Abstract
Objective
To clarify the cardiovascular mechanisms of cyanide poisoning by evaluating oxygen transport characteristics using a canine model.
Methods
A prospective controlled experiment was performed at a hospital‐based animal laboratory. Five male beagle (17 (2) kg) dogs were anesthetised with α‐chloralose, paralysed with pancuronium bromide and mechanically ventilated. Potassium cyanide was infused at 0.045 mg/kg/min for 110 min. Heart rate, blood pressure, cardiac output, oxygen delivery (DO2), oxygen consumption (VO2) and oxygen extraction ratio (OER) were measured every 10 min for 140 min. DO2 was measured by an indirect calorimeter.
Results
Cyanide and lactate levels peaked at 1.52 (0.25) mg/l and 9.1 (1.5) mmol/l, respectively. Systolic blood pressure remained relatively constant whereas diastolic blood pressure decreased by 19%. Cardiac output, heart rate and DO2 increased to a maximum of 6%, 10% and 10%, respectively, at 40 min, after which they declined to a low of 32%, 28% and 30% below baseline, respectively. Stroke volume remained constant. Oxygen consumption initially increased by 5%, then decreased to 24% below baseline. The OER initially declined to 35% below baseline, then increased throughout the rest of the study.
Conclusion
Cyanide poisoning in the canine model showed two phases of injury. The first (compensated) phase had a mechanism consistent with a traditional global oxygen consumption defect. The second (decompensated) phase had a mechanism consistent with heart failure. This heart failure was due to bradycardia. These data suggest chronotropy as an avenue of further study in the temporary treatment of cyanide poisoning.
Cyanide is a potentially lethal poison that may occur through inhalation (house fires),1,2 ingestion (1978 Jonestown massacre)3,4 or intravenous administration (byproduct of nitroprusside).5 In 2003, there were 273 cases of cyanide toxicity in the US.6 There has been recent concern over its potential as a weapon of mass destruction. In November 2003, the Department of Homeland Security issued a bulletin warning specifically of the threat of an attack of cyanide gas released through ventilation ducts.7 Although no cyanide attacks have occurred in the US, these incidents highlight the potential impact of cyanide poisoning.
Cyanide inhibits cytochrome oxidase aa3, leading to interference with the oxidative metabolism and cellular use of oxygen. This in vitro mechanism has been extensively studied for over 50 years.8,9,10,11 In the cardiovascular system (in vivo), inhibition of cytochrome oxidase aa3 should theoretically cause a considerable defect in global oxygen consumption. However, such an effect has never been clearly shown.12,13 Furthermore, animal studies suggest that cardiac failure, with cardiovascular collapse, may be an important pathway in cyanide poisoning.14,15,16,17,18,19 This is further supported by in vitro studies that show that cyanide is a direct myocardial suppressant.20 To clarify these mechanisms, we sought to explore cyanide's effect on the cardiovascular system by examining oxygen transport characteristics during poisoning.
Materials and methods
Study design, setting and population
We performed a prospective controlled experiment at a hospital‐based animal laboratory. Five male beagles weighing 17 (SE 2) kg were used in the study.
Study protocol
The protocol for the study was reviewed and approved by the Henry Ford Hospital Committee for Experimental Animal Care. Care was provided in accordance with the National Institutes of Health Public Health Service policy on humane care and use of laboratory animals.21
The animals were fasted overnight. They were anesthetised with 4% α‐chloralose (100 mg/kg intravenously) and paralysed with pancuronium bromide (0.15 mg/kg intravenously). The animals were orotracheally intubated and ventilated at a fixed respiratory rate of 13 breaths/min, fraction of inspired oxygen (FiO2) of 30% and a tidal volume of 10 ml/kg. Central venous access was obtained by cannulating the right external jugular vein. Fluid management consisted only of maintenance treatment (10 ml/h of 0.9 N saline). A thermodilution pulmonary artery catheter (Baxter model 9510, Irvine, California, USA) was floated into the pulmonary artery for measurement of cardiac output and pulmonary arterial pressure. Catheter placement was confirmed by haemodynamic tracings. The femoral artery was cannulated on the opposite side for measurements of blood pressure and heart rate. Rectal temperature was maintained between 37°C and 38°C by means of a water‐circulating warming blanket. After a stabilisation period of 10 min, potassium cyanide was infused at a continuous rate of 0.045 mg/kg/min after baseline parameters were established. The infusion was discontinued after 110 min. This dosage has been shown to cause toxicity in the canine model.18 All dogs were killed at the end of the study.
