Abstract
Lamivudine, a nucleoside analogue, has been used widely as an effective antiviral agent for the treatment of patients with chronic hepatitis B virus (HBV) infection. However, the YMDD motif mutation of HBV polymerase resistant to lamivudine occurs very frequently after long term therapy. We developed an oligonucleotide chip for the detection of YMDD motif mutants resistant to lamivudine and investigated the prevalence of the mutants in patients with chronic HBV infection who had not been treated by lamivudine before. Forty patients who had not been treated with lamivudine were included in this study. Serum samples were tested by the oligonucleotide chips designed for detection of wild-type YMDD motif, M552V and M552I. Samples were confirmed by restriction fragment length polymorphism (RFLP) and direct sequencing. M552I mutants were detected by the oligonucleotide chips in 7.5% (3/40) of chronic HBV infected patients (2 chronic hepatitis and 1 cirrhosis). The results were in accordance with those of RFLP. YMDD motif mutants occur as natural genome variabilities in patients with chronic HBV infection who had not been treated with lamivudine before. Oligonucleotide chip technology is a reliable and useful diagnostic tool for the detection of mutants resistant to antiviral therapy in chronic HBV infection.
Keywords: Hepatitis, Viral; Hepatitis B, Chronic; Lamivudine; Polymorphism (Genetics); YMDD Motif Mutants; Oligonucleotide Array Sequence Analysis
INTRODUCTION
Lamivudine, a nucleoside analogue, has been known to be an effective antiviral agent for hepatitis B virus (HBV) infection. The effectiveness of lamivudine therapy is indicated by serum HBV DNA clearance and the seroconversion of HBeAg to anti-HBe. According to follow-up reports of the multicenter study, seroconversion rates increased with the duration of treatment from 17% at 1 yr to 27% and 40% at 2 and 3 yr, respectively (1, 2). However, the emergence of viral mutants resistant to lamivudine is the main concern with the treatment. The resistance begins to occur after 8 months of treatment (3, 4) and the resistance rates increase with the duration of therapy from 17% at 1 yr to 39% and 57% at 2 and 3 yr, respectively (2). The lamivudine-resistant mutants affect the YMDD motif of the catalytic domain of the HBV DNA polymerase. The most common mutants have a change at codon 552 from M to V (M552V) or from M to I (M552I). Although the mutations of YMDD motif are thought to be secondary to lamivudine use, it is considered that the pretreatment mutants already exist in endemic regions like Korea (5). Oligonucleotide chip technology is a very useful tool for the rapid and accurate identification of pathogenic microbes and the detection of drug-resistant and point mutations. We developed an oligonucleotide chip for the detection of mutants resistant to lamivudine. This study aimed to determine the prevalence of natural mutants of YMDD motif by oligonucleotide chip technology in patients with chronic HBV infection who had never received treatment of any antiviral agent including lamivudine.
MATERIALS AND METHODS
Patients
This study consisted of 40 patients with chronic HBV infection who had never received any antiviral medication including lamivudine. Their average age was 29 yr (from 13 to 57 yr), and there were 38 males and 2 females. Thirty-four patients had chronic hepatitis and 6 had cirrhosis.
HBV DNA purification and target DNA preparation
As soon as blood samples were collected they were refrigerated for one hour and left to coagulate, which then were centrifuged at 3,000 rpm for 5 min to separate the sera. These sera were stored at -70℃, and HBV DNA was extracted by alkaline methods. Eight µL of 1 M NaOH and 72 µL of fresh defrosted serum were placed in a sterilized 1.5 mL test tube, which was shaken for 10 sec and then left at 37℃ for one hour. In this 80 µL mixture of serum and NaOH, 0.1 M HCl of the same amount was added and shaken for 10 sec, which was then centrifuged at 12,000 rpm for 10 min. The supernatant was transferred to a new test tube and stored at -20℃. Two µL of the DNA isolated from this process was used for each polymerase chain reaction (PCR).
Amplification of the target DNA to detect YMDD-mutant HBV was prepared by two biotin-modified primers, HBF2-biotin and HBR-biotin (Table 1). The primers and probes used in this study were oligonucleotide manufactured by the Perkin-Elmer DNA synthesizer (BioBasic, Ontario, Canada). PCR reactants consisted of 500 mM KCl, 100 mM Tris HCl (pH 9.0), 1% Triton X-100, 0.2 mM dNTP (dATP, dGTP, dTTP and dCTP), 1.5 mM MgCl2, 20 pmol primer and 1 U Taq DNA polymerase (QIAGEN, CA, U.S.A.). This mixture was constituted in total to 25 µL by adding 2 µL of template DNA. It was left to react at 95℃ for 3 min, enough to denature, followed by 30 cycles of reactions at 95℃ for 1 min, 50℃ for another minute, and at 72℃ for 1 min. The last cycle was extended for 10 min at 72℃. After these reactions, this PCR product was verified to be 180 bp by electrophoresis with 2% agarose gel, stained with ethidium bromide and visualized on a gel documentation system (gel doc 2000, Bio-Rad, CA, U.S.A.).
