Abstract
A reappraisal of the phylogenetic integrity of bitunicate ascomycete fungi belonging to or previously affiliated with the Hysteriaceae, Mytilinidiaceae, Gloniaceae and Patellariaceae is presented, based on an analysis of 121 isolates and four nuclear genes, the ribosomal large and small subunits, transcription elongation factor 1 and the second largest RNA polymerase II subunit. A geographically diverse and high density taxon sampling strategy was employed, including multiple isolates/species from the following genera: Anteaglonium (6/4), Encephalographa (1/1), Farlowiella (3/1), Gloniopsis (8/4), Glonium (4/2), Hysterium (12/5), Hysterobrevium (14/3), Hysterographium (2/1), Hysteropatella (2/2), Lophium (4/2), Mytilinidion (13/10), Oedohysterium (5/3), Ostreichnion (2/2), Patellaria (1/1), Psiloglonium (11/3), Quasiconcha (1/1), Rhytidhysteron (8/3), and 24 outgroup taxa. Sequence data indicate that although the Hysteriales are closely related to the Pleosporales, sufficient branch support exists for their separation into separate orders within the Pleosporomycetidae. The Mytilinidiales are more distantly related within the subclass and show a close association with the Gloniaceae. Although there are examples of concordance between morphological and molecular data, these are few. Molecular data instead support the premise of a large number of convergent evolutionary lineages, which do not correspond to previously held assumptions of synapomorphy relating to spore morphology. Thus, within the Hysteriaceae, the genera Gloniopsis, Glonium, Hysterium and Hysterographium are highly polyphyletic. This necessitated the transfer of two species of Hysterium to Oedohysterium gen. nov. (Od. insidens comb. nov. and Od. sinense comb. nov.), the description of a new species, Hysterium barrianum sp. nov., and the transfer of two species of Gloniopsis to Hysterobrevium gen. nov. (Hb. smilacis comb. nov. and Hb. constrictum comb. nov.). While Hysterographium, with the type Hg. fraxini, is removed from the Hysteriaceae, some of its species remain within the family, transferred here to Oedohysterium (Od. pulchrum comb. nov.), Hysterobrevium (Hb. mori comb. nov.) and Gloniopsis (Gp. subrugosa comb. nov.); the latter genus, in addition to the type, Gp. praelonga, with two new species, Gp. arciformis sp. nov. and Gp. kenyensis sp. nov. The genus Glonium is now divided into Anteaglonium (Pleosporales), Glonium (Gloniaceae), and Psiloglonium (Hysteriaceae). The hysterothecium has evolved convergently no less than five times within the Pleosporomycetidae (e.g., Anteaglonium, Farlowiella, Glonium, Hysterographium and the Hysteriaceae). Similarly, thin-walled mytilinidioid (e.g., Ostreichnion) and patellarioid (e.g., Rhytidhysteron) genera, previously in the Mytilinidiaceae and Patellariaceae, respectively, transferred here to the Hysteriaceae, have also evolved at least twice within the subclass. As such, character states traditionally considered to represent synapomorphies among these fungi, whether they relate to spore septation or the ascomata, in fact, represent symplesiomorphies, and most likely have arisen multiple times through convergent evolutionary processes in response to common selective pressures.
Keywords: Evolution, fungi, Hysteriales, Mytilinidiales, Patellariales, phylogeny, speciation, taxonomy
INTRODUCTION
Class Dothideomycetes, subphylum Pezizomycotina (Ascomycota), is currently classified into two subclasses, based on centrum type (Schoch et al. 2006, 2009b, Spatafora et al. 2006). The Dothideomycetidae is characterised by the absence of sterile centrum elements (e.g., pseudoparaphyses). This subclass includes the Dothideales, Capnodiales, and Myriangiales. The Microthyriales, and Trypetheliales, while within the Dothideomycetes, lie outside of the Dothideomycetidae (Schoch et al. 2009a). The second subclass recognised within the Dothideomycetes is the Pleosporomycetidae, characterised by a hamathecium of wide to narrow cellular to trabeculate pseudoparaphyses, which may or may not persist at maturity. This subclass currently comprises the Pleosporales, Hysteriales, and Mytilinidiales, and tentatively the Jahnulales. The Botryosphaeriales, and Patellariales, possess pseudoparaphyses, and would be expected to fall into the Pleosporomycetidae, however, at present, statistical support is weak. A greater number of orders, families, and genera still await placement, and are currently designated as incertae sedis within the Dothideomycetes (Lumbsch & Huhndorf 2007).
Fungi classified in the Hysteriaceae (Hysteriales), Mytilinidiaceae (Mytilinidiales), and Gloniaceae (Pleosporomycetidae fam. incertae sedis), possess persistent, carbonaceous ascomata that characteristically dehisce by a longitudinal suture. Recent molecular data support the inclusion of all three families within the Pleosporomycetidae (Schoch et al. 2006, Boehm et al. 2009, Mugami & Huhndorf 2009). In the Hysteriaceae ascomata are thick-walled, navicular, characteristically dehiscing by an invaginated slit or sulcus (Zogg 1962). Fungi in the Mytilinidiaceae, on the other hand, possess strongly laterally compressed, connivent, thin-walled conchate ascomata, reminiscent of miniature bivalve molluscs. These mytilinidioid ascomata typically dehisce by an evaginated slit, in some species forming a longitudinal keel or cristate apex (Barr 1990a). Fungi belonging to the Gloniaceae, have dichotomously branched, laterally anastomosed pseudothecia, that form radiating pseudo-stellate composites and dehisce by an inconspicuous, longitudinal, but evaginated slit (Boehm et al. 2009).
We are broadly interested in the evolution of character states traditionally used to define higher taxa within each family. Essentially, we wish to address whether morphological features historically used in the classification of these fungi are phylogenetically informative in the context of sequence-based phylogenies. This would have bearing on which morphological features are phylogenetically significant, and therefore useful for a natural delineation of higher taxa. Morphological character states traditionally used to classify these fungi have related primarily to features associated with (1) the pseudothecium, (2) the peridium, (3) the hamathecium, and (4) differences in ascospore symmetry (Barr 1987, 1990a). Character states within each family relate primarily to ascospore septation and pigmentation (Zogg 1962).
Due to the seemingly transitional nature of the ascoma, neither fully open nor closed, hysteriaceous fungi have been placed in the discomycetes and pyrenomycetes about equally by various mycologists throughout the 19th Century (Bisby 1923). In his Systema Mycologicum, Fries (1823) initially considered hysteriaceous fungi to belong to the pyrenomycetes and placed them in the Phacidiacei, but later (Fries 1835) placed them in his new class Discomycetes, stating: “Transitum sistunt ad Discomycetes, sed discum verum non monstrant.” Chevallier (1826) recognised the unique nature of the hysterothecium and established the Hysteriineae, which he considered as pyrenomycetes distinct from Fries' Phacidiei. Corda (1842), on the other hand, retained the Phacidiei within the Hysteriaceae, and divided the family into a number of subfamilies. De Notaris (1847) considered the Hysteriaceae to belong to the pyrenomycetes and used spore pigmentation to classify hysteriaceous fungi into the Phaeosporii and the Hyalosporii. Saccardo (1873) initially followed Fries, but later (1874) placed hysteriaceous fungi in the pyrenomycetes, and carried de Notaris' (1847) spore classification scheme further by dividing the Hysteriaceae into nine sections based on pigmentation and the morphology of spore septation (Saccardo 1883). Ellis & Everhart (1892), in their North American Pyrenomycetes, tentatively included the Hysteriaceae, but stated that they had not at first intended to do so due to the transitional nature of the hysterothecium. In Rabenhorst's Kryptogamen-Flora, Die Pilze, Rehm (1896) compromised and placed the Hysteriales as an order intermediate between the pyrenomycetes and the discomycetes.
Mytilinidioid fungi have also historically been classified within the family Hysteriaceae, due to perceived similarities in ascocarp morphology, specifically its means of longitudinal dehiscence (Fries 1823, De Notaris 1847, Saccardo 1875, 1883, Ellis & Everhart 1892, Massee 1895, Rehm 1896, von Höhnel 1918, Bisby 1923). Modern authors have likewise included mytilinidioid fungi within the Hysteriaceae, placing the family in the Pseudosphaeriales (Nannfeldt 1932, Gäumann 1949), the Dothiorales (Müller & von Arx 1950, von Arx & Müller 1954), the Dothideales (von Arx & Müller 1975), and in a separate order Hysteriales, closely related to the Pleosporales (Miller 1949, Luttrell 1955). The Hysteriales were placed in the subclass Loculoascomycetes by Luttrell (1955), due to the presence of bitunicate asci, corresponding to the Ascoloculares first proposed by Nannfeldt (1932).
Duby (1862) was the first to propose that hysteriaceous fungi be divided into two sections, the Hystériées and the Lophiées, the latter to accommodate mytilinidioid forms. One hundred years later, Zogg (1962) proposed two families: the Hysteriaceae s. str. to accommodate thick-walled hysteriaceous forms, and the Lophiaceae (Zogg 1962, von Arx & Müller 1975) to accommodate thin-walled, mytilinidioid fungi. Barr (1990a) made the argument for retention of the earlier name Mytilinidiaceae over the Lophiaceae, despite the proposal to conserve the latter (Hawksworth & Eriksson 1988). Luttrell (1953) studied ascomatal ontogeny and hamathecial development in Glonium stellatum. and concluded that the Hysteriaceae possess the pseudoparaphysate Pleospora-type centrum, in which cellular, septate pseudoparaphyses grow downwards from the cavity roof, initially anchored at both ends, and occupy the locule prior to the formation of asci (Luttrell 1951). Luttrell (1973) held a wide concept of the Hysteriales, but did not recognise the family Lophiaceae, instead proposing a subfamily, the Lophioideae, within the Hysteriaceae to accommodate mytilinidioid forms. Barr (1979) however maintained the two-family distinction. The Mytilinidiaceae was placed in the Melanommatales based on a thin-walled peridium of scleroparenchymatous cells enclosing a hamathecium of narrow trabeculate pseudoparaphyses, asci borne in a peripheral layer and with ascospores typically showing bipolar symmetry (Barr 1987, 1990a). Later, Barr & Huhndorf (2001) noted that the family was somewhat atypical of the Melanommatales, in that, as a consequence of reduced locule space attributed to lateral compression, they possess a basal, rather than peripheral, layer of asci and a reduced hamathecium at maturity. More recently, the Melanommatales have been included within the Pleosporales (Lumbsch & Huhndorf 2007). Barr (1983) eventually abandoned the Hysteriales and placed the Hysteriaceae within the Pleosporales due to the presence of cellular pseudoparaphyses, asci borne in a basal rather than peripheral layer and ascospores typically showing bipolar asymmetry. Eriksson (2006) removed the Mytilinidiaceae from the Hysteriales and considered it as Dothideomycetes et Chaetothyriomycetes incertae sedis, leaving the Hysteriaceae as the sole family in the Hysteriales.
More recently, Boehm et al. (2009) presented the first combined use of DNA and amino acid sequence data to reconstruct the phylogeny of hysteriaceous fungi. This study was based on a wide taxon sampling strategy, and employed four nuclear genes, namely the nuSSU and nuLSU, Transcription Elongation Factor 1 (TEF1) and the second largest RNA polymerase II subunit (RPB2). A number of specific conclusions were reached: (1) Multigene phylogenies provided strong support for the monophyly of the Hysteriaceae and of the Mytilinidiaceae, both within the Pleosporomycetidae. However, sequence data also indicated that both families were not closely related within the subclass. (2) Although core groups for many of the genera in the Hysteriaceae were defined, the genera Hysterium, Gloniopsis, and Hysterographium were demonstrated to be polyphyletic, with affinities not premised on spore septation and pigmentation. (3) The genus Glonium was also shown to be polyphyletic, but along two highly divergent lines. Glonium lies outside of the Hysteriaceae, and instead finds close affinities with the family Mytilinidiaceae, for which was proposed the Gloniaceae (Boehm et al. 2009), to accommodate the type, G. stellatum, and related forms. (4) The genus Psiloglonium was reinstated within the Hysteriaceae, with P. lineare as type, to accommodate didymospored species segregated from Glonium. (5) The genera Mytilinidion and Lophium formed a strongly supported clade within the Pleosporomycetidae, thus defining the monophyletic Mytilinidiaceae, adjacent to the Gloniaceae, for which was proposed the Mytilinidiales (Boehm et al. 2009). (6) The genus Farlowiella, previously in the Hysteriaceae, was placed as Pleosporomycetidae gen. incertae sedis. (7) The genus Ostreichnion, previously in the Mytilinidiaceae, was transferred to the Hysteriaceae. (8) The genus Rhytidhysteron, previously in the Patellariaceae, was transferred to the Hysteriaceae.
These taxonomic changes present a number of challenges for understanding evolution within this group of fungi. The lack of agreement between morphological character states, previously considered synapomorphic (e.g., Zogg 1962), and recent molecular data based on the nuSSU, nuLSU, TEF1 and RPB2 (Boehm et al. 2009), had resulted in a highly polyphyletic core set of genera for the Hysteriaceae (e.g., Hysterium, Hysterographium, Gloniopsis, and Glonium). This presented us with a complicated picture of past speciation events for the family, and necessitated the current reappraisal. Essentially, the challenge was to reconcile discrepancies between morphological and molecular data, in order to more accurately reflect natural phylogenetic relationships within the family. As a result, the revised Hysteriaceae bears little resemblance to the original concept of the family (Zogg 1962).
In an effort to facilitate species identification, a number of dichotomous keys are presented in the current study. These keys take into consideration taxonomic changes brought about by DNA and amino acid sequencing studies (Schoch et al. 2006, Boehm et al. 2009, Mugambi & Huhndorf 2009), and attempt to provide a morphological basis for the many new relationships revealed by molecular data. Although the keys are based on those first presented by Zogg (1962), they considerably expand upon them to include a number of new species and genera described since the original publication (e.g., Darker 1963, Tilak & Kale 1968, Barr 1975, 1990a, Barr & Blackwell 1980, Amano 1983, Speer 1986, Pande & Rao 1991, van der Linde 1992, Kantvilas & Coppins 1997, Lorenzo & Messuti 1998, Messuti & Lorenzo 1997, 2003, 2007, Vasilyeva 2000, 2001, Chlebicki & Knudsen 2001, Checa et al. 2007). In addition to incorporating new species and genera, the revised keys also take into consideration variation in ascospore measurements as presented by different authors, and include widened distribution reports as well. Additional information can be found at www.eboehm.com/.
MATERIALS AND METHODS
Taxon sampling
Fungal cultures, collection data and DNA GenBank accession numbers are listed in Table 1 - see online Supplementary Information. Fungal cultures initiated for this study were based on the isolation of individual ascospores, employing a method whereby individual ascomata were affixed to Petri plate lids suspended over potato-dextrose agar. Every 12 h the lids were rotated 45 degrees, such that after 96 h, confirmation of spore deposits could be made under a stereomicroscope using transmitted light. Discharged spores were observed microscopically to confirm identity, transferring a single ascospore to initiate an axenic culture (e.g., EB cultures and deposits with the CBS; Centraalbureau voor Schimmelcultures). In some cases, spore discharge was not obtained, necessitating DNA extraction from individual fruitbodies (e.g., all GKM, SMH, ANM and some EB accessions). Lastly, a number of original cultures, from the CBS were employed in this study, the provenance of which could not be ascertained beforehand. Confirmation of taxonomic identity was based on whether different isolates of the same species co-segregated in the final tree.
Table 1.
Taxon sampling, provenance and GenBank accession numbers.
| Species | Accession | Provenance | Genbank No. | |||
|---|---|---|---|---|---|---|
| nuSSU | nuLSU | TEF1 | RPB2 | |||
| Acrogenospora sphaerocephala | CBS 164.76 | W. Gams, Grande Tinémont, BELGIUM | GU296129 | GU301791 | GU349059 | GU371748 |
| Aliquandostipite khaoyaiensis | CBS 118232 | P. Inderbitzin (AFTOL 1364), Khao Yai NP, THAILAND | AF201453 | GU301796 | GU349048 | FJ238360 |
| Anteaglonium abbreviatum | ANM 925.1 | A.N. Miller (ILLS), Smoky Mts. TN, U.S.A. | — | GQ221877 | GQ221924 | — |
| A. globosum | SMH 5283 | S.M. Huhndorf (F), Indiana Dunes, IN, U.S.A. | — | GQ221911 | GQ221919 | — |
| ANM 925.2 | A.N. Miller (ILLS), Smoky Mts. TN, U.S.A. | — | GQ221879 | GQ221925 | — | |
| A. latirostrum | GKM L100N.2 | G.K. Mugambi (EA), Taita Hills, KENYA | — | GQ221876 | GQ221938 | — |
| GKM 1119 | G.K. Mugambi (EA), Taita Hills, KENYA | — | GQ221874 | GQ221937 | — | |
| A. parvulum | SMH 5210 | S.M. Huhndorf (F), NEW ZEALAND | — | GQ221907 | GQ221917 | — |
| Arthonia caesia | AFTOL 775 | A. Amtoft, NC, U.S.A. | — | FJ469668 | FJ469669 | FJ469670 |
| Botryosphaeria dothidea | CBS 115476 | B. Slippers (AFTOL 946), Crocifisso, SWITZERLAND | DQ677998 | DQ678051 | DQ767637 | DQ677944 |
| Byssothecium circinans | CBS 675.92 | G. Semeniuk (AFTOL 1735), SD, U.S.A. | AY016339 | AY016357 | GU349061 | DQ767646 |
| Cenococcum geophilum | HUNT A1 | K.F. LoBuglio, GenBank | L76616 | — | — | — |
| C. geophilum | CGMONT | K.F. LoBuglio, GenBank | L76617 | — | — | — |
| 010 | K.F. LoBuglio, GenBank | L76618 | — | — | — | |
| Cochliobolus heterostrophus | CBS 134.39 | K. Böning (AFTOL 54) | AY544727 | AY544645 | DQ497603 | DQ247790 |
| Delitschia winteri | CBS 225.62 | J.L. Bezerra (AFTOL 1599), Baarn, NETHERLANDS | DQ678026 | DQ678077 | DQ677922 | DQ677975 |
| Dothidea insculpta | CBS 189.58 | E. Müller (AFTOL921), Maupas, FRANCE | DQ247810 | DQ247802 | DQ471081 | AF107800 |
| D. sambuci | DAOM 231303 | S. Hambleton & B. Shoemaker (AFTOL 274) | AY544722 | AY544681 | DQ497606 | DQ522854 |
| Elsinoë veneta | CBS 150.27 | E.M. Wakefield (AFTOL 1853) | DQ767651 | DQ767658 | DQ767641 | — |
| Encephalographa elisae | EB 0347 | M. Tretiach, (BPI 879773), Prov. Trieste, Karst, ITALY | GU397358 | GU397343 | — | — |
| Farlowiella carmichaeliana | CBS 206.36 | E.W. Mason (AFTOL1787). EUROPE | AY541482 | AY541492 | DQ677931 | DQ677989 |
| F. carmichaeliana | CBS 179.73 | W. Gams, Teutoburger Wald, Neuenheerse, GERMANY | GU296148 | — | — | — |
| Gloniopsis arciformis | GKM L166A | G.K. Mugambi (BPI 879774 = Holotype), Malindi, KENYA | GU323180 | GU323211 | — | — |
| Gp. kenyensis | GKM 1010 | G.K. Mugambi (BPI 879775 = Holotype), EA, Malindi, KENYA | — | GQ221891 | — | — |
| Gp. praelonga | CBS 112415 | S. Marincowitz (PREM), Kogelberg NR, SOUTH AFRICA | FJ161134 | FJ161173 | FJ161090 | FJ161113 |
| CBS 123337 | E.W.A. Boehm (BPI 878725), NJ, U.S.A. | FJ161154 | FJ161195 | FJ161103 | — | |
| CMW 19983 | S. Marincowitz (PREM 57539), Jonkershoek, SOUTH AFRICA | FJ161152 | FJ161193 | — | — | |
| Gp. subrugosa | CBS 123346 | S. Marincowitz (BPI 878735), Gauteng, SOUTH AFRICA | FJ161170 | FJ161210 | — | FJ161131 |
| GKM 1214 | G.K. Mugambi (BPI 879776, EA), Mt. Kenya, KENYA | — | GQ221895 | GU397336 | — | |
| SMH 557 | S.M. Huhndorf (BPI 879777, F), Sancti Spiritus, CUBA | — | GQ221896 | GU397337 | — | |
| Glonium circumserpens | CBS 123342 | G. Kantvilas (BPI 878738), Warra SST, TASMANIA | FJ161168 | FJ161208 | — | — |
| G. circumserpens | CBS 123343 | G. Kantvilas (BPI 878739), Warra SST, TASMANIA | FJ161160 | FJ161200 | FJ161108 | FJ161126 |
| G. stellatum | CBS 207.34 | M.L. Lohman (No. 265), MI, U.S.A. | FJ161140 | FJ161179 | FJ161095 | |
| ANM 32 | A.N. Miller (ILLS), Smoky Mts., TN, U.S.A. | — | GQ221887 | |||
| Guignardia gaultheriae | CBS 447.70 | H.A. van der Aa (AFTOL 1784), Seattle, WA, U.S.A. | — | DQ678089 | DQ677987 | |
| Herpotrichia diffusa | CBS 250.62 | M.C. Pande (AFTOL 1588), Uttar Pradesh, INDIA | DQ678019 | DQ678071 | DQ677915 | DQ677968 |
| Hysterium angustatum | CBS 236.34 | M.L. Lohman (No. 309), WI, U.S.A. | GU397359 | FJ161180 | FJ161096 | FJ161117 |
| CBS 123334 | E.W.A. Boehm (BPI 878724), Sussex Co., NJ, U.S.A. | FJ161167 | FJ161207 | FJ161111 | FJ161129 | |
| CMW 20409 | S. Marincowitz (PREM 57585), Kleinmond, SOUTH AFRICA | FJ161153 | FJ161194 | — | — | |
| SMH 5211.0 | S.M. Huhndorf (F), NEW ZEALAND | GU397360 | GQ221905 | — | GQ221923 | |
| GKM 243A | G.K. Mugambi (EA), Malindi, KENYA | — | GQ221899 | — | — | |
| SMH 5216 | S.M. Huhndorf (F), NEW ZEALAND | — | — | — | GQ221933 | |
| ANM 85 | A.N. Miller (ILLS), Smoky Mts., TN, U.S.A. | — | GQ221898 | — | — | |
| H. barrianum | ANM 1495 | A.N. Miller (ILLS59908 = Holotype, BPI879783 = Paratype), TN, U.S.A. | GU323182 | GQ221885 | — | — |
| ANM 1442 | A.N. Miller (ILLS 59907, BPI 879784), Smoky Mts., TN, U.S.A. | GU323181 | GQ221884 | — | — | |
| H. hyalinum | CBS 237.34 | M.L. Lohman (No. 425), MA, U.S.A. | FJ161141 | FJ161181 | — | — |
| H. pulicare | CBS 123377 | E.W.A. Boehm (BPI 878723), NY, U.S.A. | FJ161161 | FJ161201 | FJ161109 | FJ161127 |
| H. vermiforme | GKM 1234 | G.K. Mugambi (BPI 879785, EA), Mt. Kenya, KENYA | — | GQ221897 | — | — |
| Hysterobrevium constrictum | SMH 5211.1 | S.M. Huhndorf (F), NEW ZEALAND | GU397361 | GQ221905 | — | — |
| Hb. mori | CBS 245.34 | M.L. Lohman (No. 6), MI, U.S.A. | FJ161143 | — | FJ161098 | — |
| CBS 123563 | E.W.A. Boehm (BPI 878731), NY, U.S.A. | FJ161155 | FJ161196 | FJ161104 | — | |
| CBS 123564 | E.W.A. Boehm (BPI 878732), NJ, U.S.A. | FJ161158 | FJ161198 | FJ161106 | — | |
| CBS 123336 | E.W.A. Boehm (BPI 878733), NJ, U.S.A. | FJ161164 | FJ161204 | — | — | |
| CBS 123335 | E.W.A. Boehm (BPI 878734), NY, U.S.A. | FJ161162 | FJ161202 | — | — | |
| SMH 5273 | S.M. Huhndorf (BPI 879787, F), IN, U.S.A. | — | GQ221910 | GQ221936 | — | |
| GKM 1013 | G.K. Mugambi (BPI 879788, EA), Malindi, KENYA | — | GU397344 | GU397338 | — | |
| SMH 5286 | G.K. Mugambi (BPI 879789, EA), MI, U.S.A. | — | GU397345 | — | — | |
| Hb. smilacis | CBS 114601 | O. Constantinescu, as Gp. curvata (Fr.) Sacc., SWEDEN | FJ161135 | FJ161174 | FJ161091 | FJ161114 |
| CBS 200.34 | M.L. Lohman (No. 29), as Gp. gerardiana Sacc., MI, U.S.A. | FJ161138 | FJ161177 | — | — | |
| CMW 18053 | S. Marincowitz (PREM 57546), Kirstenbosch, SOUTH AFRICA | FJ161150 | FJ161191 | — | — | |
| SMH 5280 | G.K. Mugambi (EA), IN, U.S.A. | GU323183 | GQ221912 | — | GU371784 | |
| GKM 426N | G.K. Mugambi (EA), Taita Hills, KENYA | — | GQ221901 | — | — | |
| Hysterographium fraxini | CBS 109.43 | H. Zogg, SWITZERLAND | FJ161132 | FJ161171 | FJ161088 | — |
| Hg. fraxini | CBS 242.34 | M.L. Lohman (No. 300), Manitoba, CANADA | — | FJ161189 | — | |
| Hysteropatella clavispora | CBS 247.34 | M.L. Lohman (No. 143), IN, U.S.A. | DQ678006 | AY541493 | DQ677901 | DQ677955 |
| Hp. elliptica | CBS 935.97 | G. Marson (AFTOL 1790), Fentange, LUXEMBOURG | EF495114 | DQ767657 | DQ767640 | DQ767647 |
| Jahnula aquatica | R68-1 | Campbell et al. 2007 | EF175633 | DF175655 | — | — |
| Leptosphaeria maculans | DAOM 229267 | S. Hambleton & B. Shoemaker (AFTOL 277), CANADA | DQ470993 | DQ470946 | DQ471062 | DQ470894 |
| Lophium elegans | EB 0366 | A. Gardiennet (BPI 879792), Til-Chatel, FRANCE | GU323184 | GU323210 | — | — |
| L. mytilinum | CBS 269.34 | M.L. Lohman (AFTOL 1609), MI, U.S.A. | DQ678030 | DQ678081 | DQ677926 | DQ677979 |
| CBS 114111 | K. & L. Holm & O Constantinescu, Uppland, SWEDEN | EF596819 | EF596819 | — | — | |
| CBS 123344 | E.W.A. Boehm (BPI 878736), NY, U.S.A. | FJ161163 | FJ161203 | FJ161110 | FJ161128 | |
| Mycosphaerella punctiformis | CBS 113265 | G. Verkley (AFTOL 942), Utrecht, NETHERLANDS | DQ471017 | DQ470968 | DQ471092 | DQ470920 |
| Myriangium duriaei | AFTOL 1304 | L. Grodsinsky (CBS 260.36), Delta del Parana, ARGENTINA | AY016347 | DQ678059 | DQ677900 | DQ677954 |
| Mytilinidion acicola | EB 0379 | A. Gardiennet (BPI 879793), Veronnes, FRANCE | GU397362 | GU397346 | — | GU397355 |
| M. acicola | EB 0349 | A. Gardiennet (BPI 879794), Fixey, Combe Laveau, FRANCE | GU323185 | GU323209 | — | GU371757 |
| M. andinense | CBS 123562 | M.I. Messuti (BPI 878737), Barrio Don Orione, ARGENTINA. | FJ161159 | FJ161199 | FJ161107 | FJ161125 |
| M. australe | CBS 301.34 | A.H. Smith & M.L. Lohman, (type culture), LA, U.S.A. | — | FJ161183 | — | — |
| M. californicum | EB 0385 | A. Gardiennet (BPI 879795), Bois de la Chamage, FRANCE | GU323186 | GU323208 | — | — |
| M. mytilinellum | CBS 303.34 | M.L. Lohman (No. 281), as M. laeviusculum, MI, U.S.A. | FJ161144 | FJ161184 | FJ161100 | FJ161119 |
| EB 0386 | A. Gardiennet (BPI 879796), Boissenois, FRANCE | GU397363 | GU397347 | — | GU397356 | |
| M. resinicola | CBS 304.34 | M.L. Lohman, No. 260, MI, U.S.A. | FJ161145 | FJ161185 | FJ161101 | FJ161120 |
| M. rhenanum | CBS 135.45 | NCTC 6434 (1945), as M. karstenii | FJ161136 | FJ161175 | FJ161092 | FJ161115 |
| EB 0341 | A. Brissard, Guesnes, FRANCE | GU323187 | GU323207 | — | — | |
| M. scolecosporum | CBS 305.34 | A.H. Smith & M.L. Lohman, WI, U.S.A. | FJ161146 | FJ161186 | FJ161102 | FJ161121 |
| M. thujarum | EB 0268 | E.W.A. Boehm (BPI 879797), NY, U.S.A. | GU323188 | GU323206 | — | — |
| M. tortile | EB 0377 | A. Gardiennet (BPI 879798), Veronnes, FRANCE | GU323189 | GU323205 | — | — |
| Oedohysterium insidens | CBS 238.34 | M.L. Lohman (No. 308) MI, U.S.A. | FJ161142 | FJ161182 | FJ161097 | FJ161118 |
| Od. insidens | ANM 1443 | A.N. Miller (BPI 879799, ILLS), Smoky Mts., TN, U.S.A. | GU323190 | GQ221882 | — | GU371785 |
| Od. pulchrum | DQ 402184 | J. Checa (DAOM 234345), Guanacaste, COSTA RICA | DQ402184 | — | — | — |
| Od. sinense | CBS 123345 | M. Gryzenhout (BPI 878730), Limpopo, SOUTH AFRICA | FJ161169 | FJ161209 | — | FJ161130 |
| EB 0339 | E.W.A. Boehm (BPI 879800), NJ, U.S.A. | GU397364 | GU397348 | GU397339 | GU397357 | |
| Opegrapha dolomitica | DUKE 0047528 | C. Gueidan (AFTOL 993), CROATIA | DQ883706 | — | DQ883732 | DQ883714 |
| Ostreichnion curtisii | CBS 198.34 | M.L. Lohman (No. 464), GA, U.S.A. | FJ161137 | FJ161176 | FJ161093 | — |
| O. sassafras | CBS 322.34 | M.L. Lohman (No. 530), NC, U.S.A. | FJ161148 | FJ161188 | — | FJ161122 |
| Patellaria atrata | CBS 958.97 | G. Marson, Wasserbillig, Bahnhof, LUXEMBOURG | GU296181 | GU301855 | GU349038 | GU371726 |
| Phoma herbarum | CBS 276.37 | AFTOL 1575 | DQ678014 | DQ678066 | DQ677909 | DQ677962 |
| Pleospora herbarum | CBS 191.86 | E.G. Simmons, AFTOL_940, Uttar Pradesh, INDIA | DQ247812 | DQ247804 | DQ471090 | DQ247794 |
| Psiloglonium araucanum | CBS 112412 | S. Marincowitz (PREM 57570), Kirstenbosch, SOUTH AFRICA | FJ161133 | FJ161172 | FJ161089 | FJ161112 |
| CMW 18760 | S. Marincowitz (PREM 57569), Kirstenbosch, SOUTH AFRICA | FJ161151 | FJ161192 | — | — | |
| CMW 17941 | S. Marincowitz (PREM 57566), Jonkershoek, SOUTH AFRICA | FJ161149 | FJ161190 | — | — | |
| P. clavisporum | CBS 123338 | E.W.A. Boehm (BPI 878726), NJ, U.S.A. | FJ161156 | FJ161197 | — | FJ161123 |
| CBS 123339 | E.W.A. Boehm (BPI 878727), NJ, U.S.A. | FJ161157 | FJ167526 | FJ161105 | FJ161124 | |
| CBS 123340 | E.W.A. Boehm (BPI 878728), NJ, U.S.A. | FJ161165 | FJ161205 | — | — | |
| CBS 123341 | E.W.A. Boehm (BPI 878729), NJ, U.S.A. | FJ161166 | FJ161206 | — | — | |
| GKM 344A | G.K. Mugambi (BPI 879801, EA), Malindi, KENYA | GU397365 | GQ221889 | — | — | |
| GKM L172A | G.K. Mugambi (EA), Malindi, KENYA | GU323192 | GU323204 | — | — | |
| P. simulans | CBS 206.34 | M.L. Lohman, MI, U.S.A. | FJ161139 | FJ161178 | FJ161094 | FJ161116 |
| ANM 1557 | A.N. Miller (BPI 879803, ILLS), Smoky Mts., TN, U.S.A. | — | GQ221873 | GQ221920 | — | |
| Quasiconcha reticulata | EB QR | M. Blackwell (RLG 14189), AZ, U.S.A. | — | GU397349 | — | — |
| Roccella fuciformis | AFTOL 126 | Diederich 15572 | AY584678 | AY584654 | — | DQ782866 |
| Rhytidhysteron hysterinum | EB 0351 | A. Gardiennet (BPI 879804), Gevrey-Chambertin, FRANCE | — | GU397350 | GU397340 | — |
| R. opuntiae | GKM 1190 | G.K. Mugambi (BPI 879805, EA), Malindi, KENYA | — | GQ221892 | GU397341 | — |
| R. rufulum | CBS 306.38 | R.K. Voorhees (AFTOL 2109), EUROPE | AF164375 | FJ469672 | GU349031 | — |
| GKM 361A | G.K. Mugambi (BPI 879806, EA), Malindi, KENYA | GU296192 | GQ221893 | GU349031 | — | |
| EB 0381 | E. Nkansah (BPI 879807), Kwame Nkrumah, GHANA | GU397366 | GU397351 | — | — | |
| EB 0382 | E. Nkansah (BPI 879808), Kwame Nkrumah, GHANA | — | GU397352 | — | — | |
| EB 0383 | E. Nkansah (BPI 879809), Kwame Nkrumah, GHANA | GU397353 | GU397367 | — | — | |
| EB 0384 | E. Nkansah (BPI 879810), Kwame Nkrumah, GHANA | GU397368 | GU397354 | — | — | |
| Scorias spongiosa | CBS 325.33 | L.H. Leonian (AFTOL 1594) | DQ678024 | DQ678075 | DQ677920 | DQ677973 |
| Simonyella variegata | AFTOL 80 | DUKE Printzen 14310a | AY584669 | — | DQ782891 | DQ782861 |
AFTOL: Assembling the Fungal Tree of Life; BPI: United States USDA ARS National Fungus Collections, Beltsville, MD; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: Forestry & Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, Republic of South Africa; DAOM: National Mycological Herbarium, Department of Agriculture, Ottawa, Ontario, Canada; DUKE: Duke University Herbarium, Durham, North Carolina; EA: National Museums of Kenya East African Herbarium, Nairobi, Kenya; F: Field Museum of Natural History, Chicago, IL; ILLS: Illinois Natural History Survey Herbarium, Champaign, IL; PREM: The South African National Collection of Fungi, National Mycological Herbarium, Pretoria, South Africa; RLG: The Robert L. Gilbertson Mycological Herbarium at the University of Arizona. Culture and specimen abbreviations: ANM: A.N. Miller; EB: E.W.A. Boehm; GKM: G.K. Mugambi, SMH: S.M. Huhndorf. GenBank accessions marked in bold represent new sequences generated in the current study.