Measurements
Cardiac output was determined by thermodilution using 10 ml injections of 5% dextrose solution (10–15°C). Cardiac output values represent the average of three consecutive measurements within 20% of each other. Stroke volume was calculated by dividing cardiac output by heart rate. VO2 was measured and recorded continuously from inspired and expired respiratory gases by open‐circuit indirect calorimetry (Deltatrac, Datex/Instrumentation, Senor Medics, Yorba Linda, California, USA). We used this method because it accounts for lung oxygen consumption and eliminates the mathematical artefact owing to coupling that occurs with the indirect Fick method.22 DO2 was calculated as the product of cardiac output and arterial oxygen content (cardiac output×((Hb×/1.39×/SaO2)+/0.003×/PaO2)]. Oxygen extraction ratio (OER) was calculated as the ratio of oxygen consumption to oxygen delivery (VO2/DO2). Heart rate, blood pressure, PAP and VO2 were measured every 10 min. A blood draw for serum cyanide level, haemoglobin, and DO2 was performed every 20 min.
Two phases were defined to help interpret the study findings: compensated and decompensated. The compensated phase was the period where cardiac output and DO2 were above baseline values. The decompensated phase was the period where cardiac output and DO2 were below baseline values.
Data analysis
For each measured variable, mean (SE) values were calculated from all five animals. The change in values between measurements was compared with baseline by analysis of variance with repeated measures. The F statistic was used to test for differences in means. A p value <0.05 was considered significant. All analyses were performed using STATA Intercooled V.8.0 database.
Results
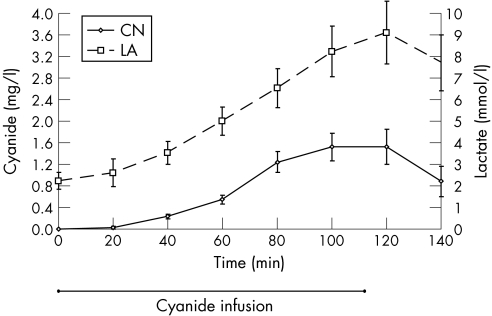
All five dogs survived the study period and provided data. Table 1 shows the Baseline haemodynamic values. The mean (SE) haemoglobin level was 13.5 (0.2) g/dl. After infusion of cyanide, there was a steady increase in cyanide and lactate levels.The cyanide level peaked at 1.52 (0.25) mg/l after the infusion was stopped (110 min) whereas lactate peaked at 9.1 (1.5) mmol/l 10 min later (120 min; fig 1). These cyanide and lactate levels are considered toxic in humans and dogs.15,23,24
Table 1 Baseline haemodynamic values (reference range)24,25.
| Haemodynamic value | Mean (SE; reference range) |
| Oxygen delivery index (DO2/BSA) | 1337 (137; 650–1100) ml/min/m2 |
| Oxygen Consumption Index (VO2/BSA) | 142 (6.6; 80–160) ml/min/m2 |
| Cardiac Index (CO/BSA) | 7.47(0.38; 3–5.3) l/min/m2 |
| Heart rate | 162(13; 150–190) beats/min |
| Pulmonary capillary wedge pressure | 8(0.7; 2–8) mm Hg |
BSA, body surface area; CO, cardiac output; DO2, oxygen delivery; VO2, oxygen consumption.
Figure 1 Cyanide and lactate levels in a canine model of poisoning. Both rise during infusion and fall after the infusion is stopped. p<0.01. Means (SE). CN, cyanide; LA, lactate.