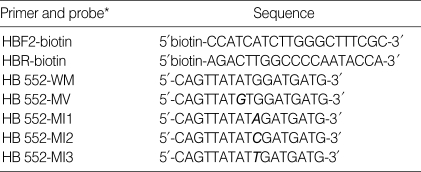
Table 1.
Primers and probes used in YMDD motif mutant detection assay
The variable sequences are in bold and italic print. W means wild type. M means mutant type.
*Characters following W or M mean amino acid type encoded by codon at mutation region. V, valine; I, isoleucine.
The design of oligonucleotide DNA chip
One probe (HB552-WM) was to detect wild-type YMDD motif and four kinds of probes (HB552-MV, HB552-MI1, HB552-MI2 and HB552-MI3) were to detect YMDD mutant on the bases of the HBV DNA polymerase sequence (Table 1). The 5'-end of each oligonucleotide probe was aminated to increase the efficacy of the immobilization of the oligonucleotide to a glass slide. The probes were diluted to 50 pmol with 3×SCC (300 mM NaCl, 30 mM Na-citrate, pH 7.0) solution. These probes were printed in duplex arrays as shown in Fig. 1, and were adhered to the silylated glass slide (Cel Associates, Texas, U.S.A.) using the Microarrayer (PLXSYS 7500 SQXL Microarryer, Cartesian Technologies, CA, U.S.A.).
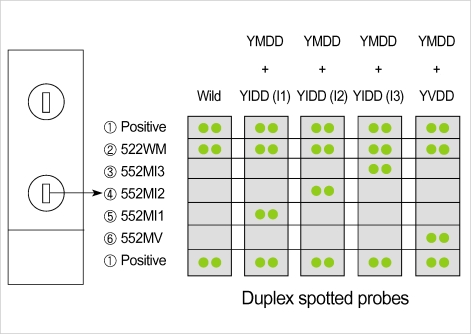
Fig. 1.
This schematic illustration shows the probe layout for the 17 mer oligonucleotide sequences noted in Table 1 and the interpretative results of oligonucleotide chips for YMDD motif mutation detection. The oligonucleotide probes are patterned as duplex arrays. ① Positive is mixture of HB552-WM, HB552-MV, HB552-MI1, HB552-MI2 and HB552-MI3 probes (Table 1), ② 522WM, ③ 552MI3, ④ 552MI2, ⑤ 552MI1 and ⑥ 552MV indicates HB552-WM, HB552-MV, HB552-MI1, HB552-MI2 and HB552-MI3 probes, respectively.
To get rid of unattached probes on the surface, the glass slide was washed with 0.2% SDS and then with distilled water twice, followed by sodium borohydride solution (300 mL of PBS, 100 mL of ethanol and 1 g of Na2BH4), and finally washed out with boiling distilled water. The slide was washed using 0.2% SDS again and then using distilled water at room temperature.
The detection of the mutants using the oligonucleotide chip
The biotin-labeled target DNA was boiled for 10 min and annealed at 4℃ for 10 min to separate the two strands. Hybridization of 2 µL of biotin-labeled ssDNA samples to the oligonucleotide chip was performed in hybridization solution, 3 µL of 20×SSPE, 1.35 µL of 22.2 M formamide, 0.5 µL of bovine serum albumin (BSA) and 0.1 µL of salmon sperm DNA containing 0.06 µL of diluted Cy3-labeled streptavidin (Amersham Pharmacia Biotech, NJ, U.S.A.). Each sampling was placed on the slide, the cover slip being applied carefully so that no air bubble was trapped inside, and was left for reaction at 40℃ for 30 min. After removing the cover slip using 2×SSC, the slide was washed out using 2×SSC and 0.2×SCC solution in this order. The GenePix 4000A (Axon Instruments, CA, U.S.A.), non-confocal laser scanner was used for oligonucleotide chip analysis (543 nm for excitation of Cy3).
Restriction fragment length polymorphism (RFLP) of YIDD (I3) mutant
To confirm the results of the oligonucleotide chip experiment, the partial polymerase gene containing YMDD was analyzed with the restriction fragment length polymorphism (RFLP) method and the PCR direct-sequencing.
