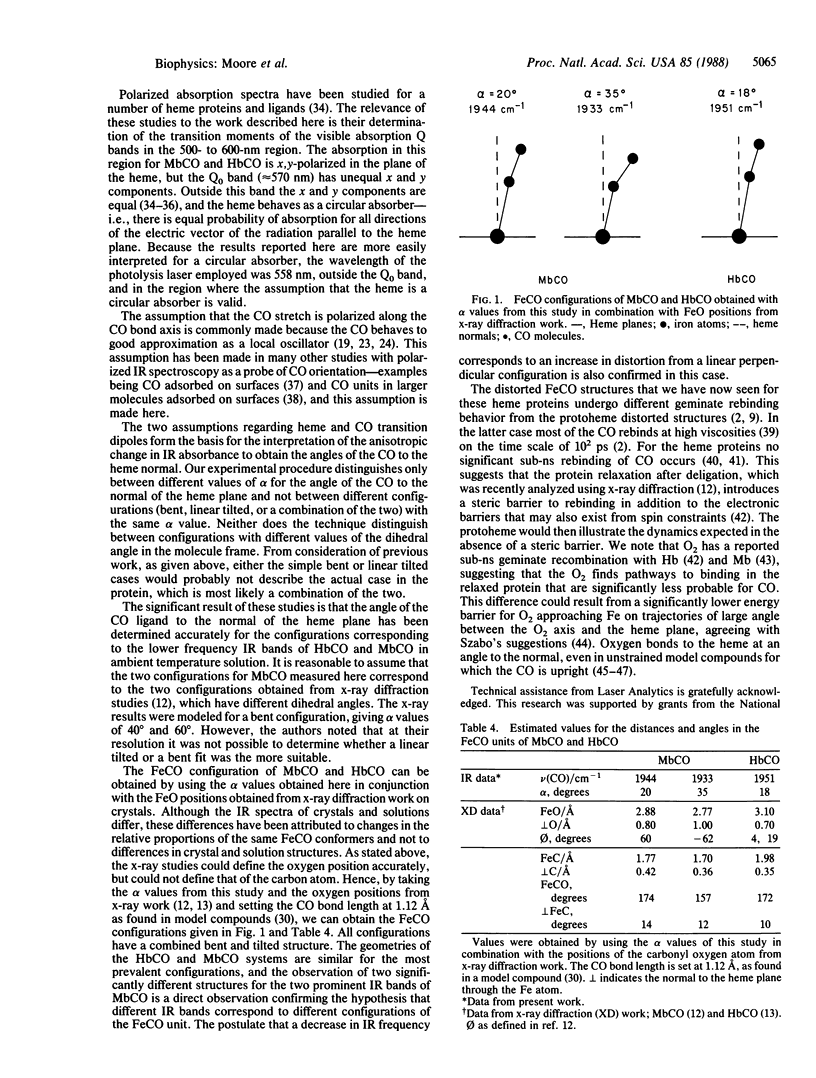
The iron-carbonyl geometries in carboxymyoglobin (MbCO) and carboxyhemoglobin (HbCO) in ambient temperature solution have been investigated using picosecond time-resolved infrared spectroscopy. Polarized infrared and visible beams were used to monitor the change in infrared absorbance of the bound CO stretch bands on photodissociation of the ligand. The ratio of the change in absorbance for perpendicular and parallel relative polarizations of the photolysis and infrared probe beams is directly related to the angle between the ligand bond axis and the normal to the heme plane. Ratios, and hence the angles, have been obtained for the configurations giving rise to the principal CO stretch infrared absorption bands of HbCO and MbCO: 18 degrees for the 1951 cm-1 band of HbCO; 20 degrees and 35 degrees, respectively, for the 1944 cm-1 and 1933 cm-1 bands of MbCO. Structures consistent with x-ray diffraction and the picosecond experiments reported here are proposed for MbCO and HbCO in which the Fe-C bond tilts to the heme normal and the Fe-C-O angle differs significantly from 180 degrees.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. M. The structure of human carbonmonoxy haemoglobin at 2.7 A resolution. J Mol Biol. 1980 Jan 15;136(2):103–128. doi: 10.1016/0022-2836(80)90308-3. [DOI] [PubMed] [Google Scholar]
- Bianconi A., Congiu-Castellano A., Durham P. J., Hasnain S. S., Phillips S. The CO bond angle of carboxymyoglobin determined by angular-resolved XANES spectroscopy. Nature. 1985 Dec 19;318(6047):685–687. doi: 10.1038/318685a0. [DOI] [PubMed] [Google Scholar]
- Brown W. E., 3rd, Sutcliffe J. W., Pulsinelli P. D. Multiple internal reflectance infrared spectra of variably hydrated hemoglobin and myoglobin films: effects of globin hydration on ligand conformer dynamics and reactivity at the heme. Biochemistry. 1983 Jun 7;22(12):2914–2923. doi: 10.1021/bi00281a021. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Ligand binding channels reflected in the resonance Raman spectra of cryogenically trapped species of myoglobin. J Biol Chem. 1987 Nov 5;262(31):14885–14890. [PubMed] [Google Scholar]
- Case D. A., Karplus M. Stereochemistry of carbon monoxide binding to myoglobin and hemoglobin. J Mol Biol. 1978 Aug 25;123(4):697–701. doi: 10.1016/0022-2836(78)90214-0. [DOI] [PubMed] [Google Scholar]
- Chance B., Fischetti R., Powers L. Structure and kinetics of the photoproduct of carboxymyoglobin at low temperatures: an X-ray absorption study. Biochemistry. 1983 Aug 2;22(16):3820–3829. doi: 10.1021/bi00285a017. [DOI] [PubMed] [Google Scholar]
- Chance M. R., Campbell B. F., Hoover R., Friedman J. M. Myoglobin recombination at low temperature. Two phases revealed by Fourier transform infrared spectroscopy. J Biol Chem. 1987 May 25;262(15):6959–6961. [PubMed] [Google Scholar]
- Chernoff D. A., Hochstrasser R. M., Steele A. W. Geminate recombination of O2 and hemoglobin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5606–5610. doi: 10.1073/pnas.77.10.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choc M. G., Caughey W. S. Evidence from infrared and 13C NMR spectra for discrete rapidly interconverting conformers at the carbon monoxide binding sites of hemoglobins A and Zurich. J Biol Chem. 1981 Feb 25;256(4):1831–1838. [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Reed C. A., Robinson W. T., Rodley G. A. Structure of an iron(II) dioxygen complex; a model for oxygen carrying hemeproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1326–1329. doi: 10.1073/pnas.71.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius P. A., Steele A. W., Chernoff D. A., Hochstrasser R. M. Different dissociation pathways and observation of an excited deoxy state in picosecond photolysis of oxy- and carboxymyoglobin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7526–7529. doi: 10.1073/pnas.78.12.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Findsen E. W., Simons P., Ondrias M. R. Response of the local heme environment of (carbonmonoxy)hemoglobin to protein dehydration. Biochemistry. 1986 Dec 2;25(24):7912–7917. doi: 10.1021/bi00372a019. [DOI] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. CO and O2 complexes of soybean leghemoglobins: pH effects upon infrared and visible spectra. Comparisons with CO and O2 complexes of myoglobin and hemoglobin. Biochemistry. 1979 Apr 3;18(7):1309–1321. doi: 10.1021/bi00574a030. [DOI] [PubMed] [Google Scholar]