An attempt was made to include a broad range of fungal isolates, belonging to or previously affiliated with the Hysteriaceae, Mytilinidiaceae, Gloniaceae and Patellariaceae (Table 1). A geographically diverse (Cuba, Europe, Ghana, Kenya, New Zealand, South Africa, Tasmania, North and South America) and high density taxon sampling strategy was employed. This included multiple isolates/species from the genera: Anteaglonium (6/4), Encephalographa (1/1), Farlowiella (3/1), Gloniopsis (8/4), Glonium (4/2), Hysterium (12/5), Hysterobrevium (14/3), Hysterographium (2/1), Hysteropatella (2/2), Lophium (4/2), Mytilinidion (13/10), Oedohysterium (5/3), Ostreichnion (2/2), Patellaria (1/1), Psiloglonium (11/3), Quasiconcha (1/1), Rhytidhysteron (8/3), and 24 outgroup taxa, for a total of 121 taxa. All cultures and the herbarium specimens from which they were derived, have been deposited and are permanently conserved in the certified public institutions given in Table 1.
Within the Pleosporales, we sampled Anteaglonium abbreviatum, A. globosum, A. latirostrum and A. parvulum, Byssothecium circinans, Cochliobolus heterostrophus, Delitschia winteri, Herpotrichia diffusa, Leptosphaeria maculans, Phoma herbarum, and Pleospora herbarum. Eight representatives from the Dothideomycetidae were included as outgroups to the Pleosporomycetidae, namely Elsinoë veneta and Myriangium duriaei (Myriangiales), Dothidea sambuci and D. insculpta (Dothideales), Mycosphaerella punctiformis and Scorias spongiosa (Capnodiales). Botryosphaeria dothidea, and Guignardia gaultheriae (Botryosphaeriales). Jahnula aquatica and Aliquandostipite khaoyaiensis (Jahnulales), were also included. Four taxa in the Arthoniomycetes, were used as outgroups to the Dothideomycetes, namely Opegrapha dolomitica, Simonyella variegata, Roccella fuciformis, and Arthonia caesia. These are not presented in Fig. 1, due to space limitations, but are presented as a full tree available on TreeBASE, as well as in Table 1.
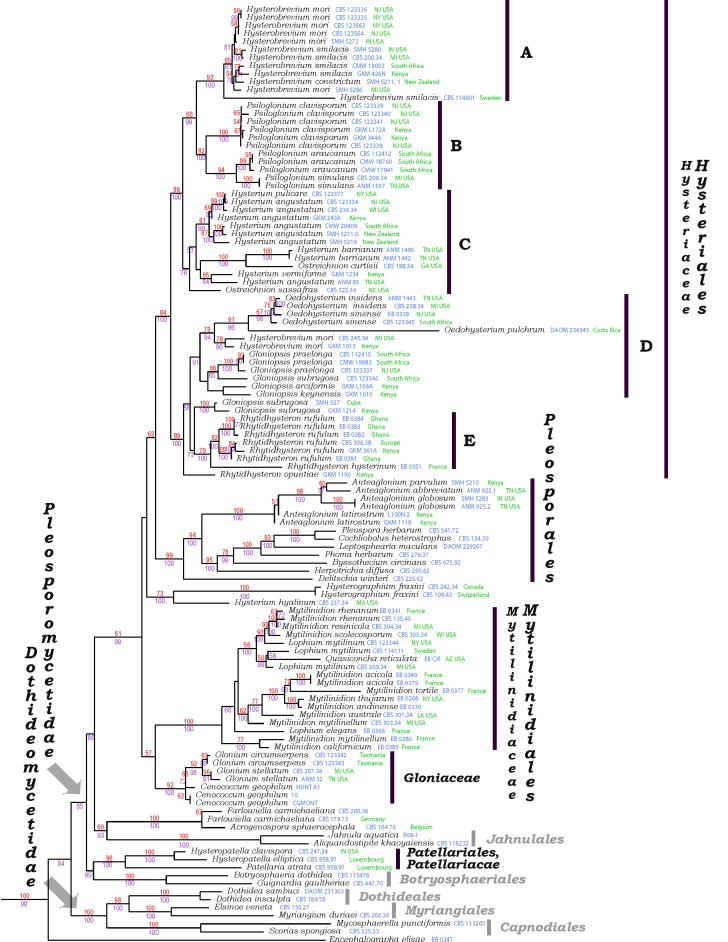
Fig. 1.
Combined ribosomal (nuSSU & nuLSU) and protein coding gene (TEF1 & RPB2) DNA phylogeny for bitunicate ascomycetes belonging to or previously affiliated with the Hysteriaceae, Mytilinidiaceae, Gloniaceae and Patellariaceae. Also included are representatives from allied groups such as the Pleosporales, Jahnulales, Patellariales, and Botryosphaeriales, as well as representatives from the Dothideales, Myriangiales and Capnodiales in the Dothideomycetidae. The Arthoniomycetes, chosen as outgroup, are not presented here due to space limitations, but are available in the full tree on TreeBASE. The tree is the highest scoring tree obtained by maximum likelihood in RAxML. Nodal values, given as percentages, are as follows: Bayesian posterior probability / maximum likelihood bootstrap. Only values above 50 % are shown.
DNA extraction, amplification and sequencing
Genomic DNA was recovered using the DNeasy® Plant Mini Kit (Qiagen Inc., Valencia, CA, U.S.A.), following the instructions of the manufacturer, but using sterile white quartz sand and a Kontes® battery-powered pestle grinder in 1.5 mL microfuge tubes. The nuSSU was amplified and double-strand sequenced using the primers NS1 and NS4 (White et al. 1990), while amplification of the nuLSU utilised the primers LROR (Rehner & Samuels 1994) and LR7 (Vilgalys & Hester 1990), in addition to the internal sequencing primers LR3R and LR16 (Moncalvo et al. 1993). Final concentrations for 50 μL PCR amplification reactions were as follows: 1 μM of each forward and reverse primer, 2 mM MgCl2, 200 μM dNTP, 1X Promega GoTaq® Flexi Reaction Buffer, 1.25 U of Promega GoTaq® Polymerase, and 2 μL template DNA diluted tenfold. For the nuSSU and nuLSU, PCR reaction parameters were as follows: a 95 °C pre-melt for 3 min, and 35 cycles of 95 °C for 20 s, 54 °C for 30 s and 72 °C for 60 s, followed by a final extension at 72 °C for 10 min. For TEF1 and RPB2, PCR amplification conditions followed those in Schoch et al. (2006). Primers used for the amplifications and sequencing of these protein coding genes were for TEF1: 983 & 2218R; and for RPB2: fRPB2-5F & fRPB2-7cR. PCR reactions were performed using PCR Master Mix Polymerase from Promega Corporation (Fitchburg, Wisconsin, U.S.A.) and run on an iCycler® from Biorad (Hercules, California, U.S.A.). For the amplification of DNA fragments used to infer the TEF1 amino acid sequence, the following conditions were used: (1) 94 °C for 2 min; (2) five cycles of 94 °C for 40 s, 55 °C for 45 s lowering by 0.8 °C per cycle and 72 °C for 90 s; (3) 30 cycles of 94 °C for 30 s, 52 °C for 45 s and 72 °C for 120 s and (4) a cycle for 10 min at 72 °C. Amplifications of DNA fragments used to infer the RPB2 amino acid sequence utilised the same cycle parameters, except for changes in steps (2) and (3) where the annealing temperatures of 55 °C and 52 °C were changed to 50 °C and 45 °C, respectively. Amplified PCR products were cleaned using the QIAquick® PCR Purification Kit (Qiagen Inc.) and resuspended in water prior to outsourcing for sequencing (Macrogen U.S.A., Inc.).
Phylogenetic analysis
DNA sequences were derived from previous studies (Schoch et al. 2006, Boehm et al. 2009, Mugambi & Huhndorf 2009), as well as from a number of new accessions generated in this study (Table 1). Sequences were aligned using default options for a simultaneous method of estimating alignments and tree phylogenies, SATé (Liu et al. 2009). Protein coding fragments were translated using BioEdit v. 7.0.1 (Hall 2004), and aligned within SATé as amino acid sequence data. These were then aligned with their respective DNA sequences using the RevTrans v. 1.4 Server (Wernersson & Pedersen 2003). Newly generated sequences were subsequently added to the core alignment with MAFFT v. 6.713 (Katoh et al. 2009). A supermatrix of four genes (nuLSU, nuSSU TEF1, RPB2) consisting of 56 % gaps and undetermined characters, across 121 taxa was obtained.
The matrix was analysed using maximum likelihood in RAxML v. 7.0.4 (Stamatakis 2006). Data was partitioned by individual gene and, where applicable, by codon, as in Schoch et al. (2009). A most likely tree was obtained after 100 successive searches in RAxML under the GTR model with gamma rate distribution across 11 partitions and starting each search from a randomised tree with a rapid hill climbing option and joint branch length optimisation. Five hundred fast bootstrap pseudoreplicates (Stamatakis et al. 2008) were run under the same conditions and these values are given above each node. The matrix analysed in this study produced 4174 distinct alignment patterns and the most likely tree had a log likelihood of -72114.22899. The average log likelihood over 100 trees was -72117.730727. Three independent Bayesian phylogenetic analyses were performed in MrBayes 3.1.2 (Huelsenbeck & Ronquist 2001) using a uniform [GTR+I+G] model. The Metropolis-coupled Markov chain Monte Carlo (MCMC) sampling approach was used to calculate posterior probabilities (PP). For each Bayesian run four Markov chains were run from a random starting tree for 5 000 000 generations and trees sampled every 100 generations. The first 50 000 generation trees were discarded as burn-in prior to convergence of four of the chains. All three runs reached a plateau that converged. One run was chosen to construct a 50 % majority rule consensus tree of all trees remaining after the burn in was discarded. Bayesian Posterior Probabilities with those equal or greater than 50 % are given below each node (Fig. 1).
RESULTS AND DISCUSSION
Phylogenetic analysis – ordinal level
At the ordinal level in the Pleosporomycetidae, molecular data indicate that the Hysteriales are closely related to the Pleosporales (Fig. 1), as was indicated in earlier studies (Schoch et al. 2006, Boehm et al. 2009). This is also confirmed by morphological evidence related to the centrum. Thus, the Hysteriales share a very similar centrum with the Pleosporales, that is, one defined by the Pleospora-type, in which cellular, septate pseudoparaphyses grow downwards from the cavity roof, initially anchored at both ends, and occupy the locule prior to the formation of asci (Luttrell 1951). However, there is also strong branch support for its separation from the Pleosporales (Boehm et al. 2009). The Hysteriales are therefore retained as defined by Luttrell (1955), to emphasise the elongated hysteriaceous locule, capable of relatively indeterminate linear growth, as distinct from the strict Pleospora-type centrum, defined as it is by constrained concentric growth. In contrast to the close association between the Hysteriales and the Pleosporales, the Mytilinidiales forms a more distant clade within the Pleosporomycetidae (Boehm et al. 2009).
Phylogenetic analysis – family level
Hysteriaceae
Although the Hysteriales receives high branch support as a monophyletic entity, distinct from the closely related Pleosporales, two major groups can be defined within the family. The first supports Clades A–C, whereas the second supports Clades D and E (Fig. 1).
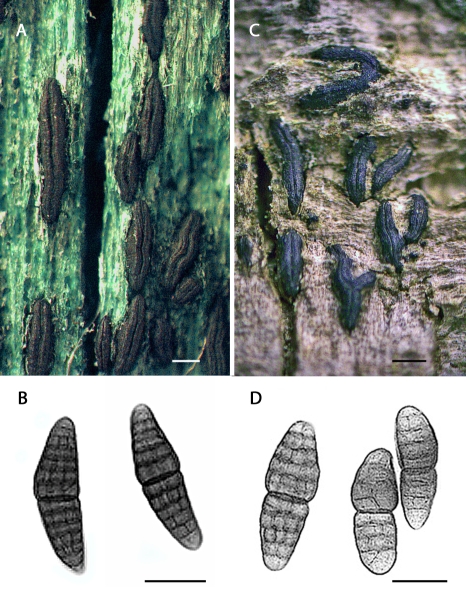
Clade A: This first clade is characterised by Hysterographium mori, with short pigmented dictyospores, Gloniopsis constricta, and Gp. smilacis, the latter two with short hyaline dictyospores. The Gp. smilacis isolates originate from highly diverse geographical sources (e.g., Sweden, South Africa, North America; Table 1), thus strongly supporting its phylogenetic placement. As these taxa are far removed from the types for their respective genera, we propose here to unite them in Hysterobrevium gen. nov., as Hb. mori comb. nov., Hb. constrictum comb. nov., and Hb. smilacis comb. nov.
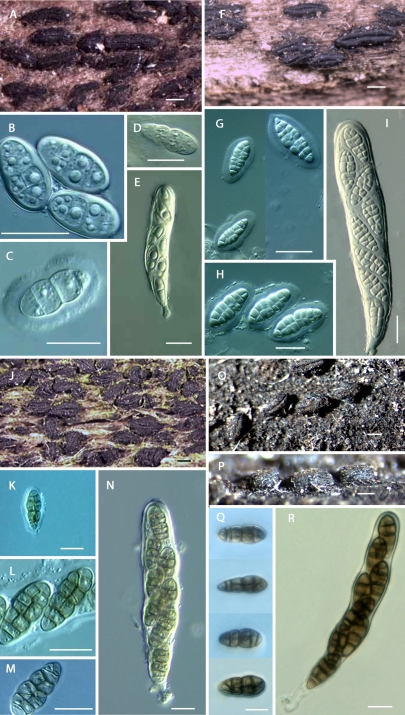
Clade B: This clade (Fig. 1) appears monophyletic for the newly reinstated genus Psiloglonium (Boehm et al. 2009), with hyaline didymospores. It includes the following species: P. simulans, P. clavisporum, and P. araucanum comb. nov. In this study, we propose a number of new combinations for the genus Psiloglonium, with P. lineare as the type (Boehm et al. 2009), to accommodate species previously classified under the genus Glonium, now in the Gloniaceae.
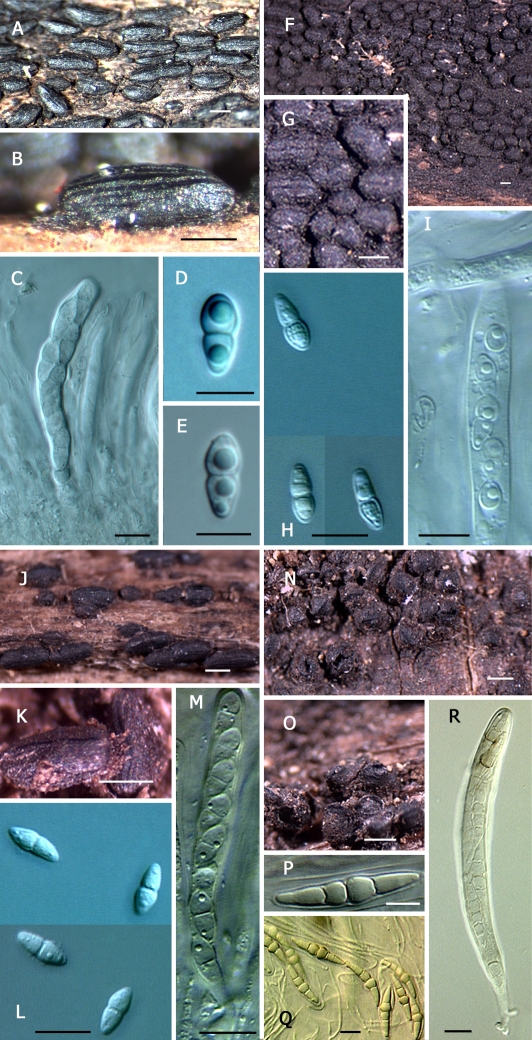
Clade C: This clade is characterised by pigmented phragmospores belonging to four species of the genus Hysterium, namely H. pulicare, H. angustatum, H. vermiforme, which have 3-septate spores, and H. barrianum sp. nov., which has 9-septate spores. Again, a geographically diverse set of isolates were surveyed (Table 1). For instance, taxon sampling for H. angustatum included isolates originating from Kenya, New Zealand, South Africa, and North America (Fig. 1). Also within this clade, but with weak bootstrap support, is Ostreichnion sassafras, and O. curtisii, previously transferred from the Mytilinidiaceae to the Hysteriaceae (Boehm et al. 2009).
Clade D: This clade is heterogeneous, but can be divided into two sub-clades. The first sub-clade includes two species formerly in the genus Hysterium, namely H. insidens and H. sinense. Molecular data indicate that these species are not related to the type species, H. pulicare, nor to related species within Clade C. Morphology also supports this separation, as H. insidens and H. sinense both possess phragmospores with a swollen supra-median cell. We therefore propose Oedohysterium gen. nov., to accommodate Od. insidens comb. nov. and Od. sinense comb. nov. Also grouping in Clade D is Hysterographium pulchrum. Despite the fact that Hg. pulchrum possesses dictyospores, we propose to unite it within Oedohysterium, as Od. pulchrum comb. nov., on account that it too possesses a swollen supra-median cell. Also present in this subclade are two isolates of Hb. mori, distant from the other Hb. mori accessions in Clade A; this anomaly will be discussed later. A separate subclade is evident in Clade D, and defines the type species for the genus Gloniopsis, namely Gp. praelonga. Closely associated with Gp. praelonga is one representative of Hg. subrugosum. Dictyospores of both species are of similar shape, size and degree of septation, differing only in the lack of pigmentation and a gelatinous sheath. We thus propose that Gp. praelonga and Hg. subrugosum be united within the same genus, proposing Gloniopsis subrugosa comb. nov. The other two representatives of Gp. subrugosa do not fall into Clade D, but lie adjacent. Lastly, an additional two species are described in Clade D, namely Gloniopsis arciformis sp. nov. and Gp. kenyensis sp. nov., both from East Africa (Table 1).
Clade E: This clade is well-supported and defines two species in the genus Rhytidhysteron, namely R. rufulum, and R. hysterinum. Taxon sampling included isolates originating from France, Ghana, Kenya and North America. This clade therefore supports the transference of the genus Rhytidhysteron from the Patellariaceae to the Hysteriaceae, as initially proposed by Boehm et al. (2009). The third species of Rhytidhysteron, R. opuntiae, is distant to the first two species, but remains adjacent to Clade E.
Mytilinidiaceae
In contrast to the Hysteriales, the family Mytilinidiaceae represents a highly monophyletic entity, defining the order Mytilinidiales (Boehm et al. 2009). The conchate nature of the fruitbody and the thin-walled peridium, seem to unite what at first may seem a disparate group of fungi into a single family (Fig. 1). In this study, we have sampled 10 of the 15 species of Mytilinidion, characterised by phragmospores and scolecospores, two of the four species of Lophium, with filiform spores, as well as the monotypic Quasiconcha, with reticulated 1-septate spores (Table 1). Although monophyletic, sequence data also indicate a complex pattern of speciation within the family, one that is not premised on past assumptions based on spore morphology (Fig. 1).
Gloniaceae
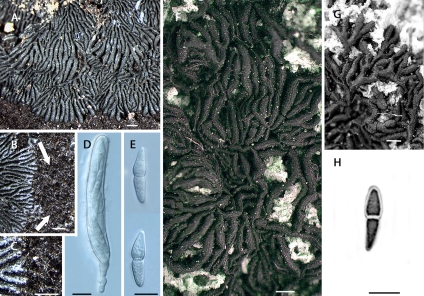
As for the monotypic family Gloniaceae (Boehm et al. 2009), based on the genus Glonium, previously classified within the Hysteriaceae (Zogg 1962), surprisingly, sequence data indicate that it finds close affinity with the Mytilinidiaceae (Fig. 1). This is based on four isolates, representing two species, Glonium stellatum and G. circumserpens. However, the Gloniaceae is not included within the Mytilinidiales, due to the highly divergent morphology associated with the genus Glonium. These include character states related to the hamathecium (persistent cellular pseudoparaphyses versus narrow trabeculate pseudoparaphyses) and to the fruitbody (dichotomously branched versus conchate), for the Gloniaceae and Mytilinidiaceae, respectively. Thus, for the present, we propose that the family Gloniaceae be considered Pleosporomycetidae fam. incertae sedis.
TAXONOMY
Hysteriaceae Chevall. 1826, Hysteriales Lindau 1897.
Fungi classified in the Hysteriaceae (Chevallier 1826) have been traditionally defined by a specialised ascocarp termed the hysterothecium (Clements 1909). Hysterothecia are dense, persistent carbonaceous structures, distinctly navicular in outline, and bear a pronounced longitudinal slit running the length of the long axis of the fruitbody. They can be immersed to erumpent to entirely superficial, solitary to gregarious, ellipsoid to greatly elongated, sometimes branched or triradiate. In vertical section, hysterothecia are globose to obovoid, typically with a thick three-layered peridium, composed of small pseudoparenchymatous cells, the outer layer heavily encrusted with pigment and often longitudinally striated on the surface, the middle layer lighter in pigmentation and the inner layer distinctly thin-walled, pallid and compressed (Barr 1987). The hamathecium is composed of persistent, narrow cellular pseudoparaphyses, often borne in a gel matrix, with tips darkened or branched at maturity above the asci. Bitunicate asci are borne in a basal layer and at maturity are typically clavate to cylindric, bearing 8 ascospores, overlapping biseriate, ranging from hyaline to dark brown, obovoid, clavate, ellipsoid or fusoid. Ascospores are highly diverse in septation and range from didymospores to phragmospores to dictyospores, at times surrounded by a gel coating, and often show bipolar asymmetry (Barr 1987). Zogg (1962) accepted the following seven genera within the Hysteriaceae: Farlowiella, Gloniella, Gloniopsis, Glonium, Hysterium, Hysterocarina, and Hysterographium.
The traditional circumspection of the Hysteriaceae was based on character states related to the hysterothecium and spore morphology (e.g., septation and pigmentation), character states previously considered synapomorphic (Zogg 1962). However, recent molecular data underscore the potential for morphology to be difficult to interpret, and even unhelpful in phylogenetic inference and reconstruction for this group of fungi (Schoch et al. 2006, Boehm et al. 2009, Mugambi & Huhndorf 2009). Thus, a number of examples of convergent evolution are presented in the current study, which relate not only to the fruitbody, but to spore morphology as well. As a result, three genera have been removed from the family (Glonium, Hysterographium and Farlowiella), based on convergence associated with the fruitbody. Additionally, within the family, several genera have their members spanning different clades (Fig. 1). This necessitated the description of two new genera (Oedohysterium and Hysterobrevium), as well as three new species, one in Hysterium and two in Gloniopsis, in addition to a number of new combinations involving Psiloglonium, Oedohysterium, Hysterobrevium and Gloniopsis. These taxonomic changes have de-emphasised both spore septation and spore pigmentation as reliable character states for deducing phylogenetic relationships within the family. Nevertheless, in the keys that follow, we have endeavoured to provide a morphological basis for the new phylogenies revealed by molecular data.
Data have also necessitated that we expand the concept of the Hysteriaceae to include thin-walled mytilinidioid forms previously in the Mytilinidiaceae (e.g., Ostreichnion), as well as patellarioid forms previously in the Patellariaceae (e.g., Rhytidhysteron). The inclusion of Ostreichnion within the Hysteriaceae was unexpected. Unlike most members of the family, the peridium in Ostreichnion is sclerenchymatoid and thin-walled, defining a fragile mytilinidioid ascoma, and with a hamathecium typified by trabeculate pseudoparaphyses (Barr 1975, 1990a). Including the genus Ostreichnion in the Hysteriaceae implies that, either morphological features within the genus need to be re-evaluated, or that the family Hysteriaceae must also encompass mytilinidioid forms. More difficult to understand perhaps is the inclusion of the genus Rhytidhysteron within the Hysteriaceae. Although included within the Patellariaceae (Kutorga & Hawksworth 1997), phylogenetic data presented here and elsewhere (Boehm et al. 2009), clearly indicate that this genus lies quite distant from other members of the Patellariaceae.