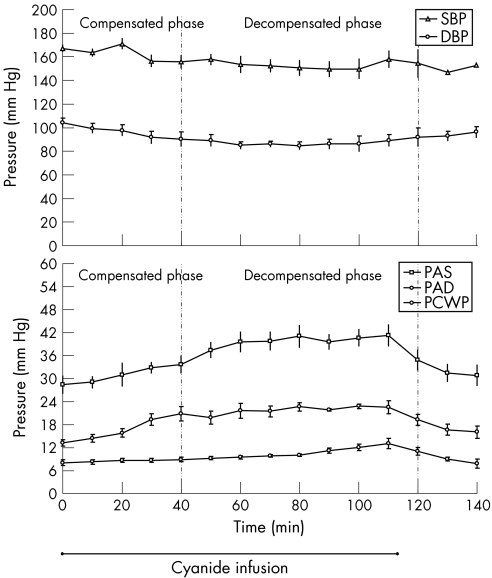
Figure 2 shows systemic and pulmonary arterial pressures. Systolic blood pressure slightly decreased throughout the cyanide infusion (12%), although this difference was not significant (p>0.05). diastolic blood pressure decreased to a nadir of 19% below baseline 80 min after infusion. Both systolic blood pressure and diastolic blood pressure increased after the infusion was stopped, although this was not significant (p>0.05 compared with nadir). Pulmonary pressures showed a steady increase throughout cyanide poisoning. Pulmonary arterial systolic, pulmonary arterial diastolic and pulmonary capillary wedge pressure increased 45%, 65% and 62% above baseline, respectively. All pulmonary pressures decreased after the cyanide infusion was stopped.
Figure 2 Systemic and pulmonary arterial pressures in a canine model of cyanide poisoning, DBP, diastolic blood pressure; PAD, pulmonary artery diastolic; PAS, pulmonary artery systolic; PCWP, pulmonary capillary wedge; SBP, systolic blood pressure, pressure. p<0.05 for all variables, except SBP. Mean (SE).
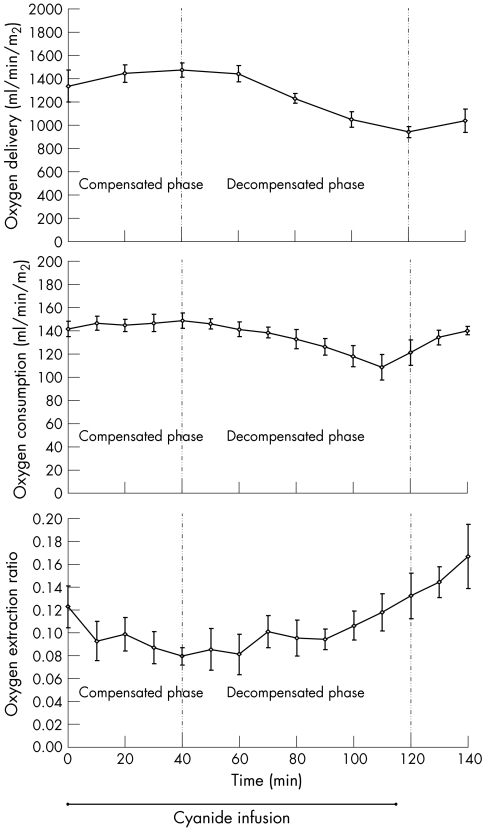
DO2 and VO2 followed a similar pattern: initially rising slightly, peaking at 40 min, dropping below baseline, and then increasing after the infusion was stopped. DO2 increased to a peak of 10% above baseline and VO2 increased to a peak of 5% above baseline. VO2 decreased to a nadir of 24% below baseline at the point the infusion was stopped. DO2 decreased to a nadir of 30% below baseline, 10 min after ending the infusion. The OER began to decrease from the outset of cyanide infusion, reaching a nadir of 35% below baseline at the 40 min mark. After this time, it steadily increased; increasing its rate of rise after the cyanide infusion was turned off at 110 min (fig 3).
