Abstract
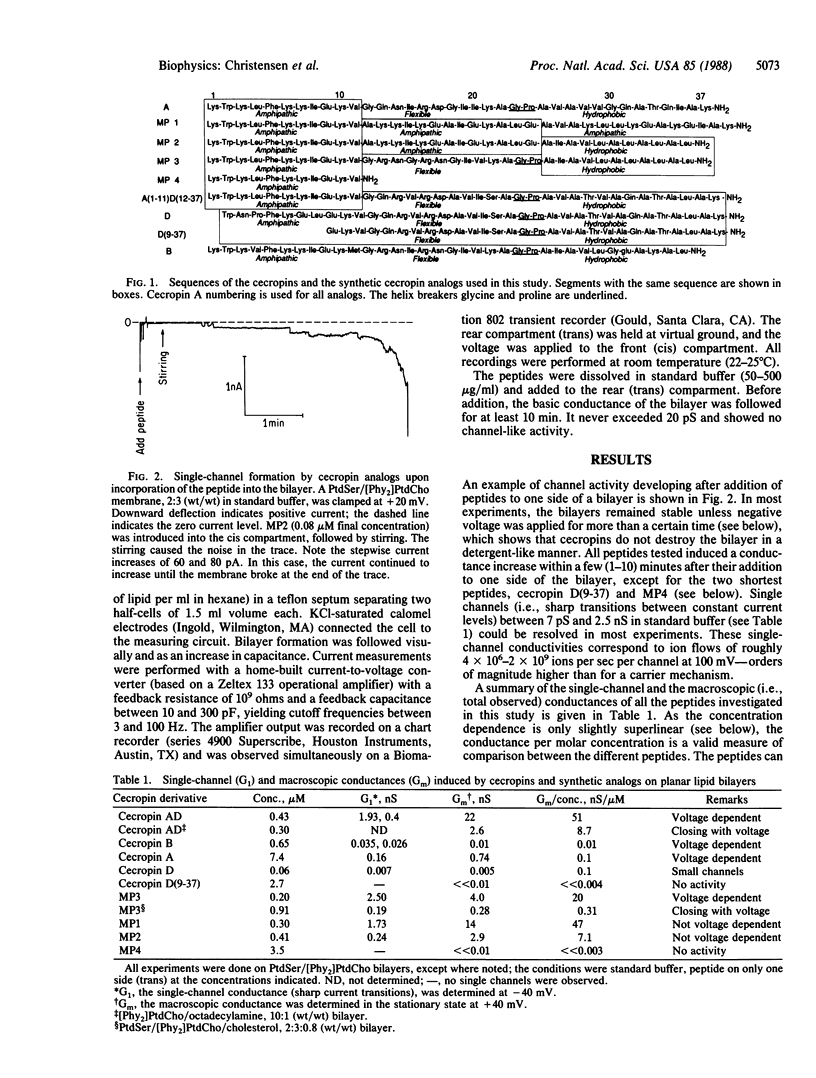
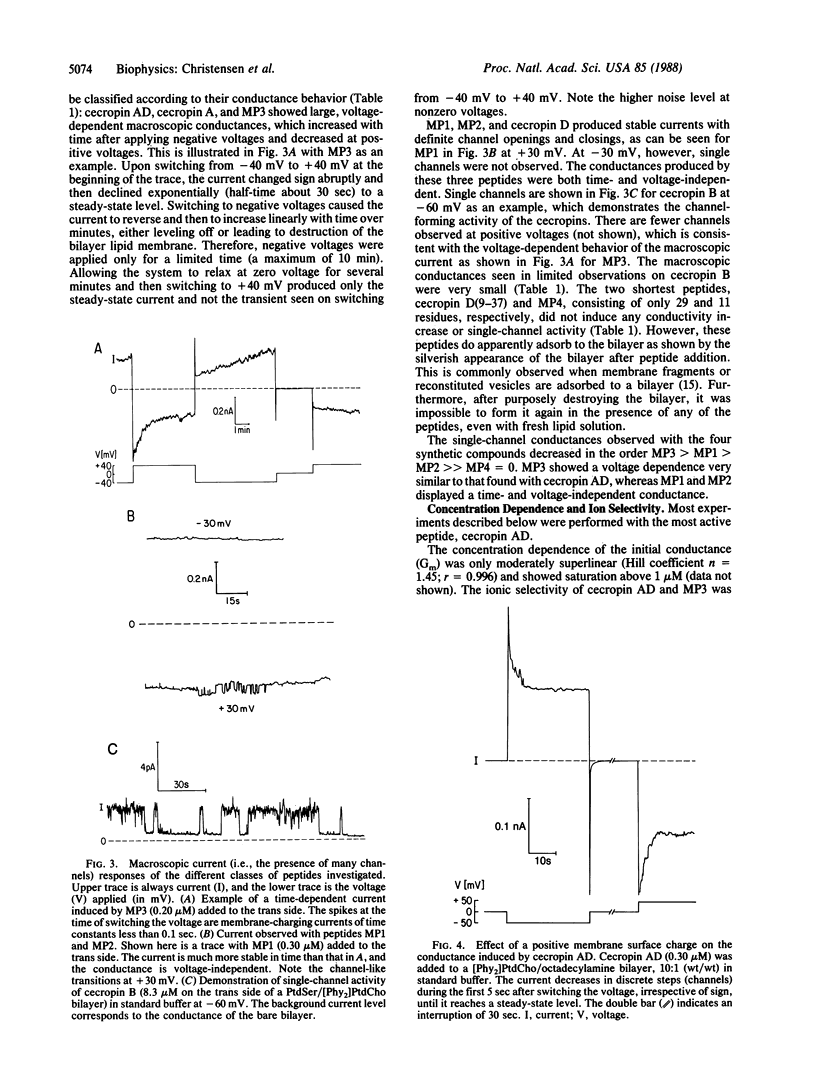
Cecropins, positively charged antibacterial peptides found in the cecropia moth, and synthetic peptide analogs form large time-variant and voltage-dependent ion channels in planar lipid membranes in the physiological range of concentration. Single-channel conductances of up to 2.5 nS (in 0.1 M NaCl) were observed, which suggests a channel diameter of 4 nm. Channels formed by the peptides cecropin AD and MP3 had a permeability ratio of Cl-/Na+ = 2:1 in 0.1 M NaCl. A comparative study of the three cecropins, cecropins A, B, and D, and of six synthetic analogs allowed determination of structural requirements for pore formation. Shorter amphipathic peptides did not form channels, although they adsorbed to the bilayer. A flexible segment between the N-terminal amphipathic region and the C-terminal more hydrophobic region of the peptide was required for the observation of a time-variant, voltage-dependent conductance. Cecropin AD was the most effective voltage-dependent pore-forming peptide and was also the most potent antibacterial peptide against several test organisms. A positive surface charge or cholesterol in the bilayer reduced the conductances caused by cecropin AD or MP3 by at least 5-fold. This behavior is consistent with the known insensitivity of eukaryotic cells to cecropins. Our observations suggest that the broad antibacterial activity of cecropins is due to formation of large pores in bacterial cell membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu D., Merrifield R. B., Steiner H., Boman H. G. N-terminal analogues of cecropin A: synthesis, antibacterial activity, and conformational properties. Biochemistry. 1985 Mar 26;24(7):1683–1688. doi: 10.1021/bi00328a017. [DOI] [PubMed] [Google Scholar]
- Andreu D., Merrifield R. B., Steiner H., Boman H. G. Solid-phase synthesis of cecropin A and related peptides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6475–6479. doi: 10.1073/pnas.80.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann G., Mueller P. A molecular model of membrane excitability. J Supramol Struct. 1974;2(5-6):538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Faye I., von Hofsten P., Kockum K., Lee J. Y., Xanthopoulos K. G., Bennich H., Engström A., Merrifield R. B., Andreu D. On the primary structures of lysozyme, cecropins and attacins from Hyalophora cecropia. Dev Comp Immunol. 