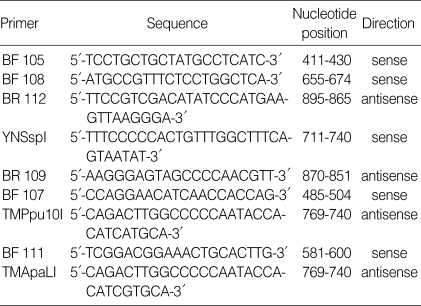
A PCR reactant solution, which consisted of 500 mM KCl, 100 mM Tris HCl (pH 9.0), 1% Triton X-100, 0.2 mM dNTP (dATP, dGTP, dTTP and dCTP), 1.5 mM MgCl2, 10 pmol primer (BF108 and BR112, Table 2) (4) and 1 U Taq DNA polymerase (QIAGEN, CA, U.S.A.), was added to 2 µL of HBV DNA purified by the alkaline method to make it 25 µL in total. This mixture solution was left to denature at 94℃ for 4 min, followed by 35 cycles of 1 min at 94℃, 1 min at 58℃ and 1 min and 30 sec at 72℃. For the last cycle, it was left for 10 min at 72℃. The second PCR was performed under the same circumstances as the first PCR using 1 µL of the first PCR product, YNSspI and BR109 primer. This product was electrophoresed with 2% agarose gel to confirm that the PCR product was about 160 bp. Six µL of this confirmed product was added to 5 units of the restriction enzyme SspI (TaKaRa, Shiga, Japan) and SspI buffer and left for reaction at 37℃ for 3 hr, which was again confirmed by electrophoresis with 4% agarose gel.
Table 2.
Primers used in sequencing and enzyme digestion assay
RFLP of YVDD mutant
As described above, the PCR was performed with BF107 and TMPpu10I primers (the first PCR) and BF111 and TMApaLI primers (the second PCR) (Table 2). This product was electrophoresed with 2% agarose gel to confirm that the PCR product was about 190 bp. Six µL of this confirmed product was added to 5 units of the restriction enzyme ApaLI (TaKaRa, Shiga, Japan) and ApaLI buffer and left for reaction at 37℃ for 3 hr, which was again confirmed by electrophoresis with 4% agarose gel.
Sequencing and sequence analysis
PCR was performed with BF105 and BR112 primers (Table 2) under the same condition as target DNA preparation. The sequence of PCR products was determined with BF111 and BR109 primers, and using the ABI prism BigDye Terminator cycle sequencing Ready Reaction Kit (Applied Biosystem Inc., CA, U.S.A.) in the automated BaseStation-51 DNA fragment analyzer (MJ Research, CA, U.S.A.).
RESULTS
Sensitivity and specificity of oligonucleotide chip
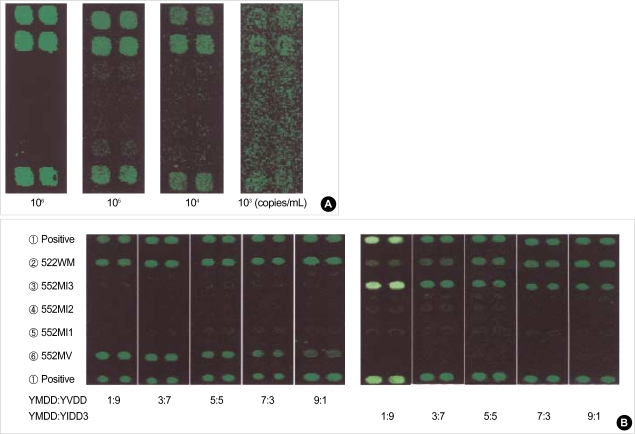
After serially diluting samples (106-103 copies/mL) with known HBV DNA concentrations by the Cobas Amplicor HBV Monitor test™, (Roche Diagnostics Systems, Meylan, France), the detection limit of the DNA chip was evaluated. The detection limit obtained by a DNA chip assay was 104 copies/mL of serum by the first PCR (Fig. 2A) and 103 copies/mL by nested PCR (data not shown). We constructed plasmids containing a copy of the YMDD, YVDD and YIDD of the polymerase gene of HBV. The YMDD, YVDD and YIDD plasmids (cloned HBV DNA) were used to determine the capacity of the DNA chip to detect wild-type HBV and YMDD motif mutant in a single sample. We could detect the YMDD motif mutant with the DNA chip when the mutant was mixed with a ten-fold-higher and -lower amount of wild-type HBV (Fig. 2B).
Fig. 2.