- Hanson J. C., Schoenborn B. P. Real space refinement of neutron diffraction data from sperm whale carbonmonoxymyoglobin. J Mol Biol. 1981 Nov 25;153(1):117–146. doi: 10.1016/0022-2836(81)90530-1. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Eaton W. A. Polarized single crystal absorption spectra of carboxy- and oxyhemoglobin. Ann N Y Acad Sci. 1973;206:210–222. doi: 10.1111/j.1749-6632.1973.tb43213.x. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden M. C., Hazard E. S., 3rd, Gibson Q. H. Protoheme-carbon monoxide geminate kinetics. Biochemistry. 1986 May 20;25(10):2786–2792. doi: 10.1021/bi00358a008. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Antonetti A., Orszag A. Femtosecond photodissociation and picosecond recombination of O2 in myoglobin: a plausible explanation for the low quantum yield in MbO2. Biochem Biophys Res Commun. 1982 Aug;107(3):803–810. doi: 10.1016/0006-291x(82)90594-0. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. An infrared study of NO bonding to heme B and hemoglobin A. Evidence for inositol hexaphosphate induced cleavage of proximal histidine to iron bonds. Biochemistry. 1976 Jan 27;15(2):388–396. doi: 10.1021/bi00647a023. [DOI] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. Infrared spectroscopy of ligands, gases, and other groups in aqueous solutions and tissue. Methods Enzymol. 1978;54:302–323. doi: 10.1016/s0076-6879(78)54021-4. [DOI] [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Powers L., Sessler J. L., Woolery G. L., Chance B. CO bond angle changes in photolysis of carboxymyoglobin. Biochemistry. 1984 Nov 6;23(23):5519–5523. doi: 10.1021/bi00318a021. [DOI] [PubMed] [Google Scholar]
- Satterlee J. D., Teintze M., Richards J. H. Spectroscopic studies of the nature of ligand bonding in carbonmonoxyhemoglobins: evidence of a specific function for histidine-E7 from infrared and nuclear magnetic resonance intensities. Biochemistry. 1978 Apr 18;17(8):1456–1462. doi: 10.1021/bi00601a015. [DOI] [PubMed] [Google Scholar]
- Scheidt W. R., Haller K. J., Fons M., Mashiko T., Reed C. A. A (carbonmonoxy)heme complex with a weak proximal bond. Molecular stereochemistry of carbonyl(deuteroporphinato)(tetrahydrofuran)iron(II). Biochemistry. 1981 Jun 9;20(12):3653–3657. doi: 10.1021/bi00515a054. [DOI] [PubMed] [Google Scholar]
- Szabo A. Kinetics of hemoglobin and transition state theory. Proc Natl Acad Sci U S A. 1978 May;75(5):2108–2111. doi: 10.1073/pnas.75.5.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubaki M., Srivastava R. B., Yu N. T. Resonance Raman investigation of carbon monoxide bonding in (carbon monoxy)hemoglobin and -myoglobin: detection of Fe-CO stretching and Fe-C-O bending vibrations and influence of the quaternary structure change. Biochemistry. 1982 Mar 16;21(6):1132–1140. doi: 10.1021/bi00535a004. [DOI] [PubMed] [Google Scholar]
- Yu N. T., Kerr E. A., Ward B., Chang C. K. Resonance Raman detection of Fe-CO stretching and Fe-C-O bending vibrations in sterically hindered carbonmonoxy "strapped hemes". A structural probe of Fe-C-O distortion. Biochemistry. 1983 Sep 13;22(19):4534–4540. doi: 10.1021/bi00288a028. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Resonance Raman studies of ligand binding. Methods Enzymol. 1986;130:350–409. doi: 10.1016/0076-6879(86)30018-1. [DOI] [PubMed] [Google Scholar]