1985 Summer;9(3):551–558. doi: 10.1016/0145-305x(85)90018-7. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Steiner H. Humoral immunity in Cecropia pupae. Curr Top Microbiol Immunol. 1981;94-95:75–91. doi: 10.1007/978-3-642-68120-2_2. [DOI] [PubMed] [Google Scholar]
- Cleveland M. V., Slatin S., Finkelstein A., Levinthal C. Structure-function relationships for a voltage-dependent ion channel: properties of COOH-terminal fragments of colicin E1. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3706–3710. doi: 10.1073/pnas.80.12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancsházy Z., Karvaly B. Incorporation of bacteriorhodopsin into a bilayer lipid membrane; a photoelectric-spectroscopic study. FEBS Lett. 1976 Dec 15;72(1):136–138. doi: 10.1016/0014-5793(76)80829-0. [DOI] [PubMed] [Google Scholar]
- Herrmann T. R., Rayfield G. W. The electrical response to light of bacteriorhodopsin in planar membranes. Biophys J. 1978 Feb;21(2):111–125. doi: 10.1016/S0006-3495(78)85512-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H. G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur J Biochem. 1980 May;106(1):7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Ilani A., Mauzerall D. The potential span of photoredox reactions of porphyrins and chlorophyll at the lipid bilayer-water interface. Biophys J. 1981 Jul;35(1):79–92. doi: 10.1016/S0006-3495(81)84775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E. C., Rosenberg I. M., Gitler C. An ion-channel forming protein produced by Entamoeba histolytica. EMBO J. 1982;1(7):801–804. doi: 10.1002/j.1460-2075.1982.tb01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina G., Mackman N., Holland I. B., Bhakdi S. Escherichia coli haemolysin forms voltage-dependent ion channels in lipid membranes. Biochim Biophys Acta. 1987 Nov 27;905(1):109–117. doi: 10.1016/0005-2736(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B., Vizioli L. D., Boman H. G. Synthesis of the antibacterial peptide cecropin A (1-33). Biochemistry. 1982 Sep 28;21(20):5020–5031. doi: 10.1021/bi00263a028. [DOI] [PubMed] [Google Scholar]