Some authors have included a number of additional genera within the Hysteriaceae. For instance, the genera Hysteropatella, Hysteroglonium, and Pseudoscypha were included in the Hysteriaceae by Eriksson (2006). In addition, the genera Hemigrapha, Graphyllium, and Encephalographa were included in the family by Kirk et al. (2001). In Boehm et al. (2009), two species belonging to Hysteropatella, namely Hp. clavispora (CBS 247.34) and Hp. elliptica (CBS 935.97), did not cluster with any of the hysteriaceous taxa surveyed. Instead, they formed a distant clade within the Pleosporomycetidae, postulated to represent the emergence of the Patellariales. In the present study, these two species of Hysteropatella continue to be distant from the Hysteriaceae, and also cluster now with Patellaria atrata (CBS 958.97). Therefore, we do not include the genus Hysteropatella within the Hysteriaceae.
Reid & Pirozynski (1966) in describing Pseudoscypha abietis on the needles of Abies balsamea did not mention the Hysteriaceae, and in fact stated that the fungus cannot be assigned to any presently known order. In their illustrations, no sterile tissue or excipulum is presented, and the bitunicate asci and pseudoparaphyses arise directly from an erumpent orange basal stromatic cushion. As such, we do not include Pseudoscypha as a member of the Hysteriaceae. As for the genus Hemigrapha, Diederich & Wedin (2000) make the argument for the inclusion of the genus in the Microthyriaceae, not the Hysteriaceae. The genus Graphyllium possesses applanate, clathrate ascospores borne in thin-walled membranous hysterothecia, at first subcuticular, later erumpent, often associated with aquatic poaceous hosts. The genus was included in the Hysteriaceae by Shoemaker & Babcock (1992) and Kirk et al. (2001), but was earlier classified in the Phaeosphaeriaceae (Barr 1987). A new species was recently described from Costa Rica (Checa et al. 2007). The unique ascospore and the lack of carbonisation or peridial wall thickness argue against the inclusion in the Hysteriaceae, but molecular data are lacking.
Key to the genera and allied genera of the Hysteriaceae
1. Ascomata apothecioid, opening widely when hydrated, fully exposing the hymenium, which may be red or black (i.e., patellarioid).............................................................................................................................................................. Rhytidhysteron 1. Hysterothecia usually remaining closed, or only opening slightly through a longitudinal fissure or sulcus to reveal a lenticular, disk-like hymenium when hydrated and mature.................................................................................................................... 2
2. Ascospores pedicellate amerospores, the upper cell pigmented and much larger than the lower, which remains un- or less-pigmented; anamorph Acrogenospora......................................................................................................................... Farlowiella Note: The genus Farlowiella has been removed from the Hysteriaceae and is currently listed as Pleosporomycetidae gen. incertae sedis (Boehm et al. 2009). 2. Ascospores not as above, didymospores, phragmospores or dictyospores, sometimes pigmented........................................................ 3
3. Didymospores small, the two cells more or less equal in size.................................................................................................................. 4 3. Ascospores not as above, phragmospores, dictyospores, +/- pigmentation, or very large didymospores (O. curtisii)............................. 7
4. Ascospores hyaline................................................................................................................................................................................... 5 4. Ascospores pigmented.................................................................................................................................................. Actidiographium
5. Didymospores less than 8 μm long..................................................................................................................................... Anteaglonium Note: The genus Anteaglonium lies within the Pleosporales (Mugambi & Huhndorf 2009), but is keyed out here with Psiloglonium. 5. Didymospores longer than 8 μm............................................................................................................................................................... 6
6. Didymospores hyaline, borne in solitary or gregarious hysterothecia, rarely associated with a subiculum, not laterally anastomosed to form radiating stellate composites............................................................................................................. Psiloglonium Note: One species of Anteaglonium, A. latirostrum, will key out here, but belongs in the Pleosporales (Mugambi & Huhndorf 2009) and is also keyed out in the Psiloglonium key. 6. Didymospores hyaline, borne in modified hysterothecia, usually associated with a subiculum, strongly laterally anastomosed along their length to form radiating stellate composites........................................................................................ Glonium Note: The genus Glonium has been transferred from the Hysteriaceae to the Gloniaceae, currently listed as fam. incertae sedis within the Pleosporomycetidae (Boehm et al. 2009).
7. Ascospores transversely septate phragmospores, or if with dictyospores then also with red pigmentation............................................. 8 7. Ascospores transversely and longitudinally septate dictyospores, or very large didymospores (O. curtisii).......................................... 10
8. Ascospores hyaline phragmospores............................................................................................................................................ Gloniella 8. Ascospores pigmented phragmospores or in one case (Od. pulchrum) with pigmented dictyospores and red pigmentation in the hamathecium............................................................................................................................................................. 9
9. Phragmospores 3-septate or rarely more, but without swollen supra-median cell(s)............................................................... Hysterium 9. Phragmospores with swollen supra-median cell, usually more than 3-septate, in one case with pigmented dictyospores and red centrum pigmentation (Od. pulchrum)........................................................................................... Oedohysterium
10. Dictyospores hyaline, +/- gelatinous sheath, or pigmented, but short, less than 25 μm in length.................................. Hysterobrevium 10. Dictyospores hyaline, +/- gelatinous sheath, or pigmented, but longer than 25 μm, or very large didymospores (O. curtisii)............................................................................................................................................................................................... 11
11. Dictyospores, if hyaline, then longer than 25 μm, or if pigmented, then measuring (22–)25–34(–45) x (6–)8–12(–17) μm, with 7–11 transverse and 1–2 vertical septa, and no red pigment associated with the hamathecium (Gp. subrugosa).......... Gloniopsis 11. Dictyospores pigmented, of different length, or if similar in length to Gp. subrugosa, then tropical with red pigment associated with the hamathecium, or very large didymospores (O. curtisii)........................................................................................... 12
12. Dictyospores or large didymospores borne in conchate, mytilinidioid, thin-walled, slerenchymatous, fragile fruitbodies.................................................................................................................................................................. Ostreichnion Note: The genus Ostreichnion, previously in the Mytilinidiaceae, was transferred to the Hysteriaceae (Boehm et al. 2009). 12. Dictyospores borne in thick-walled, navicular hysterothecia................................................................................................................... 13
13. Dictyospores pigmented, borne in typical hysterothecia, that are erumpent or sessile on the substrate..................... Hysterographium Note: The genus Hysterographium, with the type species Hg. fraxini, has been transferred out of the Hysteriaceae as Pleosporomycetidae gen. incertae sedis (Boehm et al. 2009). Residual species classified as Hysterographium, for which sequence data are lacking, are provisionally retained within the genus. 13. Hysterothecia borne within the substrate, hardly erumpent, with cristate longitudinal apex instead of a sulcus; neotropical.......................................................................................................................................................................... Hysterocarina
The genus Encephalographa was originally placed in the Hysteriaceae by Renobales & Aguirre (1990) who thought it to be lichenicolous. Tretiach & Modenesi (1999) demonstrated it to be lichenised, and maintained its placement within the Hysteriaceae. The latter authors illustrate endolithic, saxicolous, dichotomously branched, laterally anastomosed, lirelliform pseudothecia with a longitudinal sulcus, and clavate bitunicate asci bearing pigmented didymospores, highly reminiscent of the saxicolous forms of Glonium circumserpens, in the Gloniaceae. We recently were able to obtain fresh material of Encephalographa elisae from Mauro Tretiach (Dipartimento di Biologia, Università di Trieste, Trieste, Italy), and, although cultures failed, we were able to isolate DNA directly from the ascomata (EB 0347 / BPI 879773). Sequence data presented here indicate that E. elisae does not reside within the Hysteriaceae, nor within the Gloniaceae. Instead, E. elisae lies outside of the Pleosporomycetidae and Dothideomycetidae (Fig. 1).
To summarise, we accept the following genera in the Hysteriaceae: Actidiographium, Gloniella, Gloniopsis, Hysterium, Hysterobrevium, Hysterocarina, Oedohysterium, Ostreichnion, Psiloglonium, and Rhytidhysteron. Dichotomous keys are presented here for hysteriaceous fungi, with the caveat that phylogenetically unrelated taxa share the same key. Thus, despite their transference from the Hysteriaceae (Boehm et al. 2009), the genera Hysterographium, Farlowiella, Glonium and Anteaglonium (Mugambi & Huhndorf 2009), are nevertheless included in the key. This is because they typically possess ascomata that have traditionally been referred to as hysterothecia.
Hysterium Tode, Schriften Berlin. Ges. Naturf. Freunde 5: 53 (1784).
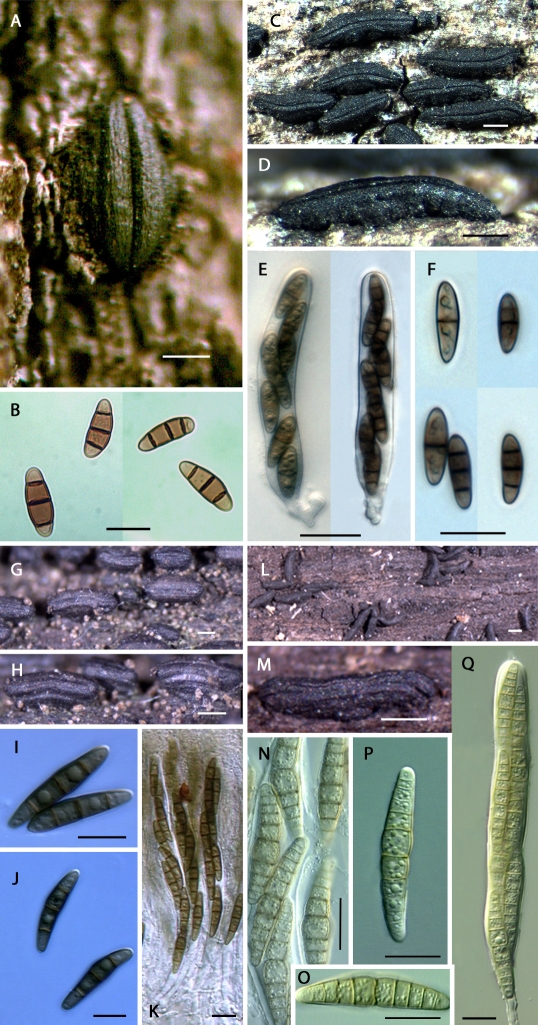
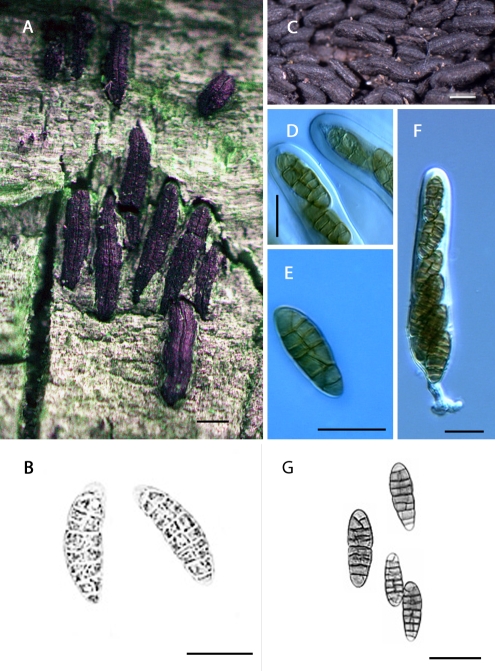
The genus Hysterium is characterised by pigmented versicolorous or concolorous asymmetric phragmospores, three- or more transversely-septate, borne in hysterothecia. A historical overview of the nomenclature of the genus was presented in Boehm et al. (2009). Zogg (1962) recognised two morphological types within the genus. Type I is characterised by 3-septate phragmospores, and includes the versicolorous type species H. pulicare (Fig. 2A–B), and its closely related concolorous counterpart, H. angustatum (Fig. 2C–F), both extremely common in the temperate zones of both hemispheres. These are followed by H. vermiforme (Fig. 2G–K), from Africa, and the much larger-spored H. macrosporum, reported from North America and China (Teng 1933). Although Zogg (1962) did not accept H. hyalinum, Lohman (1934) provided legitimacy to the epithet, noting that pigmentation is delayed in the maturation of the 3-septate ascospores (Boehm et al. 2009).
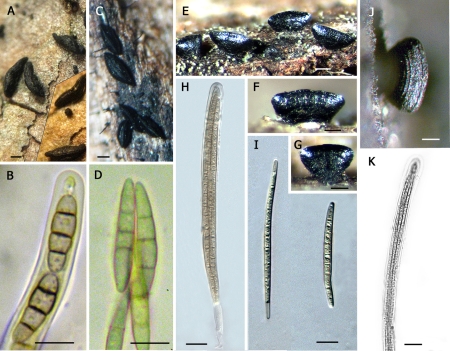
Fig. 2.
The genus Hysterium (Clade C). A–B. Hysterium pulicare [CBS 123377 (BPI 878723), U.S.A.]; C–F. Hysterium angustatum [ANM 120 (ILLS), U.S.A.; not incl.]; G–K. Hysterium vermiforme [GKM 1234 (BPI 879785), Kenya]; L–Q. Hysterium barrianum sp. nov. [ANM 1495 (ILLS 59908 = holotype), U.S.A.]. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 20 μm.
Type II corresponds to a different phragmospore, one in which, typically, there are five or more septa, and in which there exists a swollen cell, either just above the median septum (i.e., supramedian) or, rarely, some distance up from the median septum. Type II includes, by increasing spore length, the cosmopolitan H. insidens (Fig. 3A–D), the larger-spored counterpart H. sinense (Fig. 3E–H), and the unusual H. magnisporum, 7-septate, with three of the septa crowded to each end, the two central cells much larger. The latter two species are reported from China (Teng 1933) and North America (Boehm, unpubl. data). Hysterium velloziae, provisionally included by Zogg (1962), with up to 21 septa at maturity, has only been reported from Africa (van der Linde 1992).
Fig. 3.
The genus Oedohysterium (Clade D). A–D. Oedohysterium insidens [ANM 1443 (BPI 879799), U.S.A.]; E–H. Oedohysterium sinense [ANM 119 (ILLS), U.S.A.; not incl.]. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 20 μm.
An additional two species have been recently described. Hysterium asymmetricum (Checa et al. 2007) from Costa Rica, has outer centrum tissues pigmented red, and 3-septate phragmospores, showing an extended basal cell. Hysterium andinense has been recently described from the conifer Austrocedrus chilensis in Argentina (Messuti & Lorenzo 1997). However, molecular and morphological data (Boehm et al. 2009) has placed this taxon in the Mytilinidiaceae, as Mytilinidion andinense, based on CBS 123562 (BPI 878737). This brings the total number of species within the genus Hysterium to 10. An additional new species is described here.
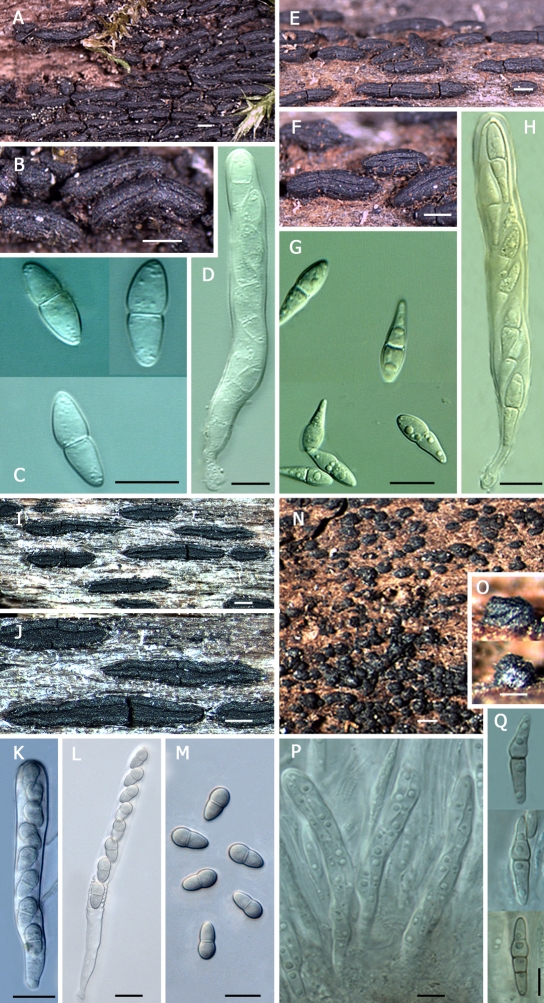
Hysterium barrianum E.W.A. Boehm, A.N. Miller, G.K. Mugambi, S.M. Huhndorf & C.L. Schoch, sp. nov. MycoBank MB515330. Fig. 2L–Q.
Etymology: Named after the late Dr Margaret E. Barr, preeminent American mycologist.
Ascomata inconspicue hysterothecioidea, modice compressa e latere in parte superiore, paulo conniventia, sulco inconspicuo angusto, latera paucis striis profundis praedita; ascomata recta vel flexuosa, sessilia, raro furcata, matura altiora quam lata, 1–2.5 mm longa, 250–450 μm alta, 200–300 μm lata. Pseudoparaphyses hyalinae, cellulares, 1–2 μm latae, supra ascos ramosae epithecium formantes. Asci bitunicati, cylindrici, breviter stipitati, (110–)125–135 x 15–20 μm. Phragmosporae fusiformes, angustae, rectae vel paulo curvatae, primum hyalinae, maturae pallide luteae, quaque cellula guttulis magnis refringentibus repleta, (7–)9(–11)-septatae, (35–)40–45(–55) x (7–)9–10(–12) μm.
Ascomata atypically hysterithecioid, somewhat laterally compressed in the upper region, slightly connivent, sulcus very shallow, existing as a narrow rim, sides laterally striate, striae few and deep, straight to flexuous, sessile on the substrate, rarely bifurcating, taller than wide at maturity: 1–2.5 mm long x 250–450 μm high, 200–300 μm wide. Pseudoparaphyses hyaline, cellular, 1–2 μm wide, branched above the ascal layer to form an epithecium. Asci bitunicate, cylindrical, short-stipitate, (110–)125–135 x 15–20 μm (n = 9). Phragmospores fusiform, narrow, hyaline and straight when young, becoming pale-yellow to lightly clear-brown, and curved when mature, highly guttulate, with guttulae large, highly refractive, present in every cell, with (7–)9(–11) septa, measuring (35–)40–45(–55) x (7–)9–10(–12) μm when mature (n = 27).
Specimens examined: U.S.A., Tennessee, Sevier Co., Great Smoky Mountains National Park, Elkmont, Little River Trail, 35° 39' 13.4'' N, 83° 34' 44.7'' W, 686 m elev., 5 Nov. 2007, A.N. Miller, S.M. Huhndorf, J.L. Crane, T.J. Atkinson, I. Promputtha, M. Grief, G.K. Mugambi, & P. Chaudhary, deposited as ILLS 59908 (ANM 1495) = holotype; BPI 879783 = paratype; Tennessee, Sevier Co., Great Smoky Mountains National Park, Chimney Tops Picnic Area, Cove Hardwood Loop Trail, 35° 38' 10.7'' N, 83° 29' 32.1'' W, 4 Nov. 2007, A.N. Miller, S.M. Huhndorf, J.L. Crane, T.J. Atkinson, I. Promputtha, M. Grief, G.K. Mugambi & P. Chaudhary, deposited as ILLS 59907 (ANM 1442), and BPI 879784.
Notes: A superficial resemblance exists between Hysterium barrianum in Clade C, with H. sinense in Clade D. The phragmospores of H. barrianum (Fig. 2N–Q) have a similar number of septa, (7–)9(–11), as those of H. sinense (Fig. 3H), the latter with (3–)5–9(–11) septa. The two species also have spores of similar length. However, the width measurements of H. barrianum, (35–)40–45(–55) x (7–)9–10(–12) μm, serve to separate it from H. sinense, (34–)38–50 x 11–15 μm. Most importantly, H. barrianum does not possess a swollen or tumid supra-median cell, as does H. sinense and the closely related H. insidens. Furthermore, H. barrianum is highly guttulate, and lightly pigmented at maturity, whereas H. sinense and H. insidens possess few if any guttulae, and are much darker in pigmentation at maturity. Lastly, molecular data place the species in different groups within the Hysteriaceae.
In this study, we were able to secure a wide taxon sampling strategy for the genus Hysterium (Table 1), including multiple isolates for seven of the eleven currently recognised species, namely: H. pulicare (1), H. angustatum (7), H. vermiforme (1), H. insidens (2), H. sinense (2), H. barrianum (2) and H. hyalinum (1). Multiple gene phylogenies indicate that the genus Hysterium is polyphyletic, along three separate lines, two within the Hysteriaceae and one, H. hyalinum, outside of the family (Fig. 1). This implies that the evolution of pigmented phragmospores borne in hysterothecia has occurred at least three times within the Pleosporomycetidae.
Sequence data indicate that Clade C contains the type species, Hysterium pulicare, as well as the closely related H. angustatum, and H. vermiforme (Fig. 1). All three taxa have 3-septate, pigmented phragmospores, corresponding to Type I. Also, within Clade C resides the newly described H. barrianum, with 9-sepate spores. None of these species has a swollen supra-median cell. Accessions of H. angustatum, originating from South Africa (CMW 20409), Kenya (GKM 243A), New Zealand (SMH 5211.0, SMH 5216) and the United States, New Jersey (CBS 123334) and Wisconsin (CBS 236.34), form a highly supported monophyletic clade with H. pulicare, collected from the United States, New York (CBS 123377). Both species possess similar pigmented 3-septate phragmospores, versicolorous in H. pulicare and concolorous in H. angustatum. Interestingly, ∼10 % of the ascospores within a given hysterothecium of H. pulicare are typically found to be concolorous (Bisby 1941). Likewise, versicolorous ascospores have also been observed in H. angustatum, stated at less than ∼5 % for a given hysterothecium (Lee & Crous 2003). Although ascospore size in H. pulicare may be twice that found in H. angustatum (Zogg 1962), a certain degree of overlap in spore length measurements exists between the two, and molecular data presented here and elsewhere (Boehm et al. 2009) indicate that they are closely related.
In this study, one of the H. angustatum accessions from Tennessee (ANM 85), did not cluster with the other surveyed H. angustatum in Clade C. Instead, ANM 85 clustered with H. vermiforme from Kenya (GKM 1234). Spore measurements of ANM 85 (ILLS) were compared to the other H. angustatum accessions from the United States (CBS 123334 / BPI 878724), Kenya (GKM 243A, EA), and New Zealand (SMH 5211.0, F) which formed the other sub-clade within Clade C. All of these specimens showed remarkably little variability in their spore morphology. Additionally, no obvious differences were noted in their fruitbody morphology. This may indicate early stages of speciation within the taxon, with sequence variation preceding morphologic change.
Grouping with the anomalous H. angustatum ANM 85, was H. vermiforme, a taxon known only from the original description by Massee in 1901 from West Africa (Ghana). The isolate included here (GKM 1234 / BPI 879785; Fig. G–K) originated from Mt. Kenya, Kenya, and possesses smaller spore measurements, (20–)25–28 x (4–)5–6 μm, than those given by Massee (1901), and reiterated by Zogg (1962), as (30–)35–40 x 12–14 μm. In other respects, however, BPI 879785 matches closely Massee's (1901) original description, and we choose here to simply expand the spore measurements for H. vermiforme to (20–)25–40 x (4–)5–14 μm, rather than describe a new species.
The 3-septate H. hyalinum (CBS 237.34) lies outside of the Hysteriaceae altogether. It falls in a small, isolated, but well-supported clade along with the type species of Hysterographium, namely Hg. fraxini. Since only one isolate is represented, it is premature to draw conclusions. Molecular data indicate that the remaining two species of Hysterium in our survey, namely H. sinense and H. insidens, are not related to the type H. pulicare and associated species within Clade C. Rather, data indicate that they belong to Clade D. As such, we propose the following new genus to accommodate these taxa.
Oedohysterium E.W.A. Boehm & C.L. Schoch, gen. nov. MycoBank MB515421.
Etymology: Greek, Oedo- meaning swollen, referring to the swollen supra-median cell of the ascospores and Hys- from Hysterium.
Hysterothecia solitaria vel gregaria, iuvenia erumpentia, deinde superficialia, navicularia, nonnumquam linearia, plus minusve parallela, neque confluentia, nonnumquam angulo inserta, raro flexuosa vel furcata, plerumque utrinque obtuse, et fissura longitudinali prominente praedita. Latitudo altitudine minor vel major. Peridium crassum, carbonaceum, maturum fragile, per longitudinem striatum, basim versus incrassatum, sursum attenuatum, bistratosum. Pseudoparaphyses cellulares, 1–2.5 μm latae, hyalinae, septatae, sursum ramosae, vulgo epithecium pigmentatum ascos obtegens formantes. Asci cylindrici vel clavati, bitunicati. Ascosporae irregulariter biseriatae, phragmoseptatae (dictyoseptatae), fusiformes, curvatae, utrinque angustatae, ad septum medium constrictae, (4–)6–8(–11) septis divisae, primum pallide luteae, deinde brunnescentes. Cellula (raro duo cellulae) ascosporarum supramediana conspicue inflata. Anamorphe ad Septonema pertinens.
Hysterothecia isolated to gregarious, erumpent when young, superficial when mature, navicular, sometimes linear in more or less parallel rows, but non confluent laterally, or sometimes situated at angles, rarely flexuous or bifurcating, usually with obtuse ends, and with a prominent longitudinal slit. Sometimes, taller than wide, other times wider than tall. Peridium thick, carbonaceous, brittle with age, longitudinally striated on the margins, thickened towards base, less thick apically, composed of two to three distinct layers, the inner compressed and pallid, the outer thickened and pigmented. Pseudoparaphyses cellular, 1–2.5 μm wide, hyaline, septate, branched above, forming a usually pigmented epithecium above the asci. Asci cylindrical to clavate, usually short stipitate, and bitunicate. Ascospores irregularly biseriate in ascus, typically phragmospores, in one case dictyospores, curved, fusiform, with tapering apices, constricted at the median septum, with (4–)6–8(–11) septa, at first hyaline-yellow, then pigmented sepia to brown at maturity. Genus characterised by a swollen or tumid supra-median cell, rarely with two cells swollen. Anamorph: Septonema.
Type species: Oedohysterium insidens (Schwein.) E.W.A. Boehm & C.L. Schoch, comb. nov.
Oedohysterium insidens (Schwein.) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515422. Fig. 3A–D. Basionym: Hysterium insidens Schwein., Trans. Amer. Philos. Soc., New Series 4(2): 244. 1832.
≡ Hysterographium insidens (Schwein.) Sacc., Syll. Fung. 2: 778. 1883.
= Hysterium complanatum Duby, Mém. Soc. Phys. Genève 16(1): 38. 1862.
= Hysterium depressum Berk. & M.A. Curtis, Grevillea 4(29): 10. 1875.
= Hysterium fusigerum Berk. & M.A. Curtis, Grevillea 4(29): 11. 1875 (as `fusiger').
= Hysterium berengeri Sacc., Syll. Fung. 2: 751. 1883.
= Hysterium janusiae Rehm, Hedwigia 37: 299. 1898.
= Hysterium apiculatum Starbäck, Bidrag Kungl. Svenska Vetensk.-Akad. Hist. 25(1): 19. 1899.
= Hysterium batucense Speg., Revista Fac. Agron. Univ. Nac. La Plata 6(1): 116. 1910.
= Hysterium andicola Speg., Anal. Mus. Nac. Hist. Nat. B. Aires 23: 85. 1912.
= Hysterium atlantis Maire, Mém. Soc. Sci. Nat. Maroc. 45: 35. 1937.
= Hysterium lavandulae Urries, Ann. Jard. Bot. Madrid 1: 64. 1941.
Hysterothecia isolated to gregarious, variably erumpent to sessile, 0.5–2.5 mm long, 0.2–0.5 mm high, lying parallel, but not confluent laterally, generally in line with the grain of the wood, and striated laterally with age. Pseudoparaphyses hyaline, cellular, 1–2.0 μm wide, walls thickened at apices, forming an epithecium, borne in mucilage, above the ascal layer, often encrusted with dark, pigmented crystals. Asci bitunicate, cylindrical, 8-spored, irregularly biseriate, 130–150 x 15–24 μm, short stipitate, and with a prominent apical nasse, especially when young. Ascospores phragmospores transversely (4–)6–8(–11)-septate, constricted at the median septum, inequilateral, slightly curved, at first hyaline-yellow, then brown at maturity, with a prominent swollen supra-median cell. If 5-septate, then swollen cell located at the second position; if 6-septate, then often the third from the top, measuring (20–)23–28(–38) x (5–)7–10(–13) μm. Principally North- and South-America, and Europe (Italy), from bark and old wood of Pinus, Larix, Castanea, Quercus, Eucalyptus, Fraxinus, Aspidosperma, and Lavandula (Zogg 1962). Also reported from South Africa (van der Linde, 1992). Anamorph: Septonema spilomeum.
Oedohysterium sinense (Teng) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515423. Fig. 3E–H. Basionym: Hysterium sinense Teng, Sinensia 4: 134. 1933.
= Hysterium macrosporum Teng, Sinensia 4: 134. 1933, non Peck, Rep. (Annual) New York State Mus. Nat. Hist. 26: 83. 1874 (1873).