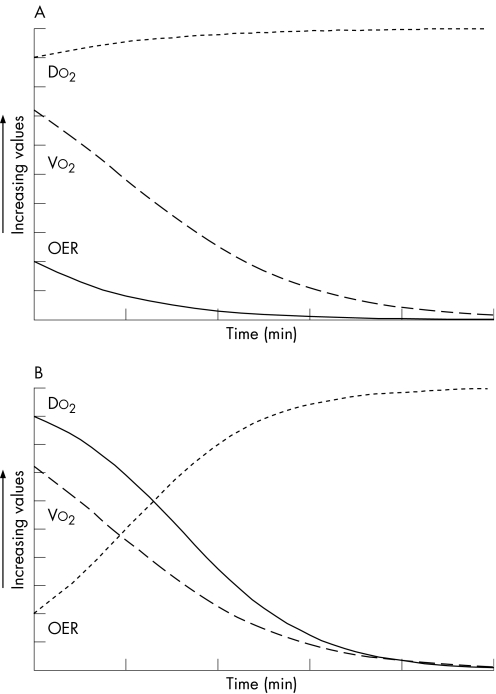
Figure 3 Oxygen transport characteristics in a canine model of cyanide poisoning. The compensated phase represents a period of increasing oxygen delivery. The decompensated phase represents a period of decreasing oxygen delivery. p<0.01 for each variable. Means (SE).
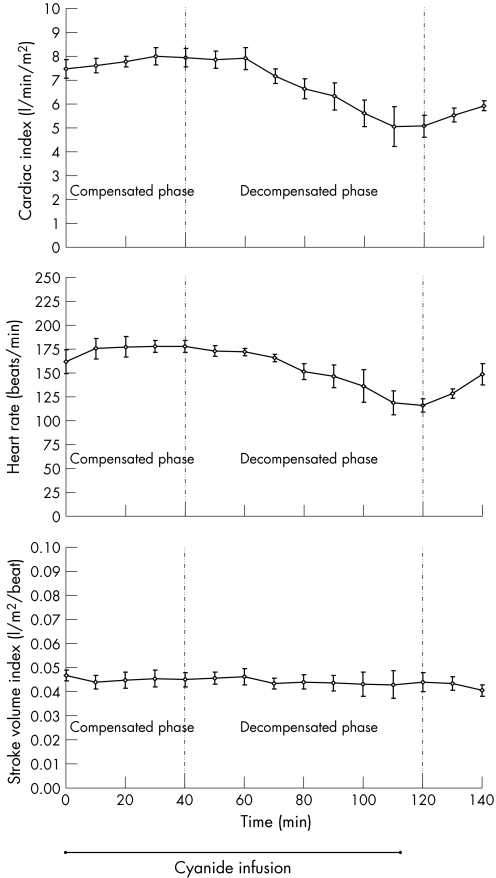
Figure 4 shows cardiac output and its determinants: stroke volume and heart rate. Heart rate followed the exact same trend as cardiac output, with an initial rise, followed by a decrease. In fact, heart rate entirely accounted for the change in cardiac output, as stroke volume was unchanged throughout the cyanide poisoning.
Figure 4 Relationship of determinants of cardiac output in canine model of cyanide poisoning: heart rate and stroke volume. Heart rate accounts for the change in cardiac output. The compensated phase represents a period of increasing oxygen delivery. The decompensated phase represents a period of decreasing oxygen delivery. p<0.01 for cardiac index and heart rate. Stroke volume index unchanged. p>0.90 Means (SE). bpm, beats/min.
Discussion
In this study, we have shown that in a canine model, cyanide poisoning affects the cardiovascular system in two distinct phases. The first phase possesses features of a hyperdynamic state. Heart rate, cardiac output, DO2 and VO2 are all raised. Stroke volume is constant and OER is depressed. The second phase follows a pattern consistent with cardiovascular failure. Heart rate, cardiac output, DO2 and VO2 are all depressed. Stroke volume remains constant whereas OER is raised.
