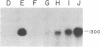
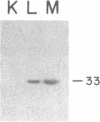
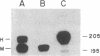
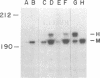
Abstract
Glyceraldehyde-3-phosphate dehydrogenase [GAPDH; D-glyceraldehyde-3-phosphate:NAD+ oxidoreductase (phosphorylating), EC 1.2.1.12] mRNA levels are induced by physiologic concentrations of insulin in cultured 3T3-F442A adipocyte and H35 hepatoma cell lines. To examine the mechanism by which insulin regulates GAPDH mRNA levels in these two insulin-sensitive tissues, we have isolated a functional human GAPDH gene. When stably transfected and expressed in 3T3-F442A preadipocytes and H35 hepatoma cells, the intact human GAPDH gene is induced 10-fold by insulin in 3T3-F442A adipocytes and 3-fold by insulin in H35 hepatoma lines, which is similar to the induction obtained with the endogenous gene. A human GAPDH-chloramphenicol acetyltransferase construct, containing sequences -487 to +20 of the human gene fused to the chloramphenicol acetyltransferase gene, is regulated by insulin in stably transfected 3T3 adipocytes and stably or transiently transfected H35 hepatoma cell lines, whereas the Rous sarcoma virus-chloramphenicol acetyltransferase fusion protein is not. Thus, the inductive effect of insulin on human GAPDH gene expression is mediated through cis-acting sequences located between -487 and +20 of the human GAPDH gene.
Full text
PDF


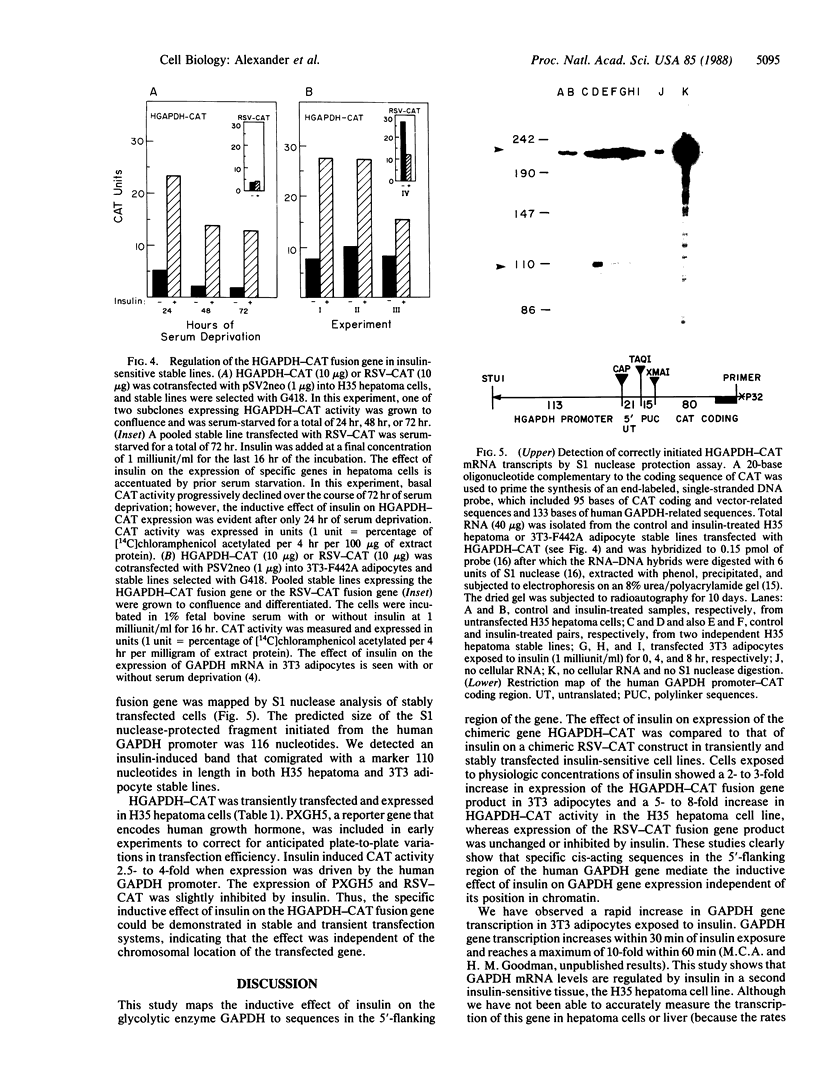

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M., Curtis G., Avruch J., Goodman H. M. Insulin regulation of protein biosynthesis in differentiated 3T3 adipocytes. Regulation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1985 Oct 5;260(22):11978–11985. [PubMed] [Google Scholar]
- Benham F. J., Hodgkinson S., Davies K. E. A glyceraldehyde-3-phosphate dehydrogenase pseudogene on the short arm of the human X chromosomes defines a multigene family. EMBO J. 1984 Nov;3(11):2635–2640. doi: 10.1002/j.1460-2075.1984.tb02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Gunn J. M., Tilghman S. M., Hanson R. W., Reshef L., Ballard F. J. Effects of cyclic adenosine monophosphate, dexamethasone and insulin on phosphoenolpyruvate carboxykinase synthesis in Reuber H-35 hepatoma cells. Biochemistry. 1975 Jun 3;14(11):2350–2357. doi: 10.1021/bi00682a012. [DOI] [PubMed] [Google Scholar]
- Hanauer A., Mandel J. L. The glyceraldehyde 3 phosphate dehydrogenase gene family: structure of a human cDNA and of an X chromosome linked pseudogene; amazing complexity of the gene family in mouse. EMBO J. 1984 Nov;3(11):2627–2633. doi: 10.1002/j.1460-2075.1984.tb02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M. A., Quinn P. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin's effects oppose those of cAMP and dexamethasone. J Biol Chem. 1987 Nov 5;262(31):14917–14920. [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Meisler M. H. Tissue-specific and insulin-dependent expression of a pancreatic amylase gene in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):326–334. doi: 10.1128/mcb.7.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Riaad-El Sabouty S., Dani C., Marty L., Jeanteur P. Unusual abundance of vertebrate 3-phosphate dehydrogenase pseudogenes. 1984 Nov 29-Dec 5Nature. 312(5993):469–471. doi: 10.1038/312469a0. [DOI] [PubMed] [Google Scholar]
- Rosen O. M. After insulin binds. Science. 1987 Sep 18;237(4821):1452–1458. doi: 10.1126/science.2442814. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Cripe T. P., Koch S. R., Andreone T. L., Petersen D. D., Beale E. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. The dominant role of insulin. J Biol Chem. 1984 Dec 25;259(24):15242–15251. [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C., Burke K., Chou M. J., Zeldis J. B., Yang C. S., Lee C. S., Isselbacher K. J., Wands J. R., Goodman H. M. Tight clustering of human hepatitis B virus integration sites in hepatomas near a triple-stranded region. J Virol. 1987 Nov;61(11):3491–3498. doi: 10.1128/jvi.61.11.3491-3498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Spiegelman B. M., Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980 Sep 25;255(18):8811–8818. [PubMed] [Google Scholar]
- Taub R., Roy A., Dieter R., Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987 Aug 5;262(22):10893–10897. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita S., Melmed S. Insulin regulation of rat growth hormone gene transcription. J Clin Invest. 1986 Oct;78(4):1008–1014. doi: 10.1172/JCI112654. [DOI] [PMC free article] [PubMed] [Google Scholar]