Hysterothecia scattered to subgregarious, linear, sometimes parallel but non-confluent laterally, more often lying at irregular angles, depending on the grain of the substrate, striated in age, usually of a similar size (2–3.5 mm in length), that is, maturing synchronously in a given colony. Pseudoparaphyses hyaline to pale-yellow, cellular, 2–2.5 μm wide, apically branched, walls of even thickness along length, forming a darkened gelatinous epithecium above the ascal layer, +/- encrusted with pigmented crystals. Asci bitunicate, cylindrical, 8-spored, irregularly biseriate, 140–170 x 26–30 μm, short-stipitate, ascospores biseriate to subseriate in ascus, with a prominent apical nasse, especially when young, but sometimes persisting through maturity. Ascospores large, fusiform, asymmetric, curved phragmospores, at first hyaline, then pale-yellow to -brown, finally deep brown at maturity, with (3–)5–9(–11) septa, with a medial septal constriction, measuring (34–)38–50 x 11–15 μm, and, like Od. insidens, with a prominent swollen or tumid supra-median cell, usually located just above the median septum. From North America (Boehm, unpubl. data), Europe (Zogg 1962), China (Teng 1933), and South Africa (van der Linde 1992), on decorticated hardwood trees and structures (e.g., aged fence posts).
Notes: Species of Oedohysterium belonging to Clade D are characterised by elongate asymmetric spores with more than 3 septa, typically showing a swollen or tumid supra-median cell. In this study, two single-ascospore isolates of Od. sinense, one from South Africa (CBS 123345 / BPI 878730), and one from the United States, New Jersey (EB 0339 / BPI 879800), cluster with two isolates of Od. insidens, both from the United States, Massachusetts (CBS 238.34) and Tennessee (ANM 1443 / BPI 879799). Both species have remarkably similar phragmospores (e.g., Fig. 3D versus Fig. 3H). As these two taxa belong to Clade D and are far removed from the type species, H. pulicare, in Clade C, we propose that they be accommodated in the new genus Oedohysterium. An additional new combination is proposed below.
Oedohysterium pulchrum (Checa, Shoemaker & Umaña) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515424.
Basionym: Hysterographium pulchrum Checa, Shoemaker & Umaña, Mycologia 99: 289. 2007.
Notes: The newly described Hg. pulchrum from Costa Rica (Checa et al. 2007) also falls within Clade D (Fig. 1) and is here transferred to Oedohysterium, as Od. pulchrum (DQ 402184 / DAOM 234345). This is because molecular data indicate a close association with the two species of Oedohysterium, Od. insidens and Od. sinense. At first surprising, on further consideration, this sub-clade forms a natural assemblage premised on morphological features. The spores of all three taxa show a remarkable degree of similarity in morphology, which includes being similarly pigmented, slightly curved and fusiform, with a common number of transverse septa. The sole difference is the presence of one or two vertical septa in Od. pulchrum, a feature noted by the authors to be absent in some spores (Checa et al. 2007). Most importantly, like Od. insidens and Od. sinense, Od. pulchrum also possesses a swollen supra-median cell. Interestingly, a striking resemblance to the phragmospores of Od. insidens can be seen for those spores of Od. pulchrum that do not possess vertical septa (Checa et al. 2007). This is based on similarities in shape (e.g., curved and fusiform), size [(20–)23–28(–38) x (5–)7–10(–13) μm versus 22–25(–27) x 5–6 μm], and in the number of transverse septa (4–)6–8(–11) versus (5–)6, for Od. insidens and Od. pulchrum, respectively. As molecular data indicate that the presence or absence of vertical septa should be considered a sympleisiomorphic character state within the Hysteriaceae (Boehm et al. 2009), we feel justified in including both phragmospores and dictyospores within the genus Oedohysterium.
Key to the species of Hysterium and Oedohysterium
1. Phragmospores mainly 3-septate............................................................................................................................................................. 2 1. Phragmospores concolorous, more than 3-septate, in one instance pigmented dictyospores with 1-2 vertical septa (Od. pulchrum).......................................................................................................................................................................................... 7
2. Phragmospores either versicolorous or delayed concolorous.................................................................................................................. 3 2. Phragmospores truly concolorous (sepia to dark brown in colour)........................................................................................................... 4
3. Terminal cell mainly remaining hyaline with inner spore cells pigmented brown (versicolorous); ascospores 20–40 x 6–12 μm; cosmopolitan........................................................................................................................... H. pulicare 3. Phragmospores tardily pigmented, often remaining hyaline for quite some time after discharge, but eventually becoming uniformly concolorous; 20–26(–28) x 6–8.5 μm; North America.................................................... H. hyalinum Note: Currently recognised as Pleosporomycetidae sp. incertae sedis (Boehm et al. 2009).
4. Phragmospores 3-septate, 28 μm or less in length.................................................................................................................................. 5 4. Phragmospores 3-septate, longer than 28 μm.......................................................................................................................................... 6
5. Phragmospores (12–)14–21(–28) x (3–)4–8(–10) μm, firmly 3-septate, no septal constrictions; end-cells obtuse; cosmopolitan...................................................................................................................................................................... H. angustatum 5. Phragmospores (14–)15–18(–20) x 5–7 μm; 3- (rarely 2- or 4)-septate; prominently constricted at first-formed septum; basal cell extended; red hamathecial pigment; neotropical.......................................................................................... H. asymmetricum
6. Phragmospores fusoid, slightly curved, guttulate; (20–)25–40 x (4–)5–14 μm; West and East Africa............................... H. vermiforme 6. Phragmospores fusoid, curved, highly guttulate; 40–57 x 11–15 μm; on Pinus, North America and China......... H. macrosporum Peck
7. Phragmospores or dictyospores (4-) 6- to 8- (11-) celled, fusiform in outline, with +/- swollen supra-median cell(s)............................... 8 7. Phragmospores with more than 11 septa, fusiform, pale brown, (13–)14–15(–21)-septate, (35–)45–50(–60) x (10–)12–13(–14) μm; Africa...................................................................................................................... H. velloziae
8. Swollen supra-median cell(s) present, either phragmospores or dictyospores (Oedohysterium)............................................................. 9 8. Phragmospores only, no swollen supra-median cells(s) present............................................................................................................ 11
9. Dictyospores lightly pigmented, 22–25(–27) x 5–6 μm, with (5–)6 transverse and 1 vertical septum in either cell or both cells adjacent to the primary septum, absent in some spores, with a swollen supra-median cell; typically with red pigment in the hamathecium; neotropical (Costa Rica)........................................................................... Od. pulchrum 9. With no red pigment present................................................................................................................................................................... 10
10. Phragmospores with (4–)6–8(–11) septa, slightly curved, fusiform, at first hyaline-yellow then reddish brown at maturity, if 5-septate, showing a swollen cell at the second position, if 6-septate, often the third from the top, +/- median septal constriction, (20–)23–28(–38) x (5–)7–10(–13) μm; cosmopolitan........................................................... Od. insidens 10. Phragmospores larger, fusiform, straight to curved, at first hyaline, then yellow or pale brown, finally deep brown; swollen supra-median cell(s) present, (3–)5–9(–11) septa, with median septal constriction; (34–)38–50 x 11–15 μm; cosmopolitan.................................................................................................................................. Od. sinense
11. Phragmospores fusiform, narrow, straight to very slightly curved, pale hyaline at first, then pale-yellow at maturity, with highly refractive guttules, in every cell, with (7–)9(–11) septa, no supra-median swollen cell(s), (35–)40–45(–55) x (7–)9–10(–12) μm; North America.......................................................................................................... H. barrianum 11. Phragmospores oblong, wide, slightly curved, bulging on one side, nearly hyaline and 1-septate at first, becoming clear brown and 7-septate, septa highly asymmetric, (2–)3 of the septa close to each end, the two central cells much larger; 48–67 x 15–20 μm; China and North America......................................................... H. magnisporum
We choose to provide the following dichotomous key whereby all hysteriaceous fungi, bearing transversely septate pigmented phragmospores (including Od. pulchrum with dictyospores) are identified together, with the caveat that unrelated taxa appear in the same key.
Gloniella Sacc., Syll. Fung. 2: 765 (1883).
The genus Gloniella was established by Saccardo (1883) to accommodate hysteriaceous fungi that possess hyaline phragmospores, from 3- to 9-septate. Zogg (1962) recognised six species: three collected on ferns from Europe and the Mediterranean, namely Gl. adianti on Adiantum, and Gl. graphidioidea and Gl. normandina, both on Pteridium. Zogg also accepted Gl. sardoa from Populus in Europe, Gl. typhae on Typha, the latter described from Europe (Zogg 1962) and Chile (Lorenzo & Messuti 1998), and Gl. bambusae on Bambusa from Brazil. Since then, an additional three species have been described: Gl. gracilis from Costa Rica (Checa et al. 2007), Gl. corticola from India (Pande & Rao 1991), and Gl. clavatispora from South Africa (Steinke & Hyde 1997). More recently, Barr (2009) recognised Gl. abietina on Abies from Idaho, and Gl. lapponica on Arctostaphylos from Washington. A number of species in the key may be conspecific, since reported spore measurements are identical or nearly so.
Key to the species of Gloniella
1. Ascospores 3-septate, shorter than 15 μm............................................................................................................................................... 2 1. Ascospores 3- or more-septate, and longer.............................................................................................................................................. 3
2. Ascospores 10–15 x 5–6 μm; India........................................................................................................................................ Gl. corticola 2. Ascospores 12–14 x 4–5 μm; on Typha, Europe...................................................................................................................... Gl. typhae
3. On ferns in Europe.................................................................................................................................................................................... 4 3. Not on ferns.............................................................................................................................................................................................. 6
4. Ascospores (2–)3(–4)-septate, (11–)15–20(–23) x 3–5 μm; on Adiantum, Europe.................................................................. Gl. adianti 4. Ascospores (3–)5(–7)-septate, slightly longer.......................................................................................................................................... 5
5. Ascospores (3–)5-septate, (15–)18–20(–22) x 4–5 μm; on Pteridium, Europe............................................................. Gl. graphidoidea 5. Ascospores 5–7-septate, (22–)25–27(–30) x 3–4 μm; on Pteridium, Europe.................................................................. Gl. normandina
6. Ascospores 1–3-septate, 36–39 x 10 μm; on Arctostaphylos, Western North America....................................................... Gl. lapponica 6. Ascospores with more septa..................................................................................................................................................................... 7
7. Ascospores 3(–5) septate, 20–27 um x 7–8 μm; on Abies grandis, Western North America................................................. Gl. abietina 7. Ascospores with more septa..................................................................................................................................................................... 8
8. Ascospores (6–)7(–8)-septate, (16–)18–21(–26) x 6–7(–8) μm; on Populus, Europe.............................................................. Gl. sardoa 8. Ascospores larger..................................................................................................................................................................................... 9
9. Ascospores (5–)6(–8)-septate, (18–)37(–41) x 10–11.5 μm, hyaline, smooth; on Avicennia marina, South Africa........ Gl. clavatispora 9. Ascospores smaller, neotropical............................................................................................................................................................. 10
10. Ascospores 6–7-septate, 32–37(–40) x 4–6 μm; Costa Rica.................................................................................................. Gl. gracilis 10. Ascospores (5–)6–7-septate, (28–)32–38(–44) x (3–)4–8(–9) μm; on Bambusa, Brazil.................................................... Gl. bambusae
Hysterographium Corda, Icon. Fung. 5: 34. 1842.
= Hysteriopsis Speg., Revista Fac. Agron. Univ. Nac. La Plata 2: 308. 1907.
= Polhysterium Speg., Anales Mus. Nac. Buenos Aires 23: 87. 1912.
= Fragosoa Cif., in Ciferri & Fragoso, Bol. Real Soc. Esp. Hist. Nat., Secc. Biol. 26(3-4): 194. 1926.
Although the genus Hysterographium has been removed from the Hysteriaceae (Boehm et al. 2009), and is currently recognised as Pleosporomycetidae gen. incertae sedis, it is included here. This is because it forms the basis for a number of new combinations within the family. The genus is characterised by pigmented dictyospores, with one to several longitudinal septa, ovoid to ellipsoid-fusoid, relatively broad, usually constricted at the first-formed septum. Zogg (1962) extensively revised the synonymy of the genus and accepted four species: Hysterographium flexuosum (Fig. 4A–B) and Hg. fraxini (Fig. 4C–D), the type, both with large dictyospores, prominently constricted at the median septum, the former with slightly longer, narrower spores. Zogg (1962) also accepted Hg. mori and Hg. subrugosum, with smaller, fewer-celled dictyospores, short and squat in the former, longer and more slender in the latter, both also constricted at the median septum.
Fig. 4.
The genus Hysterographium. A–B. Hysterographium flexuosum (EB 0098, U.S.A.; not incl.); C–D. Hysterographium fraxini (EB 0100, U.S.A.; not incl.). Scale bar (habitat) = 1 mm; Scale bar (spores) = 20 μm.
Since then, an additional three species have been described: Hysterographium minus from Japan (Amano 1983), Hg. spinicola from South Africa, recollected from the thorns of Acacia and validated by van der Linde (1992), with a brick-red epithecium and spores only slightly longer than those of Hg. mori, and, lastly, Hg. pulchrum from Costa Rica, also with a red pigment in the hamathecium (Checa et al. 2007), here transferred to Oedohysterium, as Od. pulchrum.
Four of the seven species were surveyed in the present study, with multiple isolates (Table 1): Hysterographium mori (8), Hg. subrugosum (3), Hg. fraxini (2) and Od. pulchrum (1), falling into no fewer than three separate clades, two within the Hysteriaceae (Clades A and D) and one far removed from the family (Fig. 1). The latter clade includes the type species for the genus Hysterographium, namely Hg. fraxini, represented by isolates from Switzerland (CBS 109.43), deposited by Zogg in 1943, and from Canada (CBS 242.34), deposited by Lohman in 1934. Hysterographium fraxini forms a well-supported clade distant from the Hysteriaceae, but remains within the Pleosporomycetidae (Fig. 1). As this is substantiated by two geographically disparate isolates from two different continents, deposited by two reputable workers, it is significant. The implication is that the genus Hysterographium must follow the type species and be removed from the Hysteriaceae (Boehm et al. 2009). Species with pigmented dictyospores remaining within the Hysteriaceae, previously classified in Hysterographium, must therefore be accommodated in other genera. In this study, these would include the following species, for which we have sequence data: Hysterographium mori, Hg. subrugosum, and Hg. pulchrum (= Od. pulchrum). The remaining species for which we do not have sequence data, namely Hg. minus, Hg. spinicola and Hg. flexuosum, must remain as species of Hysterographium, until such time that sequence data are available. We therefore propose the following new genus.
Hysterobrevium E.W.A. Boehm & C.L. Schoch, gen. nov. MycoBank MB515329.
Etymology: Hystero- from Hysterographium, Latin brevis, short, referring to the spores of the type, Hb. mori.
Hysterothecia navicularia, fissura longitudinali prominente praedita, utrinque acuminata vel obtusa, linearia vel flexuosa, solitaria vel gregaria, vulgo per longitudinem striata, nonnumquam erecta, quasi stipitata, superficialia vel partim in substrato immersa. Asci bitunicati, cyindrici vel clavati. Dictyosporae pigmentatae vel hyalinae, plerumque breviores quam 25 μm, ad septum medium constrictae; ascosporae hyalinae vel luteae iuvenes vulgo strato mucido circumdatae; pigmentatae pallide brunneae, pariete levi; ascosporae ovoideae vel obovoideae, apice obtuso vel acuminato, 3–4(–6) septis transversalibus et 1–2 longitudinalibus divisae.
Hysterothecia navicular, with a prominent longitudinal slit, variable with acuminate to obtuse ends, linear to flexuous, solitary to densely gregarious, surface usually longitudinally striate, sometimes erect, superficial, almost stipitate, to erumpent and partially embedded in substrate, the latter especially when gregarious. Asci bitunicate, cylindrical to clavate. Ascospores pigmented or hyaline dictyospores, usually less than 25 μm long, constricted at least at the median septum. If hyaline to pale-yellow, then typically associated with a gelatinous sheath when young, dissipating with age. If pigmented then lightly so, transparent clear brown, walls smooth; ascospores generally ovoid to obovoid, with either obtuse or acuminate ends, 3–4(–6) transverse septa, and 1–2 longitudinal septa, these mostly associated with the two central cells, but highly variable and sometimes at oblique angles in the end cells.
Type species: Hysterobrevium mori (Schwein.) E.W.A. Boehm & C.L. Schoch, comb. nov.
Hysterobrevium mori (Schwein.) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515335. Fig. 5J–R. Basionym: Hysterium mori Schwein., Trans. Amer. Philos. Soc. 4(2): 244. 1832.
Fig. 5.
The genus Hysterobrevium (Clade A). A–E. Hysterobrevium constrictum [SMH 5211.1 (F), New Zealand]; F–I. Hysterobrevium smilacis [GKM 426N (EA), Kenya]; L–N. Hysterobrevium mori [SMH 5273 (BPI 879787), U.S.A.]; O–R. Hysterobrevium mori [ANM 43 (ILLS), U.S.A.; not incl]. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 10 μm.
≡ Hysterographium mori (Schwein.) Rehm, Ascomyceten No. 363. 1876.
= Hysterium grammodes De Not., Giorn. Bot. Ital. 2 (7–8): 55. 1847.
≡ Hysterographium grammodes (De Not.) Sacc., Syll. Fung. 2: 782. 1883.
= Hysterium rousselii De Not., Piren. Ister. 2(7–8): 19. 1847.
≡ Hysterographium rousselii (De Not.) Sacc., Syll. Fung. 2: 779. 1883.
= Hysterium vulgare De Not., Piren. Ister. 2(7–8): 18. 1847.
= Hysterium australe Duby, Mém. Soc. Phys. Genève 16(1): 44. 1862.
= Hysterium lesquereuxii Duby, Mém. Soc. Phys. Genève 16(1): 41. 1862.
≡ Hysterographium lesquereuxii (Duby) Sacc., Syll. Fung. 2: 779. 1883.
= Hysterium gerardi Cooke & Peck, Bull. Buffalo Soc. Nat. Sci. 3: 33. 1875.
≡ Hysterographium gerardi (Cooke & Peck) Sacc., Syll. Fung. 2: 783. 1883.
= Hysterium viticolum Cooke & Peck, Bull. Buffalo Soc. Nat. Sci. 3: 33. 1875.
≡ Hysterographium viticola (Cooke & Peck) Rehm, Ascomyc. No. 316, in Sacc., Syll. Fung. 2: 782. 1883.
= Hysterium variabile Cooke & Peck, Bull. Buffalo Soc. Nat. Sci. 3: 33. 1875.
≡ Hysterographium variabile (Cooke & Peck) Sacc., Syll. Fung. 2: 780. 1883.
= Hysterium formosum Cooke, in Harkness & Cooke, Grevillea 7: 3. 1878.
≡ Hysterographium formosum (Cooke) Sacc., Syll. Fung. 2: 783. 1883.
= Hysterium putaminum Cooke, Grevillea 7: 48. 1878.
≡ Hysterographium putaminum (Cooke) Sacc., Syll. Fung. 2: 783. 1883.
= Hysterographium portenum Speg., Anales Soc. Ci. Argent., Secc. Santa Fe. 9(4): 185. 1880.
= Hysterographium grammodes var. minus Sacc., Syll. Fung. 2: 783. 1883.
= Hysterographium pumilionis Rehm, Discom. 1(3): 21. 1887.
= Hysterographium guaraniticum Speg., Anales Soc. Ci. Argent., Secc. Santa Fe. 26(1): 56. 1888.
= Hysterographium punctiforme Pat., Bull. Soc. Mycol. France 4: 120. 1888.
= Hysterographium ruborum Cooke, in Rehm, Ascom., No. 918. 1888.
= Hysterium insulare P. Karst. & Har., Rev. Mycol. Toulouse No. 47: 1890.
= Hysterographium incisum Ellis & Everh., Bull. Torrey Bot. Club 24: 462. 1897.
= Hysterographium ziziphi Pat., Cat. Rais. Pl. Cell. Tunisie: 112. 1897 (as “zizyphi”).
= Hysterographium rousselii (De Not.) Sacc. var. piri Feltgen, Vorst. Pilz. Luxemb. Nachtr. 3: 111. 1903.
Hysterothecia erumpent-superficial, ellipsoidal, oblong, linear or cylindrical, 1–2(–3.5) mm long, 220–275(–440) μm wide, by 190–330 μm high, mostly straight and lying parallel, but not confluent laterally, often gregarious and crowded so as to cover the substrate, longitudinally striate in age, navicular with tapering ends. Two types of hysterothecial aggregations regularly observed, depending on substrate: (1) Colonies on weathered, whitened decorticated hardwood often forming large oval colonies, with acuminate ends, measuring 5–15 cm in length, with hysterothecia gregarious in the center, densely packed in longitudinal formations, showing multiple stages of development, and darkening the adjacent substrate; when young, prior to emergence of hysterothecia, smaller colonies are seen, but still presenting darkened oval patches, often with coelomycetous anamorph present. (2) Colonies on bark (i.e., corticolous) less gregarious, not darkening the substrate, hysterothecia often situated at angles, rather than in parallel orientation. Peridium 30–60 μm thick medially, to 100+ μm at the base, distinctly three-layered in cross-section, the outer layer darkly pigmented, the middle less so, and the inner layer, thin-walled, pallid and compressed. Pseudoparaphyses cellular, septate, persistent, 1–2 μm wide, hyaline, thickened apically, branched and forming an epithecium in a gelatinous matrix above the ascal layer. Asci cylindrical to clavate, bitunicate, short-stipitate, (50–)80–110 x 10–18 μm. Ascospores pigmented, thin-walled dictyospores, obovoid, ends obtuse, 3–(5–7)-septate, with 1–2(–3) vertical septa usually associated with mid-cells, but on occasion also present obliquely in end cells, constricted at the median septum, sometimes, when fully hydrated, at additional, more distal septa, measuring (12–)14–22(–26) x (5–)7–10(–11) μm. Anamorph coelomycetous, Aposphaeria-like in nature, in culture conidiomata as irregular locules, with conidiogenous cells 8–10 x 1.5–2 μm; conidia (2–)2.5–3.5(–4) x 1–2 μm (Lohman 1932). Cosmopolitan, on aged, usually decorticated, weathered wood or bark of Pinus, Juniperus, Salix, Ostrya, Castanea, Quercus, Ulmus, Morus, Pyrus, Amelanchier, Crataegus, Rubus, Cercocarpus, Prunus, Gleditsia, various Fabaceae, Melia, Pistacia, Cotinus, Rhus, Acer, Ziziphus, Vitis, Fraxinus, Olea, and Aspidosperma (Zogg 1962).
Hysterobrevium smilacis (Schwein.) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515336. Fig. 5F–I. Basionym: Hysterium smilacis Schwein., Schriften Naturf. Ges. Leipzig 1: 49. 1822.
≡ Gloniopsis smilacis (Schwein.) Underw. & Earle, Bull. Alabama Agric. Exp. Sta. 80: 196. 1897.
≡ Hysterographium smilacis (Schwein.) Ellis & Everh., N. Amer. Pyrenomyc. 709. 1892.
= Hysterium biforme Fr., Observ. Mycol. (Havniae) 2: 354. 1818.
≡ Gloniopsis biformis (Fr.) Sacc., Syll. Fung. 2: 773. 1883.
= Hysterium elongatum β curvatum Fr., Elench. Fung. (Greifswald) 2: 138. 1828.
= Hysterium curvatum Fr., Elench. Fung. 2: 139. 1828.
≡ Gloniopsis curvata (Fr.) Sacc., Syll. Fung. 2: 775. 1883.
= Hysterium rocheanum Duby, Mém. Soc. Phys. Genève 16: 51. 1862.
≡ Gloniopsis rocheana (Duby) Sacc., Syll. Fung. 2: 773. 1883.
= Hysterographium naviculare P. Karst., Symb. Mycol. Fenn. 6: 37. 1877.
= Hysterium gloniopsis W.R. Gerard in Peck, Rep. New York State Mus. 32: 49. 1877 (1879).
≡ Hysterographium gloniopsis (W.R. Gerard) Ellis & Everh., N. Amer. Pyrenomyc. 708. 1892.
≡ Gloniopsis gloniopsis (W.R. Gerard) House, Bull. New York State Mus. 219-220: 235. 1920.
= Gloniella scortechiniana Sacc. & Roum., Rev. Mycol. Toulouse 5: tab. 41, fig. 17. 1883.
= Gloniopsis gerardiana Sacc., Syll. Fung. 2: 774. 1883.
= Gloniopsis decipiens var. cisti Rehm, Hedwigia 25: 13. 1886.
= Gloniopsis cisti Rehm, Hedwigia 25: 13. 1896.
= Gloniopsis ambigua Sacc., Ann. Mycol. 10(3): 317. 1912.
= Gloniopsis ellisii Cash, Mycologia 31: 294. 1939.
Hysterothecia erumpent, many times surrounded at the base by ruptured epidermis or periderm, especially when borne in herbaceous stems, much less so on wood, then completely superficial, 0.5–1.5 mm long, 300–400 μm wide, 200–250 μm high, longitudinally striated. Peridium 25–50 μm wide, narrower at base within the substrate, widest at mid-point, carbonaceous and brittle when dry. Pseudoparaphyses cellular, septate, persistent, 1–1.5 μm wide, hyaline to pale yellow in mass, branched above, forming an epithecium, but not darkly pigmented, exposed surface yellow-brown. Asci cylindrical to clavate, bitunicate, short-stipitate, 70–120 x 15–25 μm at maturity. Ascospores asymmetric, hyaline to pale yellow dictyospores, with acuminate ends, and a gelatinous sheath that usually dissipates at maturity, measuring (13–)15–26(– 31) x (4–)5–9(–10) μm. Spore septation highly variable, usually 3–5(–9)-septate and with 1(–3) vertical septa, passing through multiple mid-cells, and usually prominently constricted at the median septum, when fresh and hydrated, sometimes constriced along multiple transverse septa. Anamorph coelomycetous, Aposphaeria-like. Cosmopolitan on Pinus, Chamaerops, Smilax, Populus, Salix, Juglans, Betula, Fagus, Quercus, Ficus, Pyrus, Crataegus, Rubus, Rosa, Prunus, Robinia, Butea, Pistacia, Cotinus, Acer, Cistus, Erica, and Lavandula (Zogg 1962).
Notes: Hysterobrevium mori, while falling within the Hysteriaceae, finds itself in two separate clades (Fig. 1). In Clade A, one set of North American Hb. mori isolates associates with six highly geographically diverse isolates of Hb. smilacis. The Hb. mori isolates originate from the United States, from New Jersey (CBS 123336, CBS 123564), New York (CBS 123335, CBS 123563), Indiana (SMH 5273) and Michigan (SMH 5286). The Hb. smilacis isolates originate from the United States, from Indiana (SMH 5280) and Michigan (CBS 200.34), as well as from South Africa (CMW 18053), Sweden (CBS 114601) and Kenya (GKM 426N). Dictyospores of both species are of similar shape, size and degree of septation: (12–)14–22(–26) x (5–)7–10(–11) μm, 3–(5–7)-septate, with 1–2(–3) vertical septa, for Hb. mori versus (13–) 15–26(–31) x (4–)5–9(–10) μm, 3–5(–9)-septate, with 1(–3) vertical septa, for Hb. smilacis. They differ in the absence of pigmentation and the presence of a gelatinous sheath in the latter. Thus, these two species, previously classified in two separate genera, Hysterographium and Gloniopsis, are in fact closely related, with each species far removed from the type species of their respective genera. Further support for this argument, can be found in Lohman (1933a), who found a similar Aposphaeria anamorph for both Hb. mori (as Hg. mori) and Hb. smilacis (as Gp. gerardiana) and stated that they were indistinguishable in culture. The implication is that both taxa should be united within the same genus, for which we propose Hysterobrevium.
In addition to the association with Hb. smilacis in Clade A, Hb. mori also finds itself in Clade D. As this is validated by two geographically diverse isolates, one from the United States, Michigan (CBS 245.34) and one from Kenya (GKM 1013 / BPI 879788), it is significant. Spore measurements of the Kenyan accession GKM 1013 (BPI 879788) in Clade D versus those of other Hb. mori accessions in Clade A, represented by SMH 5273 / BPI 879787, CBS 123335 / BPI 878734, and CBS 123336 / BPI 878733, failed to detect any significant morphological differences; nor were there any appreciable differences detected in their hysterothecia. The association of Hb. mori with unrelated taxa within the Hysteriaceae in Clade A and D may be significant in that Hb. mori has long been regarded as a highly variable taxon (Ellis & Everhart 1892, Lohman 1933a), resulting in the synonymy of no fewer than 30 names since its inception by Schweinitz in 1832 (Zogg 1962). Future studies may well reveal that Hb. mori contains a number of cryptic species, morphologically similar, but genetically unrelated. We propose an additional new combination below.
Hysterobrevium constrictum (N. Amano) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515337. Fig. 5A–E.
Basionym: Gloniopsis constricta N. Amano, Trans. Mycol. Soc. Japan 24: 289. 1983.