Sensitivity and specificity of the detection of the YMDD mutants using oligonucleotide chip. (A) Detection limit of the YMDD mutants by oligonucleotide chip, (B) Detection capacity of mixture of wild-type and YMDD motif mutants. YMDD: YVDD represents ratios of YMDD and YVDD; YMDD:YIDD3 represents ratios of YMDD and YIDD3.
Detection of YMDD Mutants by oligonucleotide chip
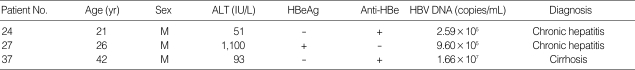
Thirty seven patients out of 40 had the wild-type YMDD mutants, and 3 of them (7.5%) had the mixed type of YMDD and YIDD (I3). Among 3 patients with any mutation (24th, 27th and 37th samples), 2 had chronic hepatitis and one had liver cirrhosis (Table 3, Fig. 3).
Table 3.
Clinical features of patients with YMDD motif mutation (YMDD+YIDD) at the time of sampling
Fig. 3.
Scanned images of oligonucleotide chip (A) wild type [YMDD], (B), (C), (D) YMDD motif mutation [YMDD+YIDD(I3)], slides of No. 24, 27 and 37, respectively.
Detection of YMDD mutants by RFLP and direct sequencing
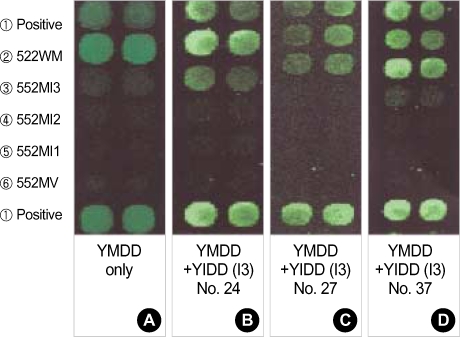
When the codon 552 of the second PCR product by the TMApaLI primer was GTG (GTGCAC), there was no band seen, and was 30 bp shorter by the limiting enzyme ApaLI, throughout the samples. When the codon 552 of the second PCR product using the YNSspI primer was ATT (AATATT), about 30 bp were cut by the limiting enzyme SspI, and this shortened band was confirmed by electrophoresis with 4% agarose gel. Out of the total samples, shortened bands were observed in sample Nos. 24, 27 and 37, which were identical to YIDD mutants (ATT) detected by the oligonucleotide chip. No shortened band was seen in the other samples (Fig. 4).
Fig. 4.
(A) YVDD RFLP analysis in 4% agarose gel does not show any band digested by the restriction enzyme ApaLI. M: 100 bp ladder. (B) YIDD RFLP analysis in 4% agarose gel shows DNA bands (arrows) digested by the restriction enzyme SspI in 3 YIDD (I3) mutants (sample Nos. 24, 27 and 37). M: B 100 bp ladder.
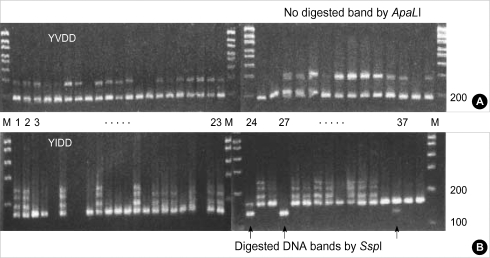
The PCR direct-sequencing method was unable to prove YMDD mutation, which was confirmed by the oligonucleotide chip (Fig. 5).
Fig. 5.
The sequences of 290 bp DNA including codon 552 represent only the wild-type (ATG) in the samples with YIDD mutants (No. 24, 27 and 37).
DISCUSSION
The YMDD motif mutant of the polymerase gene is frequently found in patients who used lamivudine on a long term basis, and it is usually reported to emerge after 8 months of therapy (2, 6, 7). The selection of a mutation in the YMDD motif is known to occur several months earlier than the phenotypic resistance, however the information about how early the genotypic resistance occurs and what causes the resistance is limited due to the small number of subjects and differences in detection methods (3, 4, 8). Hence the rate of hepatitis B resistance to lamivudine might increase further when more sensitive tests are used to detect the small amount of YMDD mutants among the wild type HBV, which would impact upon detection and monitoring the resistance.
This study demonstrated by oligonucleotide chip technology that the YMDD mutants exist naturally in the patients with chronic hepatitis B infection who had not received lamivudine before.