- Okada M., Natori S. Ionophore activity of sarcotoxin I, a bactericidal protein of Sarcophaga peregrina. Biochem J. 1985 Jul 15;229(2):453–458. doi: 10.1042/bj2290453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H., Andreu D., Merrifield R. B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988 Apr 7;939(2):260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Steiner H. Secondary structure of the cecropins: antibacterial peptides from the moth Hyalophora cecropia. FEBS Lett. 1982 Jan 25;137(2):283–287. doi: 10.1016/0014-5793(82)80368-2. [DOI] [PubMed] [Google Scholar]
- Szabo G. Dual mechanism for the action of cholesterol on membrane permeability. Nature. 1974 Nov 1;252(5478):47–49. doi: 10.1038/252047a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Kaiser E. T. The structural characterization of beta-endorphin and related peptide hormones and neurotransmitters. Pharmacol Rev. 1986 Dec;38(4):291–319. [PubMed] [Google Scholar]
- Tosteson M. T., Tosteson D. C. The sting. Melittin forms channels in lipid bilayers. Biophys J. 1981 Oct;36(1):109–116. doi: 10.1016/S0006-3495(81)84719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodle M. C., Mauzerall D. Photoinitiated ion movements in bilayer membranes containing magnesium octaethylporphyrin. Biophys J. 1986 Sep;50(3):431–439. doi: 10.1016/S0006-3495(86)83479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Blake M., Mauro A., Cohn Z. A. Properties of the major outer membrane protein from Neisseria gonorrhoeae incorporated into model lipid membranes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3831–3835. doi: 10.1073/pnas.80.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Hengartner H., Podack E. R., Cohn Z. A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Young J. D., Nathan C. F., Podack E. R., Palladino M. A., Cohn Z. A. Functional channel formation associated with cytotoxic T-cell granules. Proc Natl Acad Sci U S A. 1986 Jan;83(1):150–154. doi: 10.1073/pnas.83.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hofsten P., Faye I., Kockum K., Lee J. Y., Xanthopoulos K. G., Boman I. A., Boman H. G., Engström A., Andreu D., Merrifield R. B. Molecular cloning, cDNA sequencing, and chemical synthesis of cecropin B from Hyalophora cecropia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2240–2243. doi: 10.1073/pnas.82.8.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]