Notes: Amano (1983) described a small-spored species of Gloniopsis from Japan, Gp. constricta, noting a prominent median septal constriction. The measurements of the dictyospores were given as 10.4–13.2 x 4.4–5.8 μm, usually with 3–4 transverse and one vertical septum that passes through one to three cells. Although not mentioned (Amano 1983), the illustrations depict a very thick wall and dictyospores highly symmetric in outline and septation. Amano (1983) stated of the spores “...hyaline, later becoming brown...”, but did not mention the presence of a gelatinous sheath. He also noted that the closest resemblance is with Hb. smilacis (as Gp. curvata), the latter however with slightly larger spores. In this study, we were fortunate to obtain a specimen from New Zealand (SMH 5211.1, deposited in F; Fig. 5A–E) that corresponds to the published description given by Amano (1983), but differs on several counts. Like Gp. constricta, the hyaline dictyospores in SMH 5211.1, are highly symmetric and thick-walled, (1–)3(–4)-septate, with 1(–2) vertical septa, but the constriction at the median septum in SMH 5211.1, while present, is not prominent. Also unlike Gp. constricta, the spores in SMH 5211.1 have an obvious gelatinous sheath when young, but this quickly dissipates with age, and may be completely absent in mature specimens. In SMH 5211.1, the spores measure (18–)20(–23) x 10–12 μm, which is considerably larger than those of Gp. constricta. Nevertheless, these differences, in our opinion, are not sufficient to warrant a new species, and we choose here to simply expand the spore measurements to (11–)13–20(–23) x 5–12 μm, rather than describe a new species, proposing instead the new combination Hb. constrictum.
Gloniopsis De Not., Giorn. Bot. Ital. 2(2): 23. 1847.
A review of the nomenclatural history of the genus Gloniopsis was given in Boehm et al. (2009). The genus is characterised by hyaline to yellow dictyospores, often inequilateral, curved, in outline obovoid, ends obtuse to sub- to acuminate, multi-septate, with one or more longitudinal septa, constricted at the first-formed septum, sometimes constricted at additional septa, and usually surrounded by a gelatinous sheath, which may dissipate with age. Zogg (1962) synonymised a number of names under the type species, Gp. praelonga (Fig. 6A–B), and accepted only one additional species, namely Gp. curvata with smaller ascospores. Barr (1990a) proposed to include this latter species under the earlier name Gp. smilacis, following Cash (1939). In this study, we have transferred Gp. smilacis to Hysterobrevium, closely related to Hb. mori in Clade A. Recently, Gp. argentinensis, previously considered by Zogg (1962) as a doubtful species, was reinstated by Lorenzo & Messuti (1998). The authors state that the ascospores are 7-septate, with 1–3(–4) longitudinal septa, some passing through multiple cells, in outline widely ellipsoid, measuring 20–26 x 9–12 μm. The septation and spore measurements are nearly identical to those of Gp. praelonga, the latter 5–7(–10)-septate, with 2–3 longitudinal septa, (16–)20–32(–34) x (6–)9–12(–15) μm. We therefore synonymise Gp. argentinensis under Gp. praelonga. Lastly, Amano (1983) described an additional two species of Gloniopsis from Japan, namely Gp. macrospora and Gp. constricta, the latter transferred here to Hysterobrevium (Clade A).
Fig. 6.
The genus Gloniopsis (Clade D). A–B. Gloniopsis praelonga [CBS 123337 (BPI 878725), U.S.A.]; C–F. Gloniopsis subrugosa [GKM 1214 (BPI 879776), Kenya]; G. Gloniopsis subrugosa (CBS 123346, BPI 878735; South Africa). Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 20 μm.
Molecular data indicate that the genus Gloniopsis is polyphyletic, with the type, Gp. praelonga, belonging to Clade D (Fig. 1). Closely associated with the type, are a number of species possessing pigmented dictyospores, which would previously have been classified in the genus Hysterographium (e.g., Hysterographium subrugosum). Based on molecular data presented here, we therefore propose to emend the genus Gloniopsis, to include both hyaline and pigmented dictyospores. The following new combination is proposed, as well as two new species from Africa.
Gloniopsis subrugosa (Cooke & Ellis) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515338. Fig. 6C–G. Basionym: Hysterium subrugosum Cooke & Ellis, Grevillea 5: 54. 1876.
≡ Hysterographium subrugosum (Cooke & Ellis) Sacc., Syll. Fung. 2: 780. 1883.
= Hysterographium hiascens Rehm, Ber. Naturhist. Vereins. Augsburg 26: 780. 1881.
= Hysterographium kansense Ellis & Everh., Erythea 2: 22. 1894.
= Hysterographium cylindrosporum Rehm, Bih. Kongl. Svenska Vetensk.-Akad. Handl. 25(6): 11. 1899.
= Hysterographium minutum M.L. Lohman, Pap. Michigan Acad. Sci. 17: 267. 1933.
Hysterothecia erumpent to superficial, scattered to densely crowded, navicular, straight to flexuous, with tapered ends, surface not striated in age, but smooth to sub-rugose in texture, 1–2 mm long, 250–350 μm diam. Peridium composed of small pseudoparenchymatous cells, heavily pigmented at the surface, not showing a distinct number of layers, relatively smooth on outer surface. Pseudoparaphyses narrowly cellular, septate, 1–1.5 μm in diam., hyaline, branched above the asci, borne in a gelatinous matrix. Asci cylindrical to clavate, bitunicate, short-stipitate, 80–150 x 18–25 μm, with a prominent apical nasse, especially when young. Ascospores pigmented thin-walled, dictyospores (22–)25–34(–45) x (6–)8–12(–17) μm, mostly with 7–11 transverse and 1–2 vertical septa, hardly constricted at septa, clear brown, ends paler at times, slightly asymmetric in outline. Anamorph coelomycetous, Aposphaeria-like (Lohman 1933a). Less frequently collected, but reported from North America (Barr 1990b), Europe (Zogg 1962), Argentina (Messuti & Lorenzo 2003) and from South Africa (van der Linde 1992) as well. Old wood and bark of Populus, Quercus, Celtis, Crataegus, Rosa, and Cotinus (Zogg 1962), as well as on weathered fence posts and old planks (Boehm, unpubl. data).
Notes: In the current study, we were able to include three geographically diverse isolates of Gp. praelonga (Table 1), two from South Africa (CBS 112415 and CMW 19983 / PREM 57539), and one from the United States, New Jersey (CBS 123337 / BPI 878725). These isolates cluster together in Clade D and associate with one isolate of Gp. subrugosa from South Africa (CBS 123346 / BPI 878735). Both Gp. praelonga and Gp. subrugosa are somewhat similar in the shape, size and septation of their dictyospores, hyaline in the former (Fig. 6B), pigmented in the latter (Fig. 6G). The spores of Gp. praelonga are (16–)20–32(–34) x (6–)9–12(–15) μm, and those of Gp. subrugosa are (22–)25–34(–45) x (6–)8–12(–17) μm. Septation is also similar in both species, with 5–7(–10) transverse and 2–3 vertical septa in Gp. praelonga and 7–11 transverse and 1–2 vertical septa in Gp. subrugosa. They differ in pigmentation and the presence of a gelatinous sheath in the type. Molecular data indicate that they are closely related.
An additional two isolates of Gp. subrugosa, from Kenya (GKM 1214 / BPI 879776) and Cuba (SMH 557 / BPI 879777), are more distantly related and do not fall in Clade D. Moreover, no morphological differences were noted between these two more distantly associated isolates of Gp. subrugosa and CBS 123346 (BPI 878735) from South Africa in Clade D. Although spore morphology dictates that all three specimens of Gp. subrugosa should be classified as the same species, molecular data point to genetic heterogeneity within the taxon. This is similar to the situation in Hb. mori, mentioned earlier, which, despite identical morphologies, finds affinities in both Clades A and D. Hysterobrevium mori and, to a lesser extent, Gp. subrugosa, may represent ancestral lineages that have maintained stable morphologies, while simultaneously incurring sufficient genetic change to, in the case of Hb. mori, fall into different clades within the family. Alternatively, these isolates may represent examples of convergent evolution among genetically unrelated lineages, which produce remarkably similar ascospores and hysterothecia. Also associating with Gp. praelonga and Gp. subrugosa in Clade D are two new species from East Africa, described below.
Gloniopsis arciformis E.W.A. Boehm, G.K. Mugambi, S.M. Huhndorf & C.L. Schoch, sp. nov. MycoBank MB515331. Fig. 7A–H.
Fig. 7.
The genus Gloniopsis (Clade D). A–H. Gloniopsis arciformis sp. nov. [GKM L166A (BPI 879774 = holotype), Kenya]; I–M. Gloniopsis kenyensis sp. nov. [GKM 1010 (BPI 879775 = holotype), Kenya]. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 10 μm.
Etymology: Latin arcus, a bow or arch, referring to the arcuate or arciform dictyospores.
Hysterothecia solitaria vel pauca aggregata, recta vel flexuosa, carbonacea, plerumque erecta, conspicue applanata et altiora quam lata, (0.5–)1–2.5 mm longa, 250–350 μm lata, 400–600 μm alta, per longitudinem striata, sulco inconspicuo maturitate clauso. Peridium 40–75 μm crassum in medio, basim versus crassius, sursum tenuius, bistratosum. Pseudoparaphyses cellulares 1–1.5 μm latae, ramosae, sursum magis crassitunicatae, epithecium pigmentatum ascos obtegens formantes. Asci cylindrici vel clavati, stipite sinuoso, bitunicati, 50–75 × 14–18 μm; ascosporae irregulariter biseriatae, dictyosporae, pigmentatae, tenuitunicatae, fragiles, facile dilabentes, conspicue arcuatae, 3–5(–7)-septatae, 1–2(–3) septis verticalibus divisae; cellulis centralibus multo maioribus quam distales, ad septa haud constrictae, (10–)12–18(–22) x 6–10 μm.
Hysterothecia solitary to sparsely aggregated, straight to flexuous, carbonaceous, mainly erect, distinctly flattened and taller than wide, (0.5–)1–2.5 mm long, 250–350 μm wide, by 400–600 μm high, longitudinally striated, with an inconspicuous sulcus remaining closed at maturity. Peridium 40–75 μm thick medially, thicker towards the base, thinner towards the sulcus, composed of two layers, the inner thin, compressed and hyaline, the outer denser, and darkly pigmented. Pseudoparaphyses cellular 1–1.5 μm wide, branched and thicker-walled distally towards the top, forming a pigmented epithecium above the asci. Asci cylindrical to clavate, with a sinuous stalk, bitunicate, 50–75 x 14–18 μm (n = 7), ascospores irregularly biseriate. Ascospores pigmented, thin-walled, dictyospores, fragile, easily breaking under the slightest pressure, pronouncedly arcuate or bent (arciform), and thus highly asymmetric, 3–5(–7)-septate, with 1–2(–3) vertical septa, these mostly associated with the mid cells, which are much larger and swollen than the end-cells, no septal constrictions, measuring (10–) 12–18(–22) x 6–10 μm (n = 17).
Specimen examined: Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park, 6 Nov. 2006, G.K. Mugambi. Deposited as BPI 879774, holotype [formerly, GKM L166A (EA)].
Notes: Gloniopsis arciformis is represented by a single specimen (BPI 879774) of only ∼30 fruitbodies in the protected crevice of a small piece of decorticated hardwood, collected in Arabuko-Sokoke National Park, Malindi District, Kenya. Although the material is sparse, it does permit the description of a new species on account of the highly unusual arcuate dictyospores. Gloniopsis arciformis resides in Clade D, and is phylogenetically closely associated with two other species of Gloniopsis (Gp. praelonga and Gp. subrugosa), as well as with an additional new species described below.
Gloniopsis kenyensis E.W.A. Boehm, G.K. Mugambi, S.M. Huhndorf & C.L. Schoch, sp. nov. MycoBank MB515359. Fig. 7I–M.
Etymology: From the Latin -ensis to denote origin, from Kenya.
Hysterothecia navicularia, carbonacea, recta vel flexuosa, utrinque obtusa, dense aggregata, erumpentia, ad latera inconspicue striata vel levia, (0.5–)1–3 mm longa, 250–350 μm lata, 250–350 μm alta. Peridium prope basim ad 100 μm crassum, bivel tristratosum, stratum internum compressum, hyalinum, strata exteriora densiora et fusca. Pseudoparaphyses cellulares, septatae, 1–1.5 μm latae, sursum ramosae et anastomosantes, epithecium pigmentatum ascos obtegens formantes. Asci cylindrici vel clavati, bitunicati, 60–80 x 12–16 μm, ascosporas irregulariter biseriatas continentes. Ascosporae dictyoseptatae, pigmentatae, obovoideae, tenuitunicatae, fragiles, polis asymmetricis: apice obtuso, ad basim acuminatae vel nonnumquam protrudentes, 3(–4)-septatae, 1–2 septis verticalibus, utrinque saepe septis obliquis divisae, ad septa vix constrictae, iuvenes guttulis repletae, (12–)15–18(–19) x 5–7(–8) μm.
Hysterothecia navicular, carbonaceous, straight to flexuous, with obtuse ends, densely aggregated, erumpent, slightly striated laterally to smooth, (0.5–)1–3 mm long, 250–350 μm wide, by 250–350 μm high. Peridium up to 100 μm thick at base, composed of two to three layers, the inner thin, compressed and hyaline, the outer two progressively denser, and darkly pigmented. Pseudoparaphyses cellular, septate, 1–1.5 μm wide, branched, anastomosed distally, forming a pigmented epithecium above the asci. Asci cylindrical to clavate, bitunicate, 60–80 x 12–16 μm (n = 5), ascospores irregularly biseriate. Ascospores pigmented dictyospores, in outline obovoid, thin-walled, very fragile, spore apices asymmetric, the upper obtuse, the lower acuminate and sometimes drawn out, 3(–4[rarely])-septate, with 1–2 vertical septa, often with oblique septa in end cell, hardly constricted at the septa, highly gutulate when young, (12–)15–18(–19) x 5–7(–8) μm (n = 14). Known from only one collection, from Kenya, East Africa.
Specimen examined: Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park, 6 Apr. 2005, G.K. Mugambi. Deposited as BPI 879775, holotype; GKM 1010 (EA), paratype.
Notes: Molecular data indicate that both Gp. kenyensis and Gp. arciformis are closely associated, adjacent to Gp. praelonga and Gp. subrugosa in Clade D. The spores of all four taxa, however, are different, and thus their association would not have been predicted based on traditional morphology. The spores of Gp. kenyensis do bear a close resemblance, however, to those of Hb. mori. Both have predominantly 3-septate, thin-walled, pigmented dictyospores, with 1–2 vertical septa, often with oblique septa in the end cell. They can be differentiated on spore size: (12–)14–22(–26) x (5–)7–10(–11) μm for Hb. mori, versus (12–)15–18(–19) x 5–7(–8) μm for Gp. kenyensis. The spores of Hb. mori are usually longer and wider, and also show prominent septal constrictions, especially when fresh and hydrated. Additionally, Gp. kenyensis is highly guttulate when young, where this is rarely observed in Hb. mori. Molecular data indicate that they are not related.
To summarise, molecular data have necessitated the break up of the genus Hysterographium, because the type, Hg. fraxini, no longer resides within the Hysteriaceae (Boehm et al. 2009). This break up has resulted in: (1) the new genus Hysterobrevium, which includes both species with hyaline dictyospores, previously classified as Gloniopsis (Hb. constrictum and Hb. smilacis), and species with pigmented dictyospores, previously classified as Hysterographium (Hb. mori) in Clade A; (2) the inclusion in Gloniopsis of both hyaline (Gp. praelonga) and pigmented (Gp. subrugosa, Gp. arciformis, Gp. kenyensis) dictyospores in Clade D; (3) the inclusion in Oedohysterium of pigmented dictyospored species previously classified in Hysterographium (Od. pulchrum), also in Clade D; and, lastly, (4) the removal of Hysterographium, with the type Hg. fraxini, from the Hysteriaceae, currently placed as Pleosporomycetidae gen. incertae sedis. As the taxonomy of Hysterographium, Hysterobrevium and Gloniopsis is currently in flux, we chose to provide the following dichotomous key, whereby all hysteriaceous fungi, bearing transversely and longitudinally septate dictyospores, whether pigmented or hyaline, are identified together, with the caveat that unrelated taxa share the same key.
Key to the species of Hysterographium, Hysterobrevium and Gloniopsis
1. Dictyospores, usually shorter than 25 μm................................................................................................................................................. 2 1. Dictyospores mostly longer than 25 μm.................................................................................................................................................... 6
2. Dictyospores pigmented, thin-walled, fragile, pronouncedly arcuate or bent, 3–5(–7)-septate, with 1–2(–3) vertical septa, which are mostly associated with the mid-cells, these much larger and swollen than the end-cells, no septal constrictions, (10–)12–18(–22) x 6–10 μm; Kenya............................................................................................ Gp. arciformis 2. Not with the above combination of characters.......................................................................................................................................... 3
3. Dictyospores hyaline at maturity............................................................................................................................................................... 4 3. Dictyospores pigmented at maturity.......................................................................................................................................................... 5
4. Dictyospores highly symmetric in outline and septation, with thickened walls, gelatinous sheath present when young, absent at maturity, (1–)3(–4)-septate, with 1(–2) vertical septa, that may pass through one to two cells; (11–)13–20(–23) x 5–12 μm; Japan, New Zealand......................................................................................................... Hb. constrictum 4. Dictyospores asymmetric, with acuminate ends, with a gelatinous sheath when young, mostly 3–5(–9)-septate and with 1(–3) vertical septa, passing through multiple mid-cells, prominently constricted at the median septum, sometimes constriced at multiple septa, (13–)15–26(–31) x (4–)5–9(–10) μm; cosmopolitan.............................................. Hb. smilacis
5. Dictyospores thin-walled, obovoid, with obtuse ends, 3–(5–7)-septate, with 1–2(–3) vertical septa, usually associated with mid-cells, but occasionally present obliquely in end-cells, constricted at the median septum, sometimes at additional septa, (12–)14–22(–26) x (5–)7–10(–11) μm; cosmopolitan................................................................. Hb. mori 5. Dictyospores thin-walled, very fragile, obovoid, 3[–4(rarely)]-septate, with 1–2 vertical septa, highly gutulate when young, spore apices asymmetric, the upper obtuse, the lower acuminate and sometimes drawn out, often with oblique septa in end cell(s), hardly constricted at the septa, measuring (12–)15–18(–19) x 5–7(–8) μm; Kenya....................................... Gp. kenyensis
6. Red pigment present in hamathecium and/or centrum; dictyospores pigmented..................................................................................... 7 6. No red pigment present, spores pigmented or hyaline............................................................................................................................. 8
7. Dictyospores, 22–25(–27) x 5–6 μm, with (5–)6 transverse and 1 vertical septum in either cell or both cells adjacent to the primary septum; typically with red pigment in the hamathecium; neotropical (Costa Rica)....................... Od. pulchrum Note: Od. pulchrum is accommodated in the genus Oedohysterium and is present in both keys. 7. Dictyospores 25–28 x 11–13 μm, with 5–6 transverse and mostly one longitudinal septum; hamathecium brick-red; on Acacia thorns, South Africa...................................................................................................... Hg. spinicola
8. Dictyospores hyaline or turning brown tardily........................................................................................................................................... 9 8. Dictyospores pigmented in the ascus..................................................................................................................................................... 10
9. Dictyospores hyaline turning yellow in age, obovoid, ends usually obtuse, 5–7(–10)-septate, with 2–3 longitudinal septa, constricted at the median and often other septa, gelatinous sheath when young, (16–)20–32(–34) x (6–)9–12(–15) μm; cosmopolitan......................................................................................................... Gp. praelonga 9. Ascospores irregularly biseriate, ellipsoid, hyaline but becoming brown tardily, with the upper half generally wider than the lower half, sometimes surrounded by a gelatinous sheath, with 7–13 transverse and 1–3 longitudinal septa, constricted at the median transverse septum; 25–49 x 8–17 μm; Japan................................................................................................................................................ Gp. macrospora
10. Dictyospores usually less than 38 μm long............................................................................................................................................ 11 10. Dictyospores 30–80 μm long.................................................................................................................................................................. 12
11. Dictyospores (22–)25–34(–45) x (6–)8–12(–17) μm, mostly with 7–11 transverse and 1–2 vertical septa; cosmopolitan..................................................................................................................................................................... Gp. subrugosa 11. Dictyospores 26–38 x 10–15 μm, with 6–13 transverse and 1–3 vertical septa, obovoid, ends obtuse; Japan....................... Hg. minus
12. Dictyospores (25–)30–45(–51) x (10–)12–15(–22) μm, with 7–9 transverse and 2–3 vertical septa, obovoid, ends obtuse; cosmopolitan......................................................................................................................................... Hg. fraxini Note: Hysterographium fraxini, the type species for the genus Hysterographium, lies outside of the Hysteriaceae, as Pleosporomycetidae incertae sedis (Boehm et al. 2009). 12. Ascospore outline ellipsoid, fusoid, ends slightly acuminate, (30–)40–65(–80) x (8–)10–18(–19) μm, with 7–15 transverse and 1–3 vertical septa; cosmopolitan.............................................................................................. Hg. flexuosum
Psiloglonium Höhn., Ann. Mycol. 16: 145. 1918.
A discussion of the genus Psiloglonium (von Höhnel 1918; Petrak 1923a, b) by necessity must begin with the genus Glonium. This is because Zogg (1962) synonymised a number of species under the genus Glonium that were originally classified in Psiloglonium by von Höhnel (1918) and Petrak (1923a, b). Both Psiloglonium and Glonium possess hyaline to yellow didymospores, somewhat constricted at the septum, with obtuse or acuminate ends, typically with cells unequal in size, borne in hysterothecia.
Von Höhnel (1918) was the first to view the genus Glonium as comprised of two distinct morphological types, and stressed the importance of subicula, using it to divide the genus, at first, into two subgenera, Glonium and Psiloglonium, and, further in the same article, into two separate genera, with or without subicula, respectively. Petrak (1923a) recognised that von Höhnel (1918) had established the genus Psiloglonium, both at sub-generic and generic rank, but it was Petrak (1923a) who explicitly designated the type species for Psiloglonium as P. lineare (Fig. 8I–M), retaining G. stellatum as the type species for the genus Glonium sensu von Höhnel (1918). Petrak (1923a, b) eventually placed a number of species in Psiloglonium, all subsequently transferred to Glonium by Zogg (1962). Müller & von Arx (1950) originally accepted the genus Psiloglonium, but later reduced it to a synonym of Glonium (von Arx & Müller 1975). Lohman (1933a, 1937) also did not support Psiloglonium, based on the observation that similar anamorphs were shared between species of the two subgenera. Barr (1987), was the only modern author to retain the genus Psiloglonium, as distinct from the subiculate Glonium.
Fig. 8.
The genus Psiloglonium (Clade B). A–D. Psiloglonium simulans [ANM 1557 (BPI 879803), U.S.A.]; E–H. Psiloglonium clavisporum [GKM 344A (BPI 879801), Kenya]; I–M. Psiloglonium lineare [ANM 117 (ILLS), U.S.A.; not incl.]; N–Q. Psiloglonium araucanum [ANM 42 (ILLS), U.S.A.; not incl.]. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 10 μm.
Although von Höhnel (1918) and Petrak (1923a, b) both stressed the importance of subicula as a major morphological distinction between Psiloglonium and Glonium, Zogg (1962) noted that some species previously classified as Psiloglonium by Petrak (1923a) do in fact possess subicula on occasion (e.g., P. lineare). Zogg (1962) further noted an additional two species that were occasionally associated with subicula, namely G. pusillum and G. graphicum, stating: “...ohne Subiculum oder auf ziemlich deutlichem Subiculum sitzend...” Hence, Zogg (1962) considered subicula not to be a synapomorphic character state, and transferred those species previously classified by Petrak (1923a, b) in Psiloglonium (e.g., P. lineare, P. microspermum, P. ruthenicum, and P. finkii) to the genus Glonium.
Although Zogg (1962) did not support Psiloglonium, he did in fact recognise three distinct morphological forms within his concept of Glonium, two of which (Types I and II) we incorporate in Psiloglonium, the third (Type III) forming the basis for the Gloniaceae (Boehm et al. 2009). Zogg (1962) arranged the species of Glonium based on (1) didymospore shape: spore apices obovoid to rounded (Type I) versus spores fusiform with acuminate apices (Type II and III); and (2) the degree of complexity surrounding the architecture of the hysterothecia, simple, linear, solitary to gregarious (Types I, II) versus complex bifurcating, laterally anastomosing to form flabelliform pseudostellate composites, sometimes associated with a thin stromal crust (Type III). Thus, the genus Glonium sensu Zogg (1962) was comprised of two groups of species, one with obovoid to rounded spores apices borne in regular hysterothecia (Type I) versus those with acuminate spore apices borne in complex bifurcating or modified hysterothecia (Type III). Species belonging to Type II possess fruitbodies of Type I, but spores of Type III; the assumption was that they constituted an intermediate, perhaps transitional, morphological group. This, then, de-emphasised the presence or absence of subicula per se, as stressed by von Höhnel (1918) and Petrak (1923a, b). Nevertheless, Zogg (1962) maintained all three types within the genus Glonium. Molecular data presented here (see below), indicate that Types I & II are closely related, with Type III forming a distant clade in the Gloniaceae (Boehm et al. 2009).
Type I: This type is characterised by hysterothecia that may be solitary to gregarious, erumpent to entirely superficial, navicular to linear to highly flexuous, even triradiate, sometimes arranged in parallel orientation and confluent linearly to some degree, but never dichotomously branched, or associated with a stromal crust, as found in the Gloniaceae (Type III). These species correspond to Psiloglonium sensu von Höhnel (1918). Here, the didymospores are relatively small, hyaline, and have at least one, if not both ends, obovoid to obtuse (Type I), rather than acuminate (Types II and III). Zogg (1962) recognised five species, listed here by increasing ascospore length: Glonium abbreviatum, G. pusillum, G. lineare, G. chambianum, and G. curtisii. Barr (1975) transferred the last species to Ostreichnion, as O. curtisii in the Mytilinidiaceae, since transferred to the Hysteriaceae (Boehm et al. 2009). A sixth species, G. finkii, was included by Zogg (1962), based on ascospore shape, but placed apart in the key due to the unusual arrangement of the ascospores within the upper part of the ascus (Lohman 1937).
Psiloglonium lineare was previously reinstated within the Hysteriaceae, listing G. lineare as a synonym (Boehm et al. 2009). Here we also reinstate Psiloglonium finkii. An additional two species are included in Type I, namely G. simulans and G. clavisporum, synonymised by Zogg (1962) under G. lineare, but earlier recognised by Lohman (1932a, 1937) to be distinct from G. lineare. Boehm et al. (2009) proposed new combinations for these taxa, based on morphological as well as molecular data, as P. simulans (Fig. 8A–D) and P. clavisporum (Fig. 8E–H). To these species can also be added G. sasicola from Japan, the first report of a gelatinous sheath in the genus (Amano 1983). In this same publication Amano (1983) proposed an additional new species, G. macrosporum, also from Japan. The spore measurements were given as 13.1–16.8 x 4–5.6 μm, nearly identical to those of P. simulans at (10–)14–16(–18) x (4.5–)5–6 μm (Lohman 1937). Moreover, the illustrations given by Amano (1983) match closely those given by Lohman (1932a) for P. simulans. We therefore synonymise G. macrosporum under P. simulans.
More recently, Lorenzo & Messuti (1998), in a reappraisal of the type specimens collected by Spegazzini and Hennings from Argentina and Chile, have reinstated Glonium costesii. In a later publication, Messuti & Lorenzo (2007) synonymised G. costesii under the earlier epithet G. ephedrae. With spore measurements of 26–35 x 8–15 μm, G. ephedrae possesses the largest spores in Type I. In the same publication, Messuti & Lorenzo (2007) also accepted two additional species, G. chilense and G. uspallatense, previously considered by Zogg (1962) to be doubtful species. The spores of G. chilense measure 15–16 x (5–)7–8 μm, which places it very close to P. lineare, the latter with slightly smaller spores, (10–) 12–14(–18) x (4–)5–7(–8) μm (Zogg 1962). However, G. chilense has almost identical ascomatal and spore measurements as P. simulans, given above. We therefore synonymise G. chilense with the earlier name G. simulans, as P. simulans. For G. uspallatense, Messuti & Lorenzo (2007) gave spore measurements of 18–24 x 10–12 μm, intermediate between G. chambianum, (14–)16–18(–21) x (6–)8–9(–10) μm (Zogg 1962), and G. sasicola, 25–32 x 5–8 μm (Amano 1983).
Recently, Mugambi & Huhndorf (2009) proposed a new genus, Anteaglonium, outside of the Hysteriales but within the Pleosporales, to accommodate A. abbreviatum (Fig. 9A–E), A. globosum (Fig. 9F–I), A. parvulum (Fig. 9J–M), and A. latirostrum (Fig. 9N–R). The first three species are characterised by hyaline didymospores that belong to Type I, as defined by Zogg (1962), and are less than 8 μm in length. The fourth species, A. latirostrum, belongs to Type II (see below), with longer spores. Although phylogenetically unrelated to Psiloglonium, these species share a similar morphology and thus are included in the key below.
Fig. 9.
The genus Anteaglonium (Pleosporales). A–E. Anteaglonium abbreviatum [ANM 37 (ILLS), U.S.A.; not incl.]; F–I. Anteaglonium globosum [ANM 925.2 (ILLS), U.S.A.]; J–M. Anteaglonium parvulum [GKM 219N (EA), Kenya; not incl.]; N–R. Anteaglonium latirostrum [GKM L100N.2 (EA), Kenya]. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 5 μm.
Type II: This type is characterised by relatively large didymospores, distinctly fusoid in outline, prominently constricted at the septum, and with acuminate apices. Zogg (1962) recognised two species, namely Glonium caucasicum and the much larger-spored, neotropical G. hysterinum, to which can be added the newly described G. colihuae, on Chusquea culeou from Argentina (Lorenzo & Messuti 1998). Glonium caucasicum has recently been synonymised under the earlier name G. araucanum by Messuti & Lorenzo (2007), based on a comparison of the type specimen of G. caucasicum to Spegazini's earlier type of G. araucanum from Chile.