This relationship may be better understood under two different models of disease. The first model is a traditional theoretical model of cyanide poisoning. The underlying defect in this scenario is oxygen extraction and consumption. As time progresses, the OER steadily decreases. There may be a reflexive increase in oxygen delivery/cardiac output (by a central nervous system mechanism)26 due to relative hypoxia. VO2 steadily declines, together with oxygen extraction (fig 5). This model closely describes what we observed in the early phase of cyanide poisoning. We did not observe a significant decline in VO2, only in OER. This probably represents cardiovascular compensation for a defect in oxygen extraction.
Figure 5 Theoretical oxygen consumption—Delivery relationship in two models. (A) Oxygen consumption defect: the primary defect is a decrease in oxygen extraction ratio (OER). Oxygen consumption (VO2) decreases owing poor extraction. Increase in oxygen delivery (DO2) is a compensatory mechanism. (B) Heart failure: the primary defect is a decrease in DO2. OER increases in compensation. VO2 decreases in response to low oxygen delivery.
The second model is that seen in heart failure.9 In this model, impaired cardiac output and DO2 are the underlying defects. DO2 persistently declines as time progresses. As DO2 decreases, VO2 decreases as well, but not to the same extent. This is reflected in the increased OER (fig 5). This probably represents the cellular attempt to extract more oxygen owing limited delivery.9 This model closely describes what we observed in the late phase of cyanide poisoning.
On the basis of these two models, our findings suggest that in the early phase of cyanide poisoning, an oxygen extraction defect is the predominant mechanism. At a critical threshold of cardiovascular compensation, heart failure becomes the predominant mechanism. This heart failure is essentially due to bradycardia, as stroke volume stays constant. We expected a compensatory rise in stroke volume during the bradycardic phase, but this was not observed. Although the exact mechanism is unknown, we postulate that this was due to cyanide's blunting of the cardiac response to catecholamines.27 This relationship suggests that there is little defect in cardiac contractility, but rather a defect in automaticity. The rat model suggests that this bradycardia is due to chemoreceptor stimulation in the carotid body.28 This chemoreceptor stimulation is distinctly different from the hypoxic hypoxia of asphyxiation. Early in cyanide poisoning, this bradycardia is countered by the effects of oxygen supplementation,29,30 acidosis30 and central nervous system compensation.26
These results provide a framework for interpreting previous literature that has yielded seemingly disparate results. Our findings support previous work that has described bradycardia, hypertension or hypotension, impaired oxygen delivery and consumption associated with cyanide poisoning.2,14,15,16,17,18,19 Previous canine studies suggest that decreases in blood pressure are due to decreased cardiac output with only limited compensatory response.20,27 Our analysis introduces the importance of bradycardic heart failure in the mechanism of cyanide poisoning, with the notable preservation of contractility. Furthermore, our constant infusion allowed us to demonstrate the compensated phase of poisoning, whereas previous authors have used single high‐dose injections that focused their observations on the decompensated phase.16,17,19 Previous case reports have described tachycardia and varying degrees of blood pressure in poisoning.13,31,32,33 Given our framework, these cases probabaly represent early or recovery phases of poisoning, in which the cardiovascular system is able to compensate. Both systemic and pulmonary artery pressure changes are consistent with previous animal studies.20
Our study has several limitations. Our model of cyanide poisoning controlled for respiratory rate. In true cyanide poisoning, respiratory failure is a prominent feature. Our purpose was to isolate the cardiovascular effects of cyanide poisoning. Allowing the dogs to spontaneously ventilate would probably have lead to earlier death and the inability to appreciate the full cardiovascular effects. Furthermore, in the clinical setting, patients with severe cyanide poisoning will probably be mechanically ventilated. The second limitation is the rate and duration of exposure of the model. In true poisonings, the exposure occurs across a relatively short period of time at a higher dose. We used a continuous infusion to study cyanide exposure in a controlled methodical fashion, allowing for time to observe changes. Although our model may not correlate well with acute clinical poisoning, it may correlate with a slow graded exposure found among initial responders to a contaminated site. Finally, we did not continue cyanide poisoning until the death of the animals. As our purpose was to study the mechanism, we wanted to evaluate the mechanism both in poisoning and in recovery. Although this provided us with clear data about the mechanism, it limits our inferences about the final stages of poisoning.