YMDD mutants have a significantly low replication ability. It is thought that naturally occurring YMDD mutants occupy very small portion of total HBV, and very sensitive investigative tools are required to prove it. Oligonucleotide chip technology is a very useful tool for the rapid and accurate identification of pathogenic microbes and the detection of drug-resistant and point mutations. This method has such sensitivity that it can find rifampin-resistant tuberculosis resultant from point mutation in amounts of less than 1% (9). Oligonucleotide chip technology has been expected to be clinically applicable to detect minor variants in viral quasispecies and to increase our knowledge of spontaneous viral genome variability and selection of mutants by antiviral therapy (10). We developed the oligonucleotide chip for the detection of mutants resistant to lamivudine. In the experiment using reference sequences generated by site-directed mutagenesis, we confirmed the sensitivity and specificity of probes. The oligonucleotide chips could specifically detect mutations in the YMDD motif (codon 552) of the HBV DNA polymerase. The chips included three types of probe (five species): wild-probe (YMDD), valine-probe (YVDD) and isoleucineprobes (YIDD1, YIDD2 and YIDD3). We could accurately detect YMDD motif mutants of YMDD, YVDD, YIDD2 and YIDD3 types in sera from chronic HBV-infected patients. These chip results were in accordance with results from RFLP, sequence analysis and allele-specific PCR. We could detect easily as little as 10% of coexisting mutant viruses in wild type viruses.
The oligonucleotide chip method detected 3 YIDD mutants in the 40 patients included in this study. Only ATT (YIDD3) was detected in all three samples. This chip result was consistent with that of the RFLP analysis. The mutants were not detected by direct-sequencing and this is reasonable when taking into consideration that naturally occurring YMDD mutants are a minor portion of the total HBV and that the standard base sequence determinant method requires more than 25% of mutants among HBV.
Our study supports that oligonucleotide chip technology may have an important role as a reliable and useful diagnostic tool for the early detection of mutants resistant to lamivudine.
References
- 1.Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Chien RN, Dent J, Roman L, Edmundson S, Lai CL Asia Hepatitis Lamivudine Study Group. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Gastroenterology. 2000;119:172–180. doi: 10.1053/gast.2000.8559. [DOI] [PubMed] [Google Scholar]
- 2.Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S, Condreay LD, Chien RN On behalf of the Asia Hepatitis Lamivudine Study Group. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33:1527–1532. doi: 10.1053/jhep.2001.25084. [DOI] [PubMed] [Google Scholar]
- 3.Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J, Kuhns MC, Liang TJ, Hoofnagle JH. Long-term therapy of chronic hepatitis B with lamivudine. Hepatology. 2000;32:828–834. doi: 10.1053/jhep.2000.17912. [DOI] [PubMed] [Google Scholar]
- 4.Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, Koike H, Kobayashi M, Koida I, Arase Y, Saitoh S, Murashima N, Ikeda K, Kumada H. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711–1716. doi: 10.1002/hep.510270634. [DOI] [PubMed] [Google Scholar]
- 5.Paik YH, Chung HY, Ryu WS, Lee KS, Lee JS, Kim JH, Lee CK, Chon CY, Moon YM, Han KH. Emergence of YMDD motif mutant of hepatitis B virus during short-term lamivudine therapy in South Korea. J Hepatol. 2001;35:92–98. doi: 10.1016/s0168-8278(01)00065-4. [DOI] [PubMed] [Google Scholar]
- 6.Perrillo R, Rakela J, Dienstag J, Levy G, Martin P, Wright T, Caldwell S, Schiff E, Gish R, Villeneuve JP, Farr G, Anschuetz G, Crowther L, Brown N Lamivudine Transplant Group. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Hepatology. 1999;29:1581–1586. doi: 10.1002/hep.510290507. [DOI] [PubMed] [Google Scholar]
- 7.Liaw YF, Chien RN, Yeh CT, Tsai SL, Chu CM. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 8.Nafa S, Ahmed S, Tavan D, Pichoud C, Berby F, Stuyver L, Johnson M, Merle P, Abidi H, Trepo C, Zoulim F. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology. 2000;32:1078–1088. doi: 10.1053/jhep.2000.19619. [DOI] [PubMed] [Google Scholar]
- 9.Mikhailovich V, Lapa S, Gryadunov D, Sobolev A, Strizhkov B, Chernyh N, Skotnikova O, Irtuganova O, Moroz A, Litvinov V, Vladimirskii M, Perelman M, Chernousova L, Erokhin V, Zasedatelev A, Mirzabekov A. Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and lipase detection reaction on oligonucleotide microchips. J Clin Microbiol. 2001;39:2531–2540. doi: 10.1128/JCM.39.7.2531-2540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoulim F. Detection of hepatitis B virus resistance to antivirals. J Clin Virol. 2001;21:243–253. doi: 10.1016/s1386-6532(00)00167-0. [DOI] [PubMed] [Google Scholar]