Type III: This type corresponds to von Höhnel's (1918) and Petrak's (1923a, b) circumscription of the genus Glonium, and includes species with fusiform spores, with acuminate apices, typically producing complex laterally anastomosing hysterothecia, forming stellate composites, usually with prominent subicula, with or without stroma. Zogg (1962) included the type, G. stellatum (Fig. 12A–E), G. compactum, and G. graphicum, the later sometimes variably associated with subicula. Zogg (1962) also stated that G. compactum possesses a subiculum, much like G. stellatum, and with similar spore size, but whereas hysterothecia in G. stellatum are merely seated on the subiculum, in G. compactum the hysterothecia are embedded in and arise from a thin stromal crust, which is itself seated on subicula. Recently, a fourth species was added, based on molecular evidence (Boehm et al. 2009), namely G. circumserpens (Fig. 12F–H), from Tasmania (Kantvilas & Coppins 1997).
Fig. 12.
The Gloniaceae. A–E. Glonium stellatum [ANM 41 (ILLS), U.S.A.; not incl.], arrows in 12B, subiculum. F–H. Glonium circumserpens [CBS 123343 (BPI 878739), Tasmania]. Scale bar (habitat) = 1 mm; Scale bar (spores and asci) = 10 μm.
Sequence data presented here (Fig. 1) and elsewhere (Boehm et al. 2009, Mugambi & Huhndorf 2009), clearly indicate that the genus Glonium sensu Zogg (1962) actually comprises three entirely unrelated lineages within the Pleosporomycetidae, one within the Hysteriaceae and two forming clades outside of the family. The first lineage corresponds to Psiloglonium sensu von Höhnel (1918), and forms a highly supported monophyletic clade in this study (Clade B in Fig. 1). This clade includes: Psiloglonium clavisporum, with four single-ascospore isolates from New Jersey, the United States (CBS 123338 / BPI 878726, CBS 123339 / BPI 878727, CBS 123340 / BPI 878728 and CBS 123341 / BPI 878729), and two from Kenya (GKM 344A / BPI 879801, GKM L172A in EA), P. simulans, with two isolates from the United States, one from Michigan (CBS 206.34), deposited in 1934 by Lohman, and a more recent collection from Tennessee (ANM 1557 / BPI 879803), and, lastly, P. araucanum, with three isolates from South Africa, two from Kirstenbosch (CBS 112412 / PREM 57570, CMW 18760/ PREM 57569) and one from Jonkershoek (CMW 17941 / PREM 575566). Psiloglonium clavisporum and P. simulans belong to Type I, whereas P. araucanum belongs to Type II. Both are phylogenetically related and reside in Clade B (Fig. 1). Recently, a second lineage has been shown to be associated with the Pleosporales, now accommodated in the new genus Anteaglonium (Mugambi & Huhndorf 2009), for which we include six accessions representing four species (Table 1). The third lineage corresponds to Glonium (Type III), in the Gloniaceae (Boehm et al. 2009), for which we have included four isolates, representing two species (Table 1). We treat here all species of Glonium sensu Zogg (1962), belonging to Types I and II, outside of Anteaglonium, as belonging to Psiloglonium. Since the generic name Glonium is reserved for species in the Gloniaceae (Boehm et al. 2009), we propose eight new combinations for the genus Psiloglonium.
Psiloglonium pusillum (H. Zogg) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515327.
Basionym: Glonium pusillum H. Zogg, Beitr. Kryptogamenfl. Schweiz. 11(3): 62. 1962.
Notes: Zogg (1962) described this species as G. pusillum from Juniperus phoenicea and Pinus sylvestris from Southern France, noting that it was quite rare. Zogg (1962) stated that this species may or may not be associated with a subiculum, and hence was one of the factors behind his transfer of Petrak's (1923a, b) Psiloglonium species to Glonium. Psiloglonium pusillum has ascospores only slightly larger than those of P. abbreviatum, measuring (9–)10–12(–13) x 4–5(–6) μm. Lee & Crous (2003) also identified this fungus from Proteaceae and Restionaceae in South Africa, and Sivanesan & Hsieh (1989) reported it from Taiwan. It has also been found in North America (Boehm, unpubl. data).
Psiloglonium chambianum (Guyot) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515320.
Basionym: Glonium chambianum Guyot, Ann. Serv. Bot. Tunisie 28: 90. 1955.
Notes: Originally from North Africa, on Lonicera implexa (Caprifoliaceae), the fungus has since been reported from the Proteaceae in South Africa (Lee & Crous 2003) and Europe. Zogg (1962) gave the spore measurements for G. chambianum as (14–)16–18(–21) x (6–)8–9(–10) μm, whereas Lee & Crous (2003) gave slightly larger measurements, (18–)20–21(–23) x (4–)5–6(–7) μm. Spores ellipsoid to oblong, with upper cell broader than the lower, and with an obovoid, obtuse apex. Psiloglonium chambianum possesses larger spores than P. lineare, P. simulans, and P. clavisporum, but smaller than P. uspallatense.
Psiloglonium uspallatense (Speg.) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515321.
Basionym: Glonium uspallatense Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires. 19: 436. 1909.
Notes: Zogg (1962) listed the species a “doubtful”, but Messuti & Lorenzo (2007) reinstated G. uspallatense after locating the original holotype material. They gave the spore measurements as 18–24 x 10–12 μm, placing it intermediate between P. chambianum and P. sasicola.
Psiloglonium sasicola (N. Amano) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515322.
Basionym: Glonium sasicola N. Amano, Trans. Mycol. Soc. Japan 24: 287. 1983.
Notes: Amano (1983) described this species from dead culms of Sasa sp. (Bambusaceae) in Japan. The ascospore measurements were given as 25–32 x 5–8 μm, with a rounded apical cell, placing it between P. uspallatense and P. ephedrae. Amano (1983) further reported that ascospores of this species are associated with a gelatinous sheath, previously not known among these didymospored fungi.
Psiloglonium ephedrae (Henn.) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515323. Basionym: Glonium ephedrae Henn., Öfvers. K. Vet. Akad. Förhandl. 2: 328. 1900.
= Glonium costesi Speg., Bol., Acad. Ci., Córdoba 25: 78. 1921.
Notes: Messuti & Lorenzo (2007) reinstated G. ephedrae with the synonym G. costesi, after locating and comparing original type materials. Psiloglonium ephedrae possesses very large didymospores, measuring 26–35 x 8–15 μm, the upper cells broadly ovate. It has been collected from Ephedra andicola, and, as G. costesi, from Proustia pyrifolia in Chile.
Psiloglonium hysterinum (Rehm) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515324.
Basionym: Glonium hysterinum Rehm, Hedwigia 37: 298. 1898.
Notes: Rehm (1898) originally described a species of Glonium from Southern Brazil with large fusiform didymospores, prominently constricted at the septum, and with acuminate spore apices (“Enden zugespitzt”). The spore measurements were given as 45 x 9 μm.
Psiloglonium colihuae (Lorenzo & Messuti) E.W.A. Boehm & C.L. Schoch, comb. nov. MycoBank MB515325.
Basionym: Glonium colihuae Lorenzo & Messuti, Mycol. Res. 102: 1104. 1998.
Notes: Lorenzo & Messuti (1998) described a new species on culms of Chusquea culeou from the Argentine Nothofagus rainforests. The spore measurements were given as 30–43 x 4–9.8 μm, and, although the spores are fusiform in outline, they possess moderately acuminate apices. In comparing this species to other acuminate-spored species of Glonium, the authors noted that the greatest degree of similarity was with the slightly smaller-spored G. caucasicum.
Psiloglonium araucanum (Speg.) E.W.A. Boehm, S. Marincowitz & C.L. Schoch, comb. nov. MycoBank MB515326. Fig. 8N–Q. Basionym: Glonium araucanum Speg., Revista Fac. Agron. Univ. Nac. La Plata 6: 110. 1910.
= Gloniella caucasica Rehm, Vestn. Tiflissk. Bot. Sada 25:12. 1912.
≡ Glonium caucasicum (Rehm) H. Zogg, Beitr. Kryptogamenfl. Schweiz. 11(3): 67. 1962.
Notes: Messuti & Lorenzo (2007) transferred Glonium caucasicum to G. araucanum, after examining the types for both species. Previously, Zogg (1962) had transferred Gloniella caucasica to Glonium. Here we transfer G. araucanum to Psiloglonium. This taxon possesses fusiform spores with highly acuminate apices. Messuti & Lorenzo gave the spore measurements as 22–28 x 8–10 μm, whereas Zogg (1962) gives them as (19–)22–25(–27) x (6–) 7–9(–10) μm. Although originally European in distribution (Zogg 1962), the taxon has subsequently been collected from South (Messuti & Lorenzo 2007) and North America (Boehm unpubl. data), and from South Africa (Lee & Crous 2003).
Key to the species of Psiloglonium and Anteaglonium
1. Asci ovoid, +/- cylindrical; ascospores borne in the upper portion of the ascus, not evenly distributed; ascospores (12–)13–15 x 6–7 μm; Puerto Rico.............................................................................................................................. P. finkii 1. Asci typically cylindrical to club-shaped; ascospores in one row or distichous in the asci, but always regularly arranged for its full length......................................................................................................................................... 2
2. Ascospores obovoid, with at least one, often both, ends obtuse, typically with upper cell larger, +/- constricted at the septum (Type I)....................................................................................................................................................... 3 2. Ascospores fusiform (i.e., spindle-shaped), with both ends acuminate, usually constricted at the septum (Types II and III)................. 14
3. Ascospores small, 8 μm or less in length (Anteaglonium, in part)............................................................................................................ 4 3. Ascospores longer than 8 μm (Psiloglonium Type I)................................................................................................................................. 6
4. Ascospores 6–8 x 2.5–3 μm; hysterothecia with apices acuminate, but not associated with a darkened crust; no KOH-soluble pigments; New Zealand, East Africa, North America................................................................................... A. parvulum Note: A. parvulum lies within the Pleosporales (Mugambi & Huhndorf 2009). 4. Not with the above combination of characters.......................................................................................................................................... 5
5. Ascospores (5–)6–7(–8) x 2–3(–3.5) μm (as in A. parvulum); but hysterothecia with apices truncated, and associated with a darkened crust (tending to darken the substratum); minute amounts of soluble pigment in KOH (easily missed); Europe, East Africa, North America.............................................................................. A. abbreviatum Note: A. abbreviatum lies within the Pleosporales (Mugambi & Huhndorf 2009) 5. Ascospores 6–7 x 2–3 μm (as in A. parvulum and A. abbreviatum); but hysterothecia globose with roughened walls, an indistinct slit, and associated with sparse, short subicula, and also with short tomentum on the walls of the ascomata; like A. abbreviatum also associated with a darkened crust on substrate; producing a strong green soluble pigment in KOH; eastern and mid-western North America............................................................................................................................... A. globosum Note: A. globosum lies within the Pleosporales (Mugambi & Huhndorf 2009).
6. Ascospores (9–)10–12(–13) x 4–5(–6) μm; cosmopolitan....................................................................................................... P. pusillum 6. Ascospores slightly larger......................................................................................................................................................................... 7
7. Ascospores (10–)12–14(–18) x (4–)5–7(–8) μm; ascomata +/- confluent laterally, in parallel rows, semi-immersed to erumpent; cosmopolitan................................................................................................................................ P. lineare 7. Ascospores similar in length; ascomata not confluent laterally, usually entirely superficial...................................................................... 8
8. Ascospores (10–)14–16(–18) x (4.5–)5–6 μm; cosmopolitan................................................................................................. P. simulans 8. Ascospores slightly larger......................................................................................................................................................................... 9
9. Ascospores (15–)16–18(–20) x 5–6(–7) um; Sporidesmium stygium anamorph usually present; North and South America, Africa........................................................................................................................................ P. clavisporum 9. Ascospores slightly larger in length and breadth.................................................................................................................................... 10
10. Ascospores (14–)16–18(–21) x (6–)8–9(–10) μm; Europe, North Africa.......................................................................... P. chambianum 10. Ascospores slightly larger....................................................................................................................................................................... 10
11. Ascospores 18–24 x 10–12 μm; Argentina....................................................................................................................... P. uspallatense 11. Ascospores slightly larger....................................................................................................................................................................... 12
12. Ascospores 25–32 x 5–8 μm, with a gelatinous sheath; Japan................................................................................................ P. sasicola 12. Ascospores slightly larger....................................................................................................................................................................... 13
13. Ascospores 26–35 x 8–15 μm; Chile...................................................................................................................................... P. ephedrae 13. Ascospores (59–)62–68(–76) x 13–15 μm; North and South America...................................................................................... O. curtisii Note: The genus Ostreichnion, previously placed in the Mytilinidiaceae, has been transferred to the Hysteriaceae (Boehm et al. 2009).
14. Hysterothecia usually borne in/on subicula, typically bifurcated, forming radiating flabelliform or pseudo-stellate composites, with or without a stroma (Type III)........................................................................................................................................... Gloniaceae Note: In this study, a key to the species of the Gloniaceae is provided under that family. 14. Hysterothecia not bifurcated, forming radiating flabelliform or pseudo-stellate composites, nor with a stroma...................................... 15
15. Ascospores less than 30 μm long........................................................................................................................................................... 16 15. Ascospores more than 30 μm long......................................................................................................................................................... 17
16. Ascospores (19–)22–25(–27) x (6–)7–9(–10) μm, both ends acuminate, with a prominent septal constriction; cosmopolitan (Type II).......................................................................................................................................................... P. araucanum 16. Ascospores 22–28 x 4–6 μm, acuminate, 1-septate, hyaline and with a mucilaginous sheath when young, but acquiring additional septa and pigmentation with age, to become 3–5-septate and pale brown at maturity; Kenya.................................................................................................................................................................................. A. latirostrum Note: A. latirostrum lies within the Pleosporales (Mugambi & Huhndorf 2009).
17. Ascospores 30–43 x 4–9.8 μm; Argentina (Type II)................................................................................................................. P. colihuae 17. Ascospores about 45 x 9 μm; Brazil (Type II)...................................................................................................................... P. hysterinum
Lee & Crous (2003) identified a series of isolates from South Africa on the Restionaceae as Glonium compactum (CBS 112412, CMW 18760, CMW 17941). However, in their study they did not note the presence of subicula, nor a stromal crust. These features were stressed for this taxon by Zogg (1962). These same isolates were used in Boehm et al. (2009), and were shown to associate, with high branch support, with two species of Psiloglonium, P. clavisporum and P. simulans, distant from the other species of Glonium surveyed (e.g., G. stellatum and G. circumserpens). Thus, a new combination was proposed, Psiloglonium compactum. However, it is now realised that this new combination was made in error and is hereby retracted. It must be concluded that the South African isolates (Lee & Crous 2003) were not G. compactum, due to the absence of subicula and stroma, but rather, we suspect, the cosmopolitan P. araucanum, which has similar, but slightly smaller, fusiform acuminate didymospores. Lee & Crous (2003) give the ascospore measurements for the South African “G. compactum” as (24–)26–27(–30) x (4–)5–6(–7) μm, which matches closely those given above for P. araucanum. Furthermore, the illustrations in Lee & Crous (2003) closely match P. araucanum, and not those of G. compactum, as given by Zogg (1962). If we are correct in assuming that the South African isolates used in Boehm et al. (2009) are in fact P. araucanum (Type II) and not G. compactum (Type III), then this would provide a high degree of support for the inclusion of species with acuminate spore apices, belonging to Type II, in the genus Psiloglonium, along with species with obtuse spore apices, belonging to Type I (e.g., P. simulans and P. clavisporum). A reanalysis of the original South African herbarium specimens from which the sequences were derived (PREM 57570, PREM 57569, PREM 57566), by S. Marincowitz, has confirmed that they do indeed correspond to P. araucanum and not to G. compactum. Molecular data thus supports the association of Types I and II within the genus Psiloglonium.
In addition to the 12 currently recognised species in Psiloglonium, the following key also includes entries for the unrelated Gloniaceae, Anteaglonium and Ostreichnion curtisii.
Actidiographium Lar.N. Vassiljeva, Mikol. Fitopatol. 34 (6): 4. 2000.
Vasilyeva (2000) established the monotypic genus Actidiographium to accommodate a hysteriaceous fungus with pigmented one-septate ascospores, reminiscent of those found in Actidium in the Mytilinidiaceae. However, in Actidiographium orientale, the two-celled spores are borne in a typical thick-walled hysterothecium. The pigmented didymospores measure 13.2–16.5 x 3–4 μm. Molecular data are lacking for this taxon.
Hysterocarina H. Zogg, Ber. Schweiz. Bot. Ges. 59: 39. 1949.
Zogg (1949) erected this monotypic genus for Hysterocarina paulistae, with pigmented dictyospores as in Hysterographium, but the hysterothecia are borne within the substrate, barely erumpent at maturity, and with a cristate, slightly evaginated longitudinal keel, instead of the invaginated sulcus typical of most members of the Hysteriaceae. Described from old wood of Eucalyptus sp. in Brazil, the pigmented dictyospores measure 20–25 x 8–10 μm. The presence of an evaginated keel-like fissure in Hysterocarina is intriguing, as it seems to belong to an evolutionary trend that culminates in the Mytilinidiaceae and Gloniaceae. Clearly, molecular data are needed to resolve these issues.
Ostreichnion Duby, Mém. Soc. Phys. Genève 16: 22. 1862.
= Ostreion Sacc., Syll. Fung. 2: 765. 1883.
Since its reappraisal (Barr 1975), the genus Ostreichnion has been heterogeneous, due to the inclusion of O. curtisii an unusual taxon, from the southeastern United States (Lohman 1937) and Brazil (Zogg 1962). It is very different from the other two species of this genus, namely the type O. sassafras and O. nova-caesariense. Whereas the latter two species possess pigmented dictyospores, in O. curtisii the ascospores are 1-septate below the middle, with walls greatly thickened towards the spore apices. When mounted under different stains, the spore cytoplasm appears subdivided into numerous compartments, giving the impression of a potentially muriform structure. Lohman (1937) provided details as to the highly unusual spore germination process in this fungus, which involves a distended apical plug and numerous median germ tubes, differing from that found in species of Psiloglonium and Glonium, which send out apical germ tubes (Lohman 1931, 1932a). Ostreichnion sassafras occurs on both sides of the Atlantic, as well as in China, and has been recovered from Sassafras, Quercus, Liriodendron, and Liquidambar (Bisby 1932, Teng 1933, Barr 1975). It is unusual in having very large dictyospores, measuring (65–)76–100(–135) x 20–32 μm, with up to 27 septa, borne four to an ascus. Ostreichnion nova-caesariense is known only from the type locality in New Jersey on Pinus, and has similar, but smaller, ascospores (Barr 1975).
Key to the species of Ostreichnion
1. Ascospores mostly 1-septate, ends greatly thickened, (45–)62–80 x (10–)12–15 μm; North & South America........................ O. curtisii 1. Ascospores with both transverse and longitudinal septa.......................................................................................................................... 2
2. Ascospores measuring 35–45(–50) x 11–13 μm, with 7–13 septa, borne eight to an ascus; North America.......... O. nova-caesariense 2. Ascospores measuring (65–)76–100(–135) x 20–32 μm, with up to 27 septa, borne four to an ascus; cosmopolitan......... O. sassafras
Based on a recent four-gene analysis (Boehm et al. 2009), the genus Ostreichnion, previously in the Mytilinidiaceae (Barr 1975, 1990a), was transferred to the Hysteriaceae. This was based on sequence data derived from two of the three species (Table 1), namely O. curtisii (CBS 198.34) and O. sassafras (CBS 322.34), deposited by Lohman in 1934. Although both species find residency within Clade C (Fig. 1), their association with the genus Hysterium could not have been predicted. Given the unique nature of the ascospore in O. curtisii, considered potentially muriform, one would assume affinities with the genus Hysterographium sensu Zogg (1962), or, given its 1-septate ascospores at maturity, with Psiloglonium, where it was originally treated by Lohman (1937) as Glonium curtisii. However, molecular data suggest neither. Instead, O. curtisii shares a subclade with Hysterium barrianum, with 9-septate phragmospores (Fig. 1). Ostreichnion sassafras is more distant within Clade C. Although we recognise the genus as artificial, we present the following key, adapted from Barr (1975), to facilitate species identification.
Rhytidhysteron Speg., Anales Soc. Ci. Argent. 12: 188. 1881.
The genus Rhytidhysteron is characterised by ascomata that are at first closed and navicular (e.g., Fig. 10K), somewhat resembling those found in the Hysteriaceae, but then later opening by a longitudinal sulcus to become irregularly apothecioid at maturity, often with incurved margins (e.g., Fig. 10M) – a feature never observed in the Hysteriaceae. The peridium in Rhytidhysteron is somewhat gelatinous when wet, as compared to the hard, carbonaceous peridium found in the Hysteriaceae. Although ascomata may possess striations, in Rhytidhysteron these are perpendicular to the long axis (Fig. 10K), rather than parallel, as in the Hysteriaceae (e.g., Figs 1A, 2B, and 6A). The ascospores in Rhytidhysteron tend to be heavily pigmented and thick-walled, as opposed to lightly pigmented and thin-walled in the Hysteriaceae. These features, among others, have been used to place Rhytidhysteron within the Patellariaceae (e.g., Kutorga & Hawksworth 1997). Samuels & Müller (1979) revised the genus, providing a number of synonyms, and accepted only two species, namely the type, R. rufulum (Fig. 10E–K), with 3-septate phragmospores, and R. hysterinum (Fig. 10M), with 1-septate spores, both darkly pigmented and thick-walled. Anamorphs have been characterised as Diplodia- and Aposphaeria-like (Samuels & Müller 1979). Subsequently, another two species have been accepted in the genus, namely R. dissimile (Magnes 1997), with 5-septate phragmospores, and R. opuntiae (1990b), from the American South West, with short pigmented dictyospores (Fig. 10A–D), reminiscent of those found in Hb. mori.
Fig. 10.
The genus Rhytidhysteron (Clade E). A–D. Rhytidhysteron opuntiae [GKM 1190 (BPI 879805), Kenya]; E–J. Rhytidhysteron rufulum [GKM 361A (BPI 879806), Kenya]; K. Rhytidhysteron rufulum [EB 0382 (BPI 879808), Ghana]; L. Rhytidhysteron rufulum [EB 0381 (BPI 879807), Ghana]; M. Rhytidhysteron hysterinum [EB 0351 (BPI 879804) France, photo by Alain Gardiennet]. Scale bar (habitat) = 1 mm; Scale bar (spores and asci) = 10 μm.
Dictyospores of both R. opuntiae and Hb. mori are similar in shape, obovoid, with obtuse ends, and are also similar in size and septation. In both, the longitudinal septum is usually associated with the mid-cells, but on occasion it can be found obliquely in the end cells. However, unlike Hb. mori, the spores of R. opuntiae are thick-walled, verruculose and darkly pigmented. The most surprising morphological feature of R. opuntiae is that the spores are not borne within patellarioid ascomata, as in other members of the genus. Rather, the ascomata are hysterithecioid, that is, carbonaceous, navicular, with an invaginated longitudinal sulcus (Fig. 10A–B). In hindsight, it is remarkable that Barr (1990) recognised R. opuntiae as a member of Rhytidhysteron, transferring it from Hysterographium opuntiae, despite the presence of hysterithecioid ascomata. In this study we were fortunate to acquire an isolate of R. opuntiae from Kenya (GKM 1190 / BPI 879805). Rhytidhysteron opuntiae falls distant from R. rufulum and R. hysterinum, lying outside of Clade E altogether (Fig. 1). Although both morphological and molecular data suggest that R. opuntiae should be removed from the genus Rhytidhysteron, this is based only on a single specimen, and clearly needs to be substantiated with other isolates.
The six isolates of R. rufulum included one from Kenya (GKM 361A / BPI 879806; Fig. 10E–J), four from Ghana (EB 0381 / BPI 879807, Fig. 10L; EB 0382 / BPI 879808, Fig. 10K; EB 0383 / 879809; EB 0384 / BPI 879810), and one from Europe (CBS 306.38). Also included was one isolate of R. hysterinum from France (EB 0351 / BPI 879804). Three of the Ghanian isolates clustered together in Clade E (Fig. 1), but one (EB 0381 / BPI 879807) associated in another subclade, along with the Kenyan (GKM 361A) and European (CBS 306.38) accessions of R. rufulum. The morphology of the ascomata (Fig. 10L) of R. rufulum EB 0381 (BPI 879807) differs from other more typical specimens of R. rufulum (e.g., Fig. 10 K), although the 3-septate spores in both are identical. Finally, molecular data indicate that R. hysterinum, with 1-septate spores, falls outside of the R. rufulum subclades, while still within Clade E (Fig. 1).
Boehm et al. (2009) were the first to provide sequence data indicating that Rhytidhysteron does not lie within the Patellariaceae. Although initially based on only a single isolate of R. rufulum (CBS 306.38), the genus was tentatively noted to be associated with the Hysteriaceae. In the current study, a total of eight isolates, representing three species, clearly indicates that that the genus Rhytidhysteron belongs to the family Hysteriaceae, and not to the Patellariaceae, the latter defined in this study to include Hysteropatella clavispora (CBS 247.34), Hp. elliptica (CBS 935.97), and Patellaria atrata (CBS 958.97).
Earlier, Barr (1987) had noted the differences between Rhytidhysteron and other members of the Patellariaceae, stating: “Rhytidhysteron rufulum illustrates the problem: paraphysoids and a well-developed pseudoepithecium are conspicuous, but the structure of the peridium, thickened base of ascoma, cylindric asci, are all features attributed to members of the Hysteriaceae. When the heterogeneous family Patellariaceae is revised, Rhytidhysteron should be segregated in its own family”. Samuels & Müller (1979) also noted that “The genus does not have any close relatives in the heterogeneous Patellariaceae”. However, other authors (Bezerra & Kimbrough 1982) presented arguments against the inclusion of Rhytidhysteron within the Hysteriaceae, based on patterns of centrum development. Nevertheless, molecular data presented here, necessitate a radical reappraisal of the Hysteriaceae to include patellarioid forms.
Key to the species of Rhytidhysteron
1. Ascospores mainly 1-septate; Europe................................................................................................................................ R. hysterinum 1. Ascospores with more than one septum................................................................................................................................................... 2
2. Ascospores mainly 3-septate.................................................................................................................................................................... 3 2. Ascospores with five or more septa; Europe.......................................................................................................................... R. dissimile
3. Ascospores with three transverse, but also one or more longitudinal septa; Southwestern United States, East Africa......... R. opuntiae 3. Ascospores transversely 3-septate, with no longitudinal septa; cosmopolitan......................................................................... R. rufulum
Mytilinidiaceae Kirschst. 1924, Mytilinidiales E.W.A. Boehm, C.L. Schoch & J.W. Spatafora 2009.
= Lophiaceae H. Zogg ex Arx & E. Müll., Stud. Mycol. 9: 60. 1975.
≡ Lophiaceae H. Zogg, Beitr. Kryptogamenfl. Schweiz. 11(3): 90. 1962, nom. inval. ICBN Art. 36.
Fungi classified in the Mytilinidiaceae (Kirschstein 1924) are characterised by fragile yet persistent carbonaceous ascomata, which range from globoid to obovoid to strongly laterally compressed erect, bivalve shell-shaped (i.e., conchate) structures, standing on edge, with lateral walls more or less connivent, and extended vertically, in some species, to a prominent longitudinal keel or cristate apex. Mytilinidioid fungi possess a thin-walled, scleroparenchymatous peridium enclosing a hamathecium of narrow trabeculate pseudoparaphyses, borne in a gel matrix, which are often sparse to lacking at maturity. Bitunicate asci are borne in a basal, rarely lateral orientation within the centrum, and contain eight, rarely four, ascospores, overlapping uniseriate, biseriate or in one or two fascicles. Ascospores are diverse, ranging from scolecospores to didymospores to phragmospores or dictyospores, hyaline, soon yellow to dark brown, and generally showing bipolar symmetry (Zogg 1962, Barr 1987, 1990a). Anamorphs in the Mytilinidiaceae are primarily coelomycetous (e.g., Aposphaeria, Pyrenochaeta, Camaroglobulus, Dothiorella-like, and Sclerochaeta) and less frequently hyphomycetous (e.g., Chalara-like, Papulaspora, and Septonema) (Lohman 1932b, 1933a, b, Blackwell & Gilbertson 1985, Speer 1986). Typically temperate in distribution, mytilinidioid fungi are found in association with the wood, bark, resin, cones, scales, needles, seeds, and roots of gymnosperms.
Currently accepted genera in the Mytilinidiaceae include: Actidium, Lophium, Mytilinidion, Ostreola, and Quasiconcha, to which has recently been added Zoggium (Lohman 1932b, Zogg 1962, Darker 1963, Barr 1975, 1990a, Barr & Blackwell 1980, Vasilyeva 2001). The genus Ostreichnion, previously classified within the Mytilinidiaceae, has been removed to the Hysteriaceae (Boehm et al. 2009). The genus Glyphium, originally classified within the Mytilinidiaceae, has recently been transferred to the Chaetothyriales in the Eurotiomycetes (Lindemuth et al. 2001, Lumbsch et al. 2005). This has been restated in a number of subsequent publications (Lücking et al. 2004, Schmitt et al. 2005, Geiser et al. 2006, Kodsueb et al. 2006), including the Assembling the Fungal Tree of Life (AFTOL) Project (Lutzoni et al. 2004). A study currently in preparation (Boehm et al.) will address issues related to the phylogenetic placement of the genus Glyphium. Despite their transference out of the Mytilinidiaceae, both Ostreichnion and Glyphium are included in the current key to effectuate identification of morphologically similar fungi, regardless of whether close phylogeny is implied or not.