Our findings raise some new hypotheses for future study. Can treatment with chronotropy prevent cardiovascular collapse in cyanide poisoning? If treatment with chronotropy is successful, which method is superior: external pacing or medical (atropine) treatment? If treatment with chronotropy is successful, how long can it be sustained? Furthermore, we have not considered the neurological injury associated with cyanide poisoning. The nervous system is more sensitive to cyanide poisoning than the cardiovascular system.11 How will treatment of the cardiovascular effects of cyanide poisoning affect the neurological outcomes?
Conclusion
In summary, we have shed light on several important features of the cardiovascular effects of cyanide poisoning. First, cyanide poisoning follows two distinct phases in the canine model. The first phase is characterised by a hyperdynamic response to a defect in oxygen utilisation. This mechanism is consistent with a global oxygen consumption defect. The second phase is characterised by a decrease in DO2 and an increase in oxygen extraction, a mechanism consistent with heart failure. Moreover, the second phase is characterised by bradycardia with preserved contractility. These findings suggest further study into the role of chronotropy in the temporary treatment of cyanide poisoning.
Acknowledgements
We thank Janet J Tham, OD for assistance with manuscript editing.
Abbreviations
OER - oxygen extraction ratio
Footnotes
Competing interests: None declared.
References
- 1.Jones J, McMullen M J, Dougherty J. Toxic smoke inhalation: cyanide poisoning in fire victims. Am J Emerg Med 19875317–321. [DOI] [PubMed] [Google Scholar]
- 2.Baud F J, Barriot P, Toffis V.et al Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med 19913251761–1766. [DOI] [PubMed] [Google Scholar]
- 3.Thompson R L, Manders W W, Cowan W R. Postmortem findings of the victims of the Jonestown tragedy. J Forensic Sci 198732433–443. [PubMed] [Google Scholar]
- 4.Caravati E M, Litovitz T L. Pediatric cyanide intoxication and death from an acetonitrile‐containing cosmetic. JAMA 19882603470–3473. [PubMed] [Google Scholar]
- 5.Cottrell J E, Casthely P, Brodie J D.et al Prevention of nitroprusside‐induced cyanide toxicity with hydroxocobalamin. N Engl J Med 1978298809–811. [DOI] [PubMed] [Google Scholar]
- 6.Watson W A, Litovitz T L, Klein‐Schwartz W.et al 2003 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 200422335–404. [DOI] [PubMed] [Google Scholar]
- 7.Sperry P. Al‐Qaida terrorists to gas U.S. subways? DHS memo warns of device that uses cyanide to asphyxiate its victims. WorldNetDaily 25 November 2003
- 8.Vennesland B, Conn E E, Knowles C J.et alCyanide in biology. London: Academic, 1982233–261.
- 9.Mohsenifar Z, Amin D, Jasper A C.et al Dependence of oxygen consumption on oxygen delivery in patients with chronic congestive heart failure. Chest 198792447–450. [DOI] [PubMed] [Google Scholar]
- 10.Norris J C, Utley W A, Hume A S. Mechanism of antagonizing cyanide‐induced lethality by alpha‐ketoglutaric acid. Toxicology 199062275–283. [DOI] [PubMed] [Google Scholar]
- 11.Way J L. Cyanide intoxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol 198424451–481. [DOI] [PubMed] [Google Scholar]
- 12.Curry S C, Patrick H C. Lack of evidence for a percent saturation gap in cyanide poisoning. Ann Emerg Med 199120523–528. [DOI] [PubMed] [Google Scholar]
- 13.Yeh M M, Becker C E, Arieff A I. Is measurement of venous oxygen saturation useful in the diagnosis of cyanide poisoning? Am J Med 199293582–583. [DOI] [PubMed] [Google Scholar]
- 14.Breen P H, Isserles S A, Westley J.et al Combined carbon monoxide and cyanide poisoning: a place for treatment. Anesth Analg 199580671–677. [DOI] [PubMed] [Google Scholar]
- 15.Breen P H, Isserles S A, Westley J.et al Effect of oxygen and sodium thiosulfate during combined carbon monoxide and cyanide poisoning. Toxicol Appl Pharmacol 1995134229–234. [DOI] [PubMed] [Google Scholar]
- 16.Ivankovich A D, Braverman B, Kanuru R P.et al Cyanide antidotes and methods of their administration in dogs: a comparative study. Anesthesiology 198052210–216. [DOI] [PubMed] [Google Scholar]
- 17.Klimmek R, Fladerer H, Weger N. Circulation, respiration, and blood homeostasis in cyanide‐poisoned dogs after treatment with 4‐dimethylaminophenol or cobalt compounds. Arch Toxicol 197943121–133. [DOI] [PubMed] [Google Scholar]
- 18.Klimmek R, Roddewig C, Fladerer H.et al Cerebral blood flow, circulation, and blood homeostasis of dogs during slow cyanide poisoning and after treatment with 4‐dimethylaminophenol. Arch Toxicol 19825065–76. [DOI] [PubMed] [Google Scholar]
- 19.Klimmek R, Krettek C. Effects of amyl nitrite on circulation, respiration and blood homoeostasis in cyanide poisoning. Arch Toxicol 198862161–166. [DOI] [PubMed] [Google Scholar]
- 20.Baskin S I. The cardiac effects of cyanide. In: Baskin SI, ed. Principles of cardiac toxicology. Boca Raton: CRC Press, 1993419–430.
- 21.Office of Laboratory Animal Welfare public health service policy on humane care and use of laboratory animals. 2. Bethesda, Maryland: Department of Health and Human Services, 2002
- 22.Chittock D R, Ronco J J, Russell J A. Monitoring of oxygen transport and oxygen consumption. In: Tobin MJ, ed. Principles and practice of intensive care monitoring. New York; McGraw‐Hill 1998317–343.
- 23.Christel D, Eyer P, Hegemann M.et alPharmacokinetics of cyanide in poisoning of dogs, and the effect of 4‐dimethylaminophenol or thiosulfate. Arch Toxicol 1977;38177–189. [DOI] [PubMed]
- 24.Mitaka C, Hirata Y, Habuka K.et alAtrial natriuretic peptide infusion improves ischemic renal failure after suprarenal abdominal aortic cross‐clamping in dogs. Crit Care Med 2003;312205–2210. [DOI] [PubMed]
- 25.Haskins S, Pascoe P J, Ilkiw J E.et alReference cardiopulmonary values in normal dogs. Comp Med 2005;55156–161. [PubMed]
- 26.Krasney J A. pp. 1361–1366. [DOI] [PubMed]
- 27.Ellis S, Anderson H L. pp. 381–390. [PubMed]
- 28.Barros R C, Bonagamba L G, Okamoto‐Canesin R.et alCardiovascular responses to chemoreflex activation with potassium cyanide or hypoxic hypoxia in awake rats. Auton Neurosci 2002;97110–115. [DOI] [PubMed]
- 29.Isom G E, Way J L. pp. 57–62. [DOI] [PubMed]
- 30.Burrows G E, Liu D H, Way J L. pp. 739–748. [PubMed]
- 31.Singh B M, Coles N, Lewis P.et alThe metabolic effects of fatal cyanide poisoning. Postgrad Med J 1989;65923–925. [DOI] [PMC free article] [PubMed]
- 32.Saincher A, Swirsky N, Tenenbein M. pp. 555–557. [DOI] [PubMed]
- 33.Litovitz T L, Larkin R F, Myers R A. pp. 94–101. [DOI] [PubMed]