Key to the genera of the Mytilinidiaceae
1. Ascospores 1-septate, small, shorter than 30 μm..................................................................................................................................... 2 1. Ascospores not didymospores, or if 1-septate, then longer than 30 μm................................................................................................... 3
2. Didymospores brown, ellipsoid, symmetric, with coarsely reticulate wall; 6–8 x 5–5.5 μm................................................. Quasiconcha 2. Didymospores olive- to reddish brown, walls thin, smooth or delicately longitudinally striate, but not reticulated; longer than 10 μm........................................................................................................................................ Actidium
3. Ascospores filiform, multi-septate, about equal in length to the ascus, in some case, at maturity longer than the ascus, often spirally arranged.............................................................................................................................................................................. 4 3. Ascospores ellipsoid, fusoid, cylindrical, if scolecospores, then shorter than the ascus and not spirally arranged.................................. 6
4. Ascomata conchate, solitary to gregarious, but never forming fused, ridge-like assemblages................................................... Lophium 4. Ascomata either forming rigid, fused band- or ridge-like structures or solitary, erect, dolabrate to ligulate.............................................. 5
5. Ascomata densely gregarious, forming band- or ridge-like assemblages................................................................................... Zoggium 5. Ascomata erect, dolabrate to ligulate in outline; often with subtending hyphal strands; cosmopolitan...................................... Glyphium Note: A key to the species is not presented here.
6. Ascospores transversely septate phragmospores, or scolecospores..................................................................................... Mytilinidion 6. Ascospores dictyospores, or large and remaining 1-septate.................................................................................................................... 7
7. Ascospores ellipsoid, less than 30 μm long, with a single longitudinal septum, usually passing through the mid-cells, or spanning the entire length of the ascospore............................................................................................................................ Ostreola 7. Ascospores ellipsoid or cylindric, longer than 30 μm, with several longitudinal septa in cells or large and remaining 1-septate....................................................................................................................................................... Ostreichnion Note: The genus Ostreichnion previously classified within the Mytilinidiaceae (Barr 1990a) has been transferred to the Hysteriaceae (Boehm et al. 2009).
Actidium Fr., Observ. Mycol. 1: 190. 1815.
= Mytilinidion subgen. Bulliardella Sacc., Syll. Fung. 2: 764. 1883.
= Bulliardella (Sacc.) Paoli, Nuovo Giorn. Bot. Ital. 12:101. 1905.
= Ostreionella Seaver, Sci. Surv. Porto Rico & Virgin Islands 8(1): 77. 1926.
The genus Actidium was established by Fries (1823) to accommodate A. hysterioides, a stellate mytilinidioid fungus found on Pinus and Picea in Europe, with two-celled, symmetric ascospores, light olive- to reddish-brown, later noted to be faintly longitudinally striate (Barr 1990a). Fries (1823) noted its similarity with the genus Glonium. Zogg (1962) recognised a total of four species, namely A. hysterioides, A. baccarinii, both from Europe, A. pulchra, from China, and A. nitidum, from Europe and North America, on Pinus, Picea, Juniperus, and Thuja (Zogg 1962, Barr 1990a). Due to similarities in ascospore morphology, the genus Actidium may have affinities with other didymospored hysteriaceous genera (e.g., Actidiographium, Glonium and Psiloglonium), although molecular data are presently lacking.
Key to the species of Actidium
1. Ascomata stellate; spores 11–14 x (1.5–)2–3 μm; on Pinus, Picea, Europe.................................................................... A. hysterioides 1. Ascomata shell-shaped (conchate), not star-shaped................................................................................................................................ 2
2. Ascospores (9–)11–14(–16) x (1.5–)2–3 μm; on Pinus, Picea, Juniperus, Europe, North America......................................... A. nitidum 2. Ascospores larger..................................................................................................................................................................................... 3
3. Ascospores (16–)18–22(–24) x (3–)4–5(–6) μm; on Pinus, Picea, Thuja, Europe............................................................... A. baccarinii 3. Ascospores 23–28 x 6–7.5 μm; China...................................................................................................................................... A. pulchra
Quasiconcha M.E. Barr & M. Blackw., Mycologia 72: 1224. 1980.
The genus Quasiconcha was established by Barr & Blackwell (1980) to accommodate Q. reticulata, an unusual mytilinidioid fungus, with 1-septate, highly reticulated ascospores, borne in conchate, thin-walled ascomata, found in association with Juniperus seeds excreted in dung and the roots of two conifers from the southwestern United States (Barr & Blackwell 1980, Blackwell & Gilbertson 1985). In the present study, we were fortunate to obtain original material (RLG 141189) of Q. reticulata (Table 1) from Meredith Blackwell (Louisiana State University, Baton Rouge, LA), from which we isolated DNA (EB QR). Sequence data (Fig. 1) clearly indicate that the genus Quasiconcha belongs to the Mytilinidiaceae, in close association with Lophium, to which its fruitbodies most closely resemble.
Mytilinidion Duby, Mém. Soc. Phys. Genève 16: 34. 1862.
= Mytilidion Sacc., Atti Soc. Veneto-Trentino Sci. Nat. Padova 4: 99. 1875.
= Hypodermopsis Earle, Bull. New York Bot. Gard. 2: 345. 1902.
= Murashkinskija Petr., Hedwigia 68: 203. 1928.
The genus Mytilinidion, the type for the family Mytilinidiaceae, was established by Duby (1862) with an etymology from Mytilus, a genus of mussels. Saccardo (1883, p. 760) considered the name Mytilinidion to be linguistically incorrect and replaced it with Mytilidion. It remained for Barr (1975) to note that the name Mytilinidion had historical precedence (Rogers 1953), and therefore should replace the later name Mytilidion. Species of Mytilinidion are characterised by yellow- to reddish-brown, ellipsoid, fusoid, obovoid to elongate, transversely septate, usually symmetric, ascospores, or scolecospores, borne in thin-walled globoid to conchate pseudothecia, with lateral walls more or less connivent and extended vertically to a cristate apex. There are currently 15 recognised species, occurring on the Pinaceae, Cupressaceae, and Taxodiaceae (Lohman 1932b, Zogg 1962, Speer 1986, Barr 1990a).
Ascospore morphology can be used to discern four morphological types within the genus, listed here by increasing ascospore length: (1) Short squat phragmospores: M. acicola, M. resinae, M. decipiens, M. tortile (Fig. 11A–B), and M. resinicola; (2) Elongate phragmospores, with a spore length to width ratio of 10: 1 or less: M. californicum, M. mytilinellum (Fig. 11C–D), M. rhenanum, and M. gemmigenum; (3) Fusoid or spindle-shaped spores: M. thujarum, M. oblongisporum, and M. andinense; and (4) Highly elongated phragmospores, termed scolecospores, with a length to width ratio of 20: 1: M. scolecosporum, M. parvulum and M. australe (Fig. 11E–I). These last three scolecosporous species were postulated to form a transitional series to connect Mytilinidion with the heretofore somewhat isolated genus Lophium (Fig. 11J–K), and formed the basis for subgenus Lophiopsis, distinct from subgenus Eu-Mytilinidion sensu Lohman (Lohman 1932b), a concept accepted by Zogg (1962).
Fig. 11.
The Mytilinidiaceae. A–B. Mytilinidion tortile [EB 0377 (BPI 879798), France]; C–D. Mytilinidion mytilinellum [EB 0386 (BPI 879796), France]; E–I. Mytilinidion australe [ANM 1524 (ILLS), U.S.A.; not incl.]; J–K. Lophium mytilinum [CBS 123344 (BPI 878736), U.S.A.]. Photo credits Alain Gardiennet, Figs. A–D. Scale bar (habitat) = 500 μm; Scale bar (spores and asci) = 10 μm.
Key to the species of Mytilinidion
1. Spore length to width ratio = 10: 1 or less (phragmospores): Subgenus Eu-Mytilinidion sensu Lohman (1932b)................................... 2 1. Spore length to width ratio = approx. 20: 1 (scolecospores): Subgenus Lophiopsis sensu Lohman (1932b)....................................... 13
2. Ascomata not conchate, but erect, low and spreading at the base (scutate), seated on a shield-like process fused to the substrate, apical portion slightly connivent; ascospores 3–5(–6)-septate....................................................................................... 3 2. Ascomata conchate, standing on edge, usually with a clearly defined longitudinal cristate apex............................................................ 4
3. Ascospores 23–25 x 4–4.5(–5) μm, 3-septate; California on Sequoia............................................................................. M. californicum 3. Ascospores 14–22(–28) x (4.5–)6–8(–10) μm, 3–4–5–(–6) septate; on Juniperus, Thuja, Europe and North America..................................................................................................................................................................... M. acicola
4. Ascospores elongate phragmospores, usually not constricted at the septa............................................................................................. 5 4. Ascospores shorter, squat, or longer, but not narrowly elongated, usually constricted at median septum............................................... 7
5. Ascospores (2–)3(–5)-septate, measuring (14–)16–22(–24) x (2.5–)3–4(–5) μm; cosmopolitan..................................... M. mytilinellum 5. Ascospores longer, with more septa......................................................................................................................................................... 6
6. Ascospores 3–5(–7)-septate, measuring (24–)30–42(–50) x 3–5 μm; Europe.................................................................... M. rhenanum 6. Ascospores slightly curved, asymmetric, (3–)7–9(–11)-septate, measuring (27–)32–38(–48) x (4–)5–6(–8) μm; cosmopolitan.................................................................................................................................................................. M. gemmigenum
7. Ascospores (2–)3-septate, small, 10–13 x 4–6 μm; resinicolous on Araucaria, Brazil............................................................. M. resinae 7. Ascospores 3(–5)-septate, longer............................................................................................................................................................. 8
8. Ascospores 3-septate, slightly curved, oblong-elliptic, with obtuse ends, unconstricted, measuring (11–)13–15(–21) x 3–4(–6) μm; on Larix, Juniperus, Europe............................................................................. M. decipiens 8. Ascospores longer, or similar in length but then slightly wider.................................................................................................................. 9
9. Ascospores 3-septate, slightly curved, but oblong, fusiform, with slight constrictions, measuring (11–)14–17(–21) x 5–7(–8) μm; cosmopolitan........................................................................................................... M. tortile 9. Ascospores longer.................................................................................................................................................................................. 10
10. Ascospores 3-septate, elliptic-oblong, deeply constricted at the septa, measuring 24–26 x 8–9 μm; North America.......... M. resinicola 10. Ascospores longer, fusoid....................................................................................................................................................................... 11
11. Ascospores 3-septate, constricted at the median septum, measuring 27–33 x 7–8.5 μm; China and northwestern North America....................................................................................................................... M. oblongisporum 11. Ascospores longer.................................................................................................................................................................................. 12
12. Ascospores 3-(4–5)-septate, measuring (26–)30–34(–40) x (10–)12–13(–15) μm; on Thuja, cosmopolitan........................ M. thujarum 12. Ascospores wider, 3–7(–9)-septate, with swollen middle cells, 32–44 x 10–15 μm; on Austrocedrus chilensis, Argentina .............................................................................................................................................................................. M. andinense
13. Ascospores 5–7-septate, measuring 40–50 x 2–2.5 μm, slightly constricted at central septa; North America and Europe.......................................................................................................................................... M. scolecosporum 13. Ascospores longer, with more septa, less constricted............................................................................................................................ 14
14. Ascospores 7–9(–11)-septate, measuring (48–)54–62(–65) x 2.7–3 μm; North America...................................................... M. parvulum 14. Ascospores (10–)11–14-septate, measuring (54–)58–70(–75) x 3–4 μm; North America....................................................... M. australe
Sequence data presented here (Fig. 1), based on an analysis of 10 of the 15 currently recognised species (Table 1), do not support subgenus Lophiopsis sensu Lohman (1932b): Mytilinidion scolecosporum (CBS 305.34) does not belong to the same clade as M. australe (CBS 301.34) (Fig. 1). This implies that the scolecospore has independently evolved at least twice within the family. Data do however support the association of fusoid or spindle-shaped spores belonging to M. thujarum (EB 0268 / BPI 879797) and to M. andinense (CBS 123562 / BPI 878737), thus defining a lineage for this type of spore within the genus. On the other hand, species possessing short, squat phragmospores, namely M. acicola (EB 0349 / BPI 879794, EB 0379 / BPI 879793), M. tortile (EB 0377 / BPI 879798), and M. resinicola (CBS 304.34) display complex relationships with species possessing elongate phragmospores, such as M. californicum (EB 0385 / BPI 879795), M. mytilinellum (EB 0386 / BPI 879796, CBS 303.34) and M. rhenanum (EB 0341, CBS 135.45). This indicates that phragmospores with different length to width ratios have also evolved multiple times within the genus (Fig. 1). A manuscript currently in preparation (Boehm et al.) will address speciation events within the Mytilinidiaceae. Despite the lack of molecular support for the subgenus Lophiopsis, it is included in the key below to facilitate species identification.
Lophium Fr., Syst. Mycol. 2: 534. 1823.
= Lophidium P. Karst., Bidrag. Kännedom Finlands Natur Folk. 23: 33, 247. 1873.
The genus Lophium is characterised by fragile, conchate ascocarps, sometimes seated on a foot-like base or sessile directly on the substrate. The thin-walled scleroparenchymatous peridium encloses a basal hamathecium of narrow trabeculate pseudoparaphyses, with very elongate asci, each bearing one fascicle of transversely septate filiform ascospores, often spirally arranged. The type species, Lophium mytilinum (Fig. 11J–K), is cosmopolitan in the temperate zones and has been recorded from both sides of the Atlantic (Zogg 1962, Barr 1990a). Zogg (1962) described two additional species, namely L. elegans on Juniperus from alpine regions of France, Italy and Switzerland, and L. mayorii on Pinus and Larix from the European Alps. Like Mytilinidion, most species of Lophium have only been recovered from coniferous substrates. The exception being the recently described L. igoschinae, recovered on Dryas octopetala and D. crenulata (Rosaceae) from Russia and Greenland (Chlebicki & Knudsen 2001).
Three isolates of the type species, L. mytilinum, were surveyed (Table 1), two from the United States, one from Michigan (CBS 269.34) and one from New York (CBS 123344 / BPI 878736), and one from Sweden (CBS 114111). An additional species of Lophium, namely a single-spored isolate of L. elegans from France (EB 0366 / BPI 879792), was included as well (Table 1). Both species are morphologically similar, with L. elegans having spirally arranged spores in the ascus and L. mytilinum having them in parallel orientation (Zogg 1962). Molecular data indicate that the two species are not closely related within the family. Lophium mytilinum, with filiform ascospores, shows a close phylogenetic relationship however to the genus Quasiconcha (EB QR), with reticulated didymospores (Fig. 1). Although having dissimilar spores, the fruitbodies of both taxa are remarkably similar in their shape, size and fragility.
Key to the species of Lophium
1. Fruitbody erect, conchate, with thin-walled sclerenchymatoid peridium................................................................................................... 2 1. Fruitbody conchate, but crowded, band- or ridge-like, horizontal to recumbent and elongated; ascospores arranged parallel in the ascus, measuring (60–)80–100(–110) x 3–4(–5) μm; Europe, Russian Far East............. L. mayorii Note: Transferred to the genus Zoggium (Vasilyeva 2001).
2. Ascospores filiform, 12–15-septate, measuring 78–86 x 2.6–3 μm; on Dryas, Greenland, Russia.................................... L. igoschinae 2. Ascospores filiform, but longer; on conifers.............................................................................................................................................. 3
3. Ascospores arranged parallel in the ascus; measuring (130–)170–250(–300) x 1–2(–2.5) μm; cosmopolitan..................... L. mytilinum 3. Ascospores spirally arranged in the ascus; measuring (200–)260–280(–300) x 2 μm; Europe............................................... L. elegans
Zoggium Lar.N. Vassiljeva, Mikol. Fitopatol. 35: 17. 2001.
Zogg described Lophium mayorii on Pinus and Larix from the Swiss and French Alps, but noted that it differed from other species of Lophium in having rigid, band-forming ascomata, with a less fragile peridium as compared to Lophium and Mytilinidion. Vasilyeva (2001) found the same fungus in the Russian Far East and stated that it differed sufficiently from other species of Lophium in having gross, erumpent crowded ascomata, band- or ridge-like in appearance, as compared to the smaller, fragile, and entirely superficial fruitbodies typical of species of Lophium and made the transfer to Zoggium mayorii. Molecular data are presently lacking.
Ostreola Darker, Canad. J. Bot. 41: 1383. 1963.
Barr (1975, 1990a) recognised two genera with muriform ascospores in the Mytilinidiaceae, namely Ostreichnion and Ostreola. Darker (1963) originally established the genus Ostreola for dictyospored forms that otherwise resembled species of Mytilinidion – that is, mytilinidioid counterparts to Hysterographium sensu Zogg (1962). Barr (1990a) differentiated Ostreola from Ostreichnion by smaller ascospores in the former, and recognised two species from North America, Ot. consociata from northeastern North America, and Ot. formosa, the latter common on conifers in western North America and Europe, with spores similar to those of Hysterobrevium mori. Tilak & Kale (1968) added another two species from India, namely Ot. indica and Ot. ziziphi, surprisingly both from non-coniferous substrates. Molecular data are presently lacking for this genus.
Key to the species of Ostreola
1. Ascomata on coniferous hosts; North America, Europe........................................................................................................................... 2 1. Ascomata on non-coniferous hosts; India................................................................................................................................................. 3
2. Base of ascoma foot-like, immersed in substrate; ascospores 3–5(–7)-septate, with a longitudinal septum in the mid-cells, 14–18(–22) x 5–7 μm; on Picea, Northeastern North America.................................................................... Ot. consociata 2. Base of ascoma tapered or applanate on surface of substrate; ascospores (3–)5(–6)-septate, wider than in O. consociata, 15–21 x 6.5–9.5 μm; alpine, on Abies, Europe and Western North America................................................. Ot. formosa
3. Ascospores transversely 3–7-septate, with 2–3 longitudinal septa, slightly constricted in the middle; 24–30 x 8–9.6 μm; on culms of Maduca, India......................................................................................................................................................... Ot. indica 3. Ascospores as above but smaller, 19–23 x 6–7.5 μm; on culms of Ziziphus, India................................................................... Ot. ziziphi
Gloniaceae (Corda) E.W.A. Boehm, C.L. Schoch & J.W. Spatafora 2009, Pleosporomycetidae fam. incertae sedis.
= Gloniaceae Corda, Icon. Fung. (Abellini) 5: 34. 1842.
Corda (1842) originally proposed the Gloniaceae as an intrafamilial taxonomic rank under the family Hysteriaceae, to comprise Hysterographium and Glonium. Boehm et al. (2009) emended and restricted the circumscription and elevated the taxon to family rank. The genus Glonium was retained as circumscribed first by von Höhnel (1918) and then by Petrak (1923a). We feel justified in reinstating the Gloniaceae and, more importantly, recognising it at family rank for a single genus, because of the high support the group receives in a recent four-gene analysis (Boehm et al. 2009), and corroborated here.
Glonium Muhl., Cont. Lab. Plant Disease Sci. Fac. Agric. Gifu Univ. 101. 1813.
= Solenarium Spreng., Syst. Veg. 4(1): 376, 414. 1827.
= Psiloglonium Höhn., Ann. Mycol. 16(1): 149. 1918.
The genus Glonium is characterised by modified hysterothecia, progressively dichotomously branched, laterally anastomosed along their length to form radiating flabelliform or pseudostellate composites, usually seated upon a conspicuous brown felt-like subiculum, sometimes borne in a stroma (Zogg 1962). Hysterothecia in vertical section globose to obovoid, typically with a thick, three-layered peridium, but fragile, unlike the robust peridium of the Hysteriaceae. Luttrell (1953) described the development of the ascocarp in the type species, G. stellatum as composed of small pseudoparenchymatous cells, the outer layer heavily encrusted with pigment and often longitudinally striate on the surface, the middle layer lighter in pigmentation and the inner layer distinctly thin-walled, pallid and compressed. The hamathecium consisted of persistent narrow cellular pseudoparaphyses, often borne in a gel matrix, with tips darkened or branched at maturity. Bitunicate asci are borne in a basal layer and at maturity are typically clavate to cylindric, bearing eight ascospores, overlapping biseriate; ascospores ranging from hyaline to pale yellow, 1-septate, conspicuously constricted at the septum, fusoid in outline, with at least one end, often both, acuminate, and showing bipolar asymmetry.
Zogg (1962) listed three species that he grouped together in his key, that later formed the basis for the Gloniaceae (Boehm et al. 2009). These are the type species, G. stellatum (Fig. 12A–E), G. graphicum, and G. compactum, the latter associated with both subicula and stroma. To these three species, we can add the recently described saxicolous, terricolous and lignicolous G. circumserpens (Fig. 12F–H) from Tasmania (Kantvilas & Coppins 1997). Although von Höhnel (1918) and Petrak (1923a) stressed the importance of subiculum as a synapomorphic character state, Zogg (1962) noted that G. graphicum may or may not be associated with a subiculum. This, combined with the observation that P. lineare may also on occasion be associated with subiculum, led Zogg not to accept the genus Psiloglonium. Data presented here and elsewhere (Boehm et al. 2009), however, indicate that the synapomorphic character state is not subicula per se, but the ascomata, which are modified hysterothecia that are progressively dichotomously branched, to form radiating pseudostellate composites. This is most pronounced in G. stellatum and G. circumserpens, but may also be found to a lesser extent in G. graphicum (Zogg 1962). One distinguishing feature that separates G. stellatum from G. circumserpens is that, although both are associated with subicula, in the former this extends as a wide margin in front of the developing hysterothecia (Fig. 12A–C), whereas in G. circumserpens (Fig. 12F–G) the subicula is only associated with the under surface of the hysterothecia, closely appressed to the substrate, with only minor deviations from the long axis of the fruitbody.
Key to the species of Glonium
1. Hysterothecia associated and seated upon a thin crust-like stroma, or arising from within a stromal crust; stroma itself seated on subiculum; didymospores spindle-shaped with the upper cell slightly swollen and larger than the lower cell, measuring 24–28 x 5–6 μm; Africa..................................................................................................... G. compactum 1. Hysterothecia not associated with stroma................................................................................................................................................ 2
2. Hysterothecia somewhat branched, irregular, “graphoid”; without well-developed subiculum; didymospores oblong to spindle-shaped; upper cell pear-shaped, constricted at septum; both ends acuminate, measuring (13–)15–18(–21) x (3–)5–6 μm; on Pinus, Juniperus, Europe........................................................................... G. graphicum 2. Hysterothecia in mature specimens highly bifurcated, closely appressed to the substrate, dichotomously branched to form irregular creeping masses; usually seated upon or sitting behind a front of well-developed brown to black subiculum................................................................................................................................. 3
3. Didymospores hyaline, constricted at the septum, apices pointed, measuring (15–)16–17 x 6–7 μm; on soil (terricolous) or rock (saxicolous), or lignicolous; Tasmania.............................................................................. G. circumserpens 3. Didymospores oblong to spindle-shaped; upper cell pear-shaped, constricted at septum; both ends acuminate, measuring (18–)21–26(–28) x (4–)5–6(–7) μm; cosmopolitan............................................................................................... G. stellatum
Four isolates were surveyed for the Gloniaceae. Two of G. stellatum, from Michigan (CBS 207.34) and Tennessee (ANM 32), the United States, and two of G. circumserpens, recently isolated from wood (CBS 123342 / BPI 878738) and dolerite stone (CBS 123343 / BPI 878739) from Tasmania (Table 1). Molecular data indicate that all four isolates are closely related. Surprisingly, this clade also includes multiple isolates of Cenococcum geophilum, an ecologically important ectomycorrhizal fungus with a global distribution and a wide host range, but with no known teleomorph (LoBuglio et al. 1996).
Farlowiella Sacc., Syll. Fung. 9: 1101. 1891.
= Farlowia Sacc., Syll. Fung. 2: 727. 1883.
Recent molecular data (Schoch et al. 2006; Boehm et al. 2009) support the transference of the genus Farlowiella from the Hysteriaceae, and its current placement as Pleosporomycetidae gen. incertae sedis. The genus is characterised by 1-celled pedicellate slightly laterally compressed amerospores, the upper cell pigmented and much larger than the lower, which remains hyaline or moderately pigmented, and can be considered as an associated papilla. The hysterothecia are somewhat laterally compressed, but nonetheless thick-walled and with a prominent sunken slit. They can be solitary to gregarious, but remain erect, and elevated, presenting an almost stipitate appearance. Anamorphs have been described in the genus Acrogenospora (Goh et al. 1998). Two species are recognised, namely Farlowiella carmichaeliana from Europe (Belgium, England, Germany, Switzerland), from the bark and wood of Fagus, Quercus, Sorbus and Prunus, and F. australis known only from the original collection on Phylica arborea from Tristan da Cunha in the South Atlantic (Dennis 1955). Sequence data from two isolates of F. carmichaeliana (CBS 206.36 and CBS 179.73) indicate that this taxon lies quite distant from the Hysteriaceae (Fig. 1). An additional isolate of the anamorph, Acrogenospora sphaerocephala (CBS 164.76), further supports the current placement of the genus Farlowiella.
Key to the species of Farlowiella
1. Ascospores unequally 2-celled; upper cell pigmented, much larger than the lower cell, which is smaller and hyaline, together measuring 18–21 x 7–12 μm; Europe............................................................................................................. F. carmichaeliana 1. Ascospores as above, but smaller, 13–15 x 6–7.5 μm; Tristan da Cunha............................................................................... F. australis
CONCLUSIONS
Hysteriaceous fungi are an ancient and ecologically successful group of organisms, as attested by their wide geographic distribution on a multitude of gymnosperm and angiosperm host species. Whereas the Mytilinidiaceae are found almost exclusively on conifers, the Hysteriaceae occur primarily on angiosperms (Zogg 1962). Presumably, the Hysteriaceae underwent rapid speciation in response to the angiosperm radiation of the mid- to late-Cretaceous, 65–100 mya (Palmer et al. 2004). However, this must have occurred prior to the complete loss of continental contiguity, which occurred during the same time period. This is because we see today a remarkable degree of intraspecific stability, in both morphology and sequence data, among geographically disparate accessions (Fig. 1). For example, little morphological or sequence variation was detected in Hysterium angustatum, from North America (CBS 123334), Kenya (GKM 243A), New Zealand (SMH 5211.0), and South Africa (CMW 20409; Lee & Crous 2003). Similarly, little variation was detected in Psiloglonium clavisporum, from Kenya (GKM L172A, GKM 344A) and North America (e.g., CBS 123338), or in Oedohysterium sinense, from South Africa (CBS 123345) and North America (EB 0339). As we are presumably sampling remnants of once contiguous sexual populations, their similarity today must imply that speciation occurred prior to complete genetic isolation. The break-up of Pangea during the Triassic 200 mya, and the formation of the nascent central Atlantic Ocean, separating Gondwana from Laurasia, during the Jurassic, 150 mya, must have effectively disrupted once contiguous populations. Although most flowering plant families were established by the end of the Cretaceous, 65–70 mya, it is now believed that they diversified into their present lineages (e.g., eudicots, Magnoliids and monocots) much earlier, around 140 mya (Davies et al. 2004, Palmer et al. 2004, Moore et al. 2007). This may have allowed for remnants of once contiguous populations to colonise early angiosperm lineages, prior to the complete dissolution of continental integrity during the mid- to late-Cretaceous. Recent studies (Lücking et al. 2009), based on a recalibration of published molecular clock trees, using internally unconstrained, uniform calibration points, have suggested an origin for the fungi between 760 mya to 1.0 bya, with the origin of the Ascomycota set at 500-650 mya. Whatever the timing, hysteriaceous fungi incurred little appreciable intraspecific morphological or genetic (e.g., nuLSU, nuSSU, TEF1 and RPB2) change over significant periods of geologic time, on different continents. Thus, with the exception of Hb. mori, and perhaps, Gp. subrugosa (see below), most members of the Hysteriaceae appear to be stable species.
Sequence data indicate that Hb. mori occurs in both Clades A and D. However, analysis of Hb. mori specimens originating from each clade (e.g., CBS 123563 / BPI 878731, and others, in Clade A versus GKM 1013 / BPI 879788 in Clade D), failed to find any appreciable difference in either spore morphology (e.g., septation, pigmentation, symmetry, or measurement), substrate-choice, or features associated with the hysterothecium. Likewise, no morphological difference could be detected among genetically unrelated accessions of Gp. subrugosa, from South Africa (CBS 123346 / BPI 878735), in Clade D, versus those from Kenya (GKM 1214 / BPI 879776) and Cuba (SMH 557 / BPI 879777), outside of Clade D. These two examples illustrate a lack of correspondence between the morphospecies concept (Burnett 2003) and the genealogical concordance phylogenetic species recognition concept (Taylor et al. 2000), the latter indicating here the presence of cryptic species within the two morphospecies. Hysterobrevium mori and, to a lesser extent, Gp. subrugosa, may represent examples of convergent evolution, whereby similar ascospores borne in hysterothecia have evolved multiple times within the family. This is supported by the polyphyly inherent in the circumspection of the classical genera of the Hysteriaceae (e.g., Gloniopsis, Glonium, Hysterium, and Hysterographium), revealed by recent studies (Schoch et al. 2006, Boehm et al. 2009, Mugambi & Huhndorf 2009). Alternatively, Hb. mori and Gp. subrugosa may have retained ancestral character states, and thus may represent evolutionary lineages that did not incur appreciable morphological change, while at the same time accumulating sufficient genetic change to fall, in the case of Hb. mori, into distant clades within the family. If this is the case, then these two taxa may represent examples of speciation in progress, with genetic change preceding morphological change, thus differing from independent convergent character states. Whatever the mechanism, it is difficult to see how Hb. mori, for example, may be classified into different species, in different genera (e.g., Hysterobrevium and Oedohysterium), without a sound morphological basis. We conclude that both Hb. mori and Gp. subrugosa contain genetically unrelated, cryptic, and potentially different biological species, that can not at present be morphologically differentiated.
Although there are examples of concordance between morphological and molecular data in this study (see below), these are few. For the most part, molecular data support the premise of a large number of convergent evolutionary lineages, sharing similar spore morphologies, but that are not closely related. This resulted in a polyphyletic core set of genera for the Hysteriaceae, and presented us with a complicated picture of past speciation events within the family (Boehm et al. 2009). To achieve a natural phylogeny, that is, one based on the concordance of morphological and molecular data, required that we break-up what were once thought to be stable genera. Thus, two species of Hysterium were transferred to Oedohysterium (Od. insidens and Od. sinense), and two species of Gloniopsis to Hysterobrevium (Hb. smilacis and Hb. constrictum). While Hysterographium, with the type Hg. fraxini, was removed from the Hysteriaceae (Boehm et al. 2009), some of its species remained within the family, transferred here to Oedohysterium (Od. pulchrum), Hysterobrevium (Hb. mori) and Gloniopsis (Gp. subrugosa). New species were described (e.g., Gp. arciformis and Gp. kenyensis) which would previously have been classified in Hysterographium, but are now accommodated in Gloniopsis. Molecular data necessitated that both Gloniopsis and Hysterobrevium include hyaline and pigmented dictyospores, and the genus Oedohysterium, both phragmospores and dictyospores. This, then, de-emphasised spore morphology as a synapomorphic character state. Likewise, the genus Glonium sensu Zogg (1962) was divided into Psiloglonium in the Hysteriaceae and Glonium in the Gloniaceae (Boehm et al. 2009), and, more recently, Anteaglonium in the Pleosporales (Mugambi & Huhndorf 2009). These taxonomic changes were unexpected, as they were not premised on past assumptions of synapomorphy related to spore morphology (Zogg 1962). Although we have included here a total of 59 accessions, representing 22 species in seven genera, for the Hysteriaceae, and another 62 outside of the family (Table 1), taxon sampling may still be insufficient. Clearly, additional species and genera need to be sampled before a complete picture emerges for the family.
The quest for synapomorphic character states that correlate with molecular data was one of the goals of this study. If traditional character states associated with spore septation/pigmentation or the fruitbody (Zogg 1962) can not be relied upon to deduce phylogeny, are there other character states that can be emphasised instead? Two examples are discussed below, the first relating to spore morphology, the second to characters associated with the fruitbody. Although both Oedohysterium and Hysterium possess similar pigmented asymmetric phragmospores, species of Oedohysterium can be morphologically differentiated by the possession of an enlarged supra-median cell. Molecular data also revealed that a species previously classified as Hysterographium, namely Hg. pulchrum, belonged to Oedohysterium, despite the presence of dictyospores. Closer inspection, however, reveals that the dictyospores of Od. pulchrum also possess a swollen supra-median cell. Additionally, a certain number of spores remain as transversely septate phragmospores (Checa et al. 2007), thus reinforcing its placement within Oedohysterium, and perhaps underscoring the plasticity of spore septation configurations for this group of fungi.
The second example relates to character states associated with the fruitbody. Fruitbody morphology clearly supports the transfer of the genus Glonium out of the Hysteriaceae to its own family, the Gloniaceae, closely allied with the Mytilinidiales. The Gloniaceae possess a modified hysterothecium, one in which the frutibodies frequently bifurcate to a greater (e.g., G. stellatum and G. circumserpens) or lesser (e.g., G. graphicum) degree, the former two species with radiating stellate composites, usually seated on subicula. This is in contrast to hysterothecia found in the Hysteriaceae which may be gregarious, but are never laterally anastomosed to form radiating composites. Additionally, the morphology of the dehiscence slit found in the Gloniaceae is unlike that found in the Hysteriaceae. In the Gloniaceae, the aperture is in most cases evaginated, forming a miniscule crest, similar to the more extended version found in some species in the Mytilinidiaceae; whereas, in the Hysteriaceae, hysterothecia have deeply invaginated slits. Also, hysterothecia found in the Gloniaceae, like those in the Mytilinidiaceae, are considerably more fragile, as compared to those found within the Hysteriaceae. These character states were either not noted before (e.g., swollen supra-median cell in Oedohysterium and evaginated slit in Glonium), or were noticed, but given less taxonomic weight (e.g., modified hysterothecium in Glonium; Zogg 1962). These examples illustrate that morphological features can be found that correlate with molecular data, despite the anomalies presented by Hb. mori and Gp. subrugosa, mentioned earlier.
The hysterothecium, thick-walled, navicular, and with a prominent longitudinal slit, has long been considered synapomorphic, defining the Hysteriales. However, this type of fruitbody has evolved convergently no less than five times within the Pleosporomycetidae (e.g., Farlowiella, Glonium, Anteaglonium, Hysterographium and the Hysteriaceae). Similarly, thin-walled mytilinidioid (e.g., Ostreichnion) and patellarioid (e.g., Rhytidhysteron) ascomata have also evolved at least twice within the subclass, the genera having been transferred from the Mytilinidiaceae and Patellariaceae, respectively, to the Hysteriaceae. As such, character states relating not only to the external features of the ascoma, but to the centrum as well (e.g., cellular pseudoparaphyses versus trabeculae, etc.), previously considered to represent synapomorphies among these fungi, in fact, represent symplesiomorphies, and most likely have arisen multiple times through convergent evolutionary processes in response to common selective pressures. Similar findings have emerged for a number of other ascomycete lineages within the Pezizomycotina (e.g., Schoch et al. 2009b). One selective advantage of the hysterothecium may be spore discharge over prolonged periods of time, since some, if not most, species may be perennial (Lohman 1931, 1933a). The thick-walled peridium further contributes to xerotolerance, as many of these fungi persist on decorticated, weathered woody substrates prone to prolonged periods of desiccation. Thus, the ability to perennate, and time spore discharge with environmental conditions suitable for germination, spanning multiple seasons, may be the driving force behind the repeated evolution of the hysterothecium.
Acknowledgments
The authors wish to thank Alain Gardiennet (Veronnes, France), Gintaras Kantvilas (Tasmanian Herbarium, Hobart, Tasmania), Marieka Gryzenhout (Dept. Microbiology and Plant Pathology, Forestry and Agricultural Biotechnology Institute, University of Pretoria, South Africa), Maria Inéz Messuti and Laura Emma Lorenzo (Departamento de Botanica, Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, Quintral, Bariloche, Rio Negro, Argentina), Eunice Nkansah (Kean University, Union, NJ, U.S.A.), and Meredith Blackwell (Dept. Biological Sciences, Louisiana State University, Baton Rouge, Louisiana, U.S.A.) for kindly supplying some of the isolates used in this study (Table 1). The authors wish to thank Walter Gams (Baarn, The Netherlands) for the Latin translations, and for his numerous helpful insights into the taxonomic and nomenclatural issues raised by this work. We also wish to acknowledge Scott Redhead (National Mycological Herbarium, Agriculture and Agri-Food Canada, Ottawa, Canada) who provided helpful suggestions on the manuscript prior to submission. E.W.A. Boehm wishes to acknowledge support from the National Science Foundation (NSF) Major Research Instrumentation Grant DBI 0922603. A.N. Miller acknowledges funding from the NSF through a BS&I award (DEB0515558) and from Discover Life in America (DLIA 2005-15). Part of this work was also funded by a grant from NSF (DEB-0717476) to J.W. Spatafora (and C.L. Schoch until 2008). Work performed by C.L. Schoch after 2008 was supported in part by the Intramural Research Program of the NIH, National Library of Medicine.
Taxonomic novelties: New species: Gloniopsis arciformis E.W.A. Boehm, G.K. Mugambi, S.M. Huhndorf & C.L. Schoch, Gp. kenyensis E.W.A. Boehm, G.K. Mugambi, S.M. Huhndorf & C.L. Schoch, Hysterium barrianum E.W.A. Boehm, A.N. Miller, G.K. Mugambi, S.M. Huhndorf & C.L. Schoch. New genera: Hysterobrevium E.W.A. Boehm & C.L. Schoch, Oedohysterium E.W.A. Boehm & C.L. Schoch. New combinations: Gloniopsis subrugosa (Cooke & Ellis) E.W.A. Boehm & C.L. Schoch, Hysterobrevium constrictum (N. Amano) E.W.A. Boehm & C.L. Schoch, Hb. mori (Schwein.) E.W.A. Boehm & C.L. Schoch, Hb. smilacis (Schwein.) E.W.A. Boehm & C.L. Schoch, Oedohysterium insidens (Schwein.) E.W.A. Boehm & C.L. Schoch, Od. pulchrum (Checa, Shoemaker & Umaña) E.W.A. Boehm & C.L. Schoch, Od. sinense (Teng) E.W.A. Boehm & C.L. Schoch, Psiloglonium araucanum (Speg.) E.W.A. Boehm, S. Marincowitz & C.L. Schoch, P. chambianum (Guyot) E.W.A. Boehm & C.L. Schoch, P. colihuae (Lorenzo & Messuti) E.W.A. Boehm & C.L. Schoch, P. ephedrae (Henn.) E.W.A. Boehm & C.L. Schoch, P. hysterinum (Rehm) E.W.A. Boehm & C.L. Schoch, P. pusillum (H. Zogg) E.W.A. Boehm & C.L. Schoch, P. sasicola (N. Amano) E.W.A. Boehm & C.L. Schoch, and P. uspallatense (Speg.) E.W.A. Boehm & C.L. Schoch.
References
- Amano N (1983). Saprobic loculoascomycetous fungi from Japan 1. Hysteriaceous fungi. Transactions of the Mycological Society of Japan 24: 283–297. [Google Scholar]
- Arx JA von, Müller E (1954). Die Gattungen der amerosporen Pyrenomyceten. Beiträge zur Kryptogamenflora der Schweiz 11: 1–434. [Google Scholar]
- Arx JA von, Müller E (1975). A re-evaluation of the bitunicate Ascomycetes with keys to families and genera. Studies in Mycology 9: 1–159. [Google Scholar]
- Barr ME (1975). The genus Ostreichnion. Mycotaxon 3: 81–88. [Google Scholar]
- Barr ME (1979). A classification of loculoascomycetes. Mycologia 71: 935–957. [Google Scholar]
- Barr ME (1983). The ascomycete connection. Mycologia 75: 1–13. [Google Scholar]
- Barr ME (1987). Prodromus to class Loculoascomycetes. Hamilton I. Newell, Inc., Amherst, Massachusetts: published by the author.
- Barr ME (1990a). Melanommatales (Loculoascomycetes). North American Flora, Series II, Part 13: 1–129. [Google Scholar]
- Barr ME (1990b). Some dictyosporous genera and species of Pleosporales in North America. Memoirs of the New York Botanical Garden 62: 1–92. [Google Scholar]
- Barr ME (2009). A Nomenclator of Loculoascomycetous Fungi from the Pacific Northwest. North American Fungi 4: 1–94. [Google Scholar]
- Barr ME, Blackwell M (1980). A new genus in the Lophiaceae. Mycologia 72: 1224–1227. [Google Scholar]
- Barr ME, Huhndorf SM (2001). Loculoascomycetes. Chapter 13, In: The Mycota, Systematics and Evolution, Part A. VII. (McLaughlin DJ, McLaughlin EG, Lemke PA, eds). Springer: 283–305.
- Bezerra JL, Kimbrough JW (1982). Culture and cytological development of Rhytidhysterium rufulum on citrus. Canadian Journal of Botany 60: 568–579. [Google Scholar]
- Bisby GR (1923). The literature on the classification of the Hysteriales. Transactions of the British Mycological Society 8: 176–189. [Google Scholar]
- Bisby GR (1932). Type specimens of certain Hysteriales. Mycologia 24: 304–329. [Google Scholar]
- Bisby GR (1941). British species of Hysterium, Gloniopsis, Dichaena and Mytilidion. Transactions of the British Mycological Society 25: 127–140. [Google Scholar]
- Blackwell M, Gilbertson RL (1985). Quasiconcha reticulata and its anamorph from conifer roots. Mycologia 77: 50–54. [Google Scholar]
- Boehm EWA, Schoch CL, Spatafora JW (2009). On the evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycological Research 113: 461–479. [DOI] [PubMed] [Google Scholar]
- Burnett J (2003). Fungal Populations and Species. Oxford University Press, Oxford, U.K.
- Cash E (1939). Two species of Hysteriales on Smilax. Mycologia 31: 289–296. [Google Scholar]
- Checa J, Shoemaker RA, Umaña L (2007). Some new hysteriaceous fungi from Costa Rica. Mycologia 99: 285–290. [DOI] [PubMed] [Google Scholar]
- Chevallier FF (1826). Flore générale des environs de Paris, Vol I. Ferra Librairie-Editeur, Paris, France.
- Chlebicki A, Knudsen H (2001). Dryadicolous microfungi from Greenland. I. List of species. Acta Societatis Botanicorum Poloniae 70: 291–301. [Google Scholar]
- Clements FE (1909). The Genera of Fungi. HW Wilson Co. Publ., Minneapolis, MN, U.S.A.
- Corda ACJ (1842). Abbildungen der Pilze und Schwaemme. Icones Fungorum, Hucusque Cognitorum 5: 34. [Google Scholar]
- Darker GD (1963). A new genus of the Lophiaceae. Canadian Journal of Botany 41: 1383–1388. [Google Scholar]
- Davies JT, Barraclough TG, Chase MW, Soltis PS, Soltis DE, Savolainen V (2004). Darwin's abominable mystery: Insights from a supertree of the angiosperms. Proceedings of the National Academy of Sciences 101: 1904–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Notaris CG (1847). Prime linee di una nuova disposizione dei Pirenomiceti Isterini. Giornale Botanico Italiano 2, part I, fasc. 7–8: 5–52. [Google Scholar]
- Dennis RWG (1955). Ascomycetes from Tristan da Cunha. Results of the Norwegian Scientific Expedition to Tristan da Cunha (1937-1938) 36-38: 1–10. [Google Scholar]
- Diederich P, Wedin M (2000). The species of Hemigrapha (lichenicolous Ascomycetes, Dothideales) on Peltigerales. Nordic Journal of Botany 20: 203–214. [Google Scholar]
- Duby JE (1862). Mémoire sur la tribu des Hystérinées de la famille des Hypoxylées (Pyrénomycètes). Mémoires de la Société de Physique et Histoire Naturelle de Genève 16: 15–70. [Google Scholar]
- Ellis JB, Everhart BM (1892). The North American Pyrenomycetes. Newfield NJ, U.S.A.
- Eriksson OE (2006). Outline of Ascomycota. Myconet 12: 1–88. [Google Scholar]
- Fries EM (1823). Systema Mycologicum, sistens fungorum ordines, genera et species hucusque cognitas, II, pars II: 276–620.
- Fries EM (1835). Corpus florarum provincialium Sueciae. I. Floram scanicam scripsit Elias Fries. Uppsala.
- Gäumann EA (1949). Die Pilze, Grundzüge ihrer Entwicklungsgeschichte und Morphologie. Birkhäuser. Basel.
- Geiser DM, Gueidan C, Miadlikowska J, Lutzoni F, Kauff F, et al. (2006). Eurotiomycetes: Eurotiomycetidae and Chaetothyriomycetidae. Mycologia 98: 1053–1064. [DOI] [PubMed] [Google Scholar]
- Goh TK, Hyde KD, Tsui KM (1998). The hyphomycete genus Acrogenospora, with two new species and two new combinations. Mycological Research 102: 1309–1315. [Google Scholar]
- Hall, T. 2004. Bioedit v7.0.1. Isis Pharmaceuticals, U.S.A.
- Hawksworth DL, Eriksson OE (1988). Proposals to conserve 11 family names in the Ascomycotina (Fungi). Taxon 37: 190–193. [Google Scholar]
- Höhnel F. von (1918). Mycologische Fragmente, 272. Über die Hysteriaceen. Annales Mycologici 16: 145–154. [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Kantvilas G, Coppins BJ (1997). Melaspilea circumserpens Nyl. rediscovered and referred to Glonium, with discussion on the provenance of some of Robert Brown's lichen specimens. Lichenologist 29: 525–531. [Google Scholar]
- Katoh K, Asimenos G, Toh H (2009). Multiple alignment of DNA sequences with MAFFT. Methods in Molecular Biology 537: 39–64. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Canon PF, David JC, Stalpers JA (2001). Dictionary of the Fungi. 9th Ed. CAB International, U.K.
- Kirschstein W (1924). Beiträge zur Kenntnis der Ascomyceten. Verhandlungen des Botanischen Vereins der Provinz Brandenburg 66: 23–29. [Google Scholar]
- Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, et al. (2006). The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 98: 571–583. [DOI] [PubMed] [Google Scholar]
- Kutorga E, Hawksworth DL (1997). A reassessment of the genera referred to the family Patellariaceae (Ascomycota). Systema Ascomycetum 15: 1–110. [Google Scholar]
- Lee S, Crous PW (2003). Taxonomy and biodiversity of hysteriaceous ascomycetes in fynbos. South African Journal of Botany 69: 480–488. [Google Scholar]
- Lindau G (1897). Hysteriineae. In: Engler & Prantl, Naturliche Pflanzenfamilien. I. Teil, I. Abteilung. 1: 265-278. [Google Scholar]
- Linde EJ van der (1992). Notes on the South African Hysteriaceae (Ascomyctes: Mycotina). South African Journal of Botany 58: 491–499. [Google Scholar]
- Lindemuth R, Wirtz N, Lumbsch HT (2001). Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that loculoascomycetes (Ascomycota) are not monophyletic. Mycological Research 105: 1176–1181. [Google Scholar]
- Liu K, Raghavan S, Nelesen S, Linder CR, Warnow T (2009). Rapid and accurate large-scale coestimation of sequence alignments and phylogenetic trees. Science 324: 1561–1564. [DOI] [PubMed] [Google Scholar]
- LoBuglio KF, Berbee ML, Taylor JW (1996). Phylogenetic origins of the asexual mycorrhizal symbiont Cenococcum geophilum Fr. and other mycorrhizal fungi among the ascomycetes. Molecular Phylogenetics and Evolution 6: 287–94. [DOI] [PubMed] [Google Scholar]
- Lohman ML (1931). A study of Glonium parvulum in culture. Papers of the Michigan Academy of Science Arts & Letters 13: 141–156. [Google Scholar]
- Lohman ML (1932a). The comparative morphology of germinating ascospores in certain species of the Hysteriaceae. Papers of the Michigan Academy of Science Arts & Letters 15: 97–111. [Google Scholar]
- Lohman ML (1932b). Three new species of Mytilidion in the proposed subgenus Lophiopsis. Mycologia 24: 477–484. [Google Scholar]
- Lohman ML (1933a). Hysteriaceae: Life histories of certain species. Papers of the Michigan Academy of Science Arts & Letters 17: 229–288. [Google Scholar]
- Lohman ML (1933b). Septonema toruloideum: A stage of Mytilidion scolecosporum. Mycologia 25: 34–43. [Google Scholar]
- Lohman ML (1934). A cultural and taxonomic study of Hysterium hyalinum. Papers of the Michigan Academy of Science Arts & Letters 19: 133–140. [Google Scholar]
- Lohman ML (1937). Studies in the genus Glonium as represented in the Southeast. Bulletin of the Torrey Botanical Club 64: 57–73. [Google Scholar]
- Lorenzo LE, Messuti MI (1998). Noteworthy Hysteriaceae from southern South America. Mycological Research 102: 1101–1107. [Google Scholar]
- Lücking R, Stuart BL, Lumbsch HT (2004). Phylogenetic relationships of Gomphillaceae and Asterothyriaceae: evidence from a combined Bayesian analysis of nuclear and mitochondrial sequences. Mycologia 96: 283–294. [DOI] [PubMed] [Google Scholar]
- Lücking R, Huhndorf S, Pfister DH, Plata ER, Lumbsch HT (2009). Fungi evolved right on track. Mycologia 101: 810–822. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Huhndorf SM (2007). Outline of the Ascomycota. Myconet 13: 1–58. [Google Scholar]
- Lumbsch HT, Schmitt I, Lindemuth R, Miller A, Mangold A, Fernando F, Huhndorf S (2005). Performance of four ribosomal DNA regions to infer higher-level phylogenetic relationships of inoperculate euascomycetes (Leotiomyceta). Molecular Phylogenetics and Evolution 34: 512–524. [DOI] [PubMed] [Google Scholar]
- Luttrell ES (1951). Taxonomy of the Pyrenomycetes. University of Missouri Studies in Science 24: 1–120. [Google Scholar]
- Luttrell ES (1953). Development of the ascocarp in Glonium stellatum. American Journal of Botany 40: 626–633. [Google Scholar]
- Luttrell ES (1955). The ascostromatic ascomycetes. Mycologia 47: 511–532. [Google Scholar]
- Luttrell ES (1973). Loculoascomycetes. In: The Fungi: an advanced treatise. IVA. Ainsworth GC, Sparrow FK, Sussman AS, eds. London, Academic Press: 135–219.
- Lutzoni F, Kauff F, Cox CJ, McLaughlin DJ, Celio G, et al. (2004). Assembling the fungal tree of life: Progress, classification, and evlutiion of subcellular traits. American Journal of Botany 91: 1446–1480. [DOI] [PubMed] [Google Scholar]
- Magnes M (1997). Weltmonographie der Triblidiaceae. Bibliotheca Mycologica 165: 127–130. [Google Scholar]
- Massee G (1895). British Fungus Flora, A Classified Textbook of Mycology, Vol IV. George Bell & Sons, London & New York, U.S.A.
- Massee G (1901). Fungi exotici III. Royal Botanic Gardens, Kew, Bulletin No.175– 177: 150–169. [Google Scholar]
- Messuti MI, Lorenzo LE (1997). A new species of Hysterium from Patagonia, Argentina. Mycological Research 101: 302–304. [Google Scholar]
- Messuti MI, Lorenzo LE (2003). Notes on the genus Hysterographium (Ascomycota, Hysteriaceae) in southern South America. Nova Hedwigia 76: 451–458. [Google Scholar]
- Messuti MI, Lorenzo LE (2007). Taxonomy of Glonium (Hysteriales, Ascomycota) in southern Argentina and Chile. Nova Hedwigia 84: 521–528. [Google Scholar]
- Miller JH (1949). A revision of the classification of the Ascomycetes with special emphasis on the Pyrenomycetes. Mycologia 41: 99–127. [Google Scholar]
- Moncalvo JM, Rehner SA, Vilgalys R (1993). Systematics of Lyophyllum section Difformia based on evidence from culture studies and ribosomal DNA sequences. Mycologia 85: 788–794. [Google Scholar]
- Moore MJ, Bell CD, Soltis PS, Soltis DE. 2007. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proceedings of the National Academy of Sciences, U.S.A. 104: 19363–19368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugambi, G.K. and S.M. Huhndorf. 2009. Parallel evolution of hysterothecial ascomata in ascolocularous fungi (Ascomycota, Fungi). Systematics and Biodiversity 7: 453–464. [Google Scholar]
- Müller E, von Arx JA (1950). Einige Aspekte zur Systematik pseudosphärialer Ascomyceten. Berichte der Schweizerischen Botanischen Gesellschaft 60: 329–397. [Google Scholar]
- Nannfeldt JA (1932). Studien über die Morphologie und Systematik der nicht-lichenisierten, inoperkulaten Discomyceten. Nova Acta Regiae Societatis Scientiarum Uppsaliensis IV, 8: 1–368. [Google Scholar]
- Palmer JD, Soltis DE, Chase MW. 2004. The plant tree of life: An overview and some points of view. American Journal of Botany 91: 1437–1445. [DOI] [PubMed] [Google Scholar]
- Pande A, Rao VG (1991). On three hysteriaceous fungi from peninsular India. Geobios new Reports 10: 62–64. [Google Scholar]
- Petrak F (1923a). Mykologische Notizen VI. No. 226. Über die Gattung Glonium Muhl. Annales Mycologici 21: 225–227. [Google Scholar]
- Petrak F (1923b). Mykologische Notizen VI, Nr. 284. Psiloglonium finkii nov. sp. Annales Mycologici 21: 308–309. [Google Scholar]
- Rehm H (1896). Ascomyceten: Hysteriaceen und Discomyceten, In: L. Rabenhorst's Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. 2nd Ed, Eduard Kummer, Leipzig 3: 1–56. [Google Scholar]
- Rehm H (1898). Beiträge zur Pilzflora von Südamerika. V. Hysteriaceae. Hedwigia 37: 296–302. [Google Scholar]
- Rehner SA, Samuels GJ (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. [Google Scholar]
- Reid J, Pirozynski KA (1966). A new loculoascomycete on Abies balsamea (L.) Mill. Canadian Journal of Botany 44: 351–354. [Google Scholar]
- Renobales G, Aguirre B (1990). The nomenclature and systematic position of the genus Encephalographa. Systema Ascomycetum 8: 87–92. [Google Scholar]
- Rogers DP (1953). Disposition of nomina generica conservanda for fungi. Taxon 2: 29–32. [Google Scholar]
- Saccardo PA (1873). Mycologiae Venetae Specimen. Atti della Società Veneto-Trentina di Scienze Naturali Padova 2: 53–264. [Google Scholar]
- Saccardo PA (1875). Conspectus generum pyrenomycetum italicorum additis speciebus fungorum Venetorum novis vel criticis, systemate carpologico dispositorum. Atti della Società Veneto-Trentina di Scienze Naturali Padova 4: 77–100. [Google Scholar]
- Saccardo PA (1883). Sylloge Fungorum. 2: 1–815. Patavii, Italy. [Google Scholar]
- Samuels GJ, Müller E (1979). Life-history studies of Brazilian Ascomycetes. 7. Rhytidhysteron rufulum and the genus Eutryblidiella. Sydowia 32: 277–292. [Google Scholar]
- Schmitt I, Mueller G, Lumbsch HT (2005). Ascoma morphology is homoplaseous and phylogenetically misleading in some pyrenocarpous lichens. Mycologia 97: 362–374. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. (2009a). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Sung G-H, López-Giráldez F, Townsend JP, Miadlikowska J, et al. (2009b). The Ascomycota Tree of Life: A phylum wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Sung GH, Volkmann-Kohlmeyer B, Kohlmeyer J, Spatafora JW (2007). Marine fungal lineages in the Hypocreomycetidae. Mycological Research 111: 154–162. [DOI] [PubMed] [Google Scholar]
- Shoemaker RA, Babcock CE (1992). Applanodictyosporus Pleosporales: Clathrospora, Comoclathris, Graphillium, Macrospora, and Platysporoides. Canadian Journal of Botany 70: 1617–1658. [Google Scholar]
- Sivanesan A, Hsieh WH (1989). New species and new records of ascomycetes from Taiwan. Mycological Research 93: 340–351. [Google Scholar]
- Spatafora JW, Johnson D, Sung G-H, Hosaka K, O'Rourke B, et al. (2006). A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia 98: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Speer EO (1986). A propos de champignons du Brésil III. Mytilidion resinae sp. nov. (Hysteriales) et sa forme conidienne, Camaroglobulus resinae gen. et spec. nov. (Sphaeropsidales). Bulletin Trimestriel de la Société de Mycologie de France 102: 97–100. [Google Scholar]
- Stamatakis A (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–90. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J (2008). A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Steinke TD, Hyde KD (1997). Gloniella clavatispora, sp. nov. from Avicennia marina in South Africa. Mycoscience 38: 7–9. [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, et al. (2000). Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32. [DOI] [PubMed] [Google Scholar]
- Teng SC (1933). Notes on Hysteriales from China. Sinensia 4: 129–144. [Google Scholar]
- Tilak ST, Kale SB (1968). Contribution to the genus Ostreola. Indian Phytopathology 21: 289–293. [Google Scholar]
- Tretiach M, Modenesi P (1999). Critical notes on the lichen genus Encephalographa A. Massal. (?Hysteriaceae). Nova Hedwigia 68: 527–544. [Google Scholar]
- Vasilyeva LN (1999a). Hysteriaceous fungi in the Russian Far East I. Hysterium. Mikologiya i Fitopatologiya 33: 225–227. [Google Scholar]
- Vasilyeva LN (1999b). Hysteriaceous fungi in the Russian Far East II. The genus Hysterographium. Mikologiya i Fitopatologiya 33: 297–301. [Google Scholar]
- Vasilyeva LN (2000). Hysteriaceous fungi in the Russian Far East III. Glonium and Actidiographium. Mikologiya i Fitopatologiya 34: 3–5. [Google Scholar]
- Vasilyeva LN (2001). Hysteriaceous fungi in the Russian Far East IV. Glyphium, Lophium and Mytilinidion. Mikologiya i Fitopatologiya 35: 15–18. [Google Scholar]
- Vilgalys R, Hester M (1990). Rapid identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernersson R, Pedersen AG (2003). RevTrans: Multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Research 31: 3537–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WhiteTJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A guide to methods and applications. (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds.). Academic Press, New York: 315–322.
- Zogg H (1949). Beiträge zur Kenntnis der brasilianischen Hysteriaceen. Berichte der Schweizerischen botanischen Gesellschaft 59: 39–42. [Google Scholar]
- Zogg H (1962). Die Hysteriaceae s. str. und Lophiaceae, unter besonderer Berücksichtigung der mitteleuropäischen Formen. Beiträge zur Kryptogamenflora der Schweiz, Band 11: 1–190. [Google Scholar]