Abstract
The classification of Pleosporales has posed major challenges due to the lack of clear understanding of the importance of the morphological characters used to distinguish between different groups in the order. This has resulted in varied taxonomic treatments of many families in the group including Melanommataceae and Lophiostomataceae. In this study we employ two nuclear DNA gene markers, nuclear ribosomal large subunit DNA and translation elongation factor 1-alpha in order to examine the molecular phylogenetics of Pleosporales with strong emphasis on the families Melanommataceae and Lophiostomataceae. Phylogenetic analyses recovered Melanommataceae, Lophiostomataceae, Hypsostromataceae, and a few others as strongly supported clades within the Pleosporales. Melanommataceae as currently circumscribed was found to be polyphyletic. The genera Byssosphaeria, Melanomma, and Pseudotrichia were recovered within the family, while others such as Ostropella and Xenolophium nested outside in a weakly supported group along with Platystomum compressum and Pseudotrichia guatopoensis that may correspond to the family Platystomaceae. The genus Byssosphaeria was recovered as a strongly supported group within the Melanommataceae while Melanomma was weakly supported with unclear relationships among the species. The genera Herpotrichia and Bertiella were also found to belong in the Melanommataceae. Lophiostomataceae occurs as a strongly supported group but its concept is here expanded to include a new genus Misturatosphaeria that bears morphology traditionally not known to occur in the family. The strongly supported clade of Misturatosphaeria contains nine species that have gregarious, papillate ascomata with lighter coloured apices and plugged ostioles and that vary in ascospore morphology from 1- to 3-septate to muriform. Along with a strongly supported Lophiostoma clade, also within the family are Thyridaria macrostomoides based on new sequences from Kenyan collections and Massariosphaeria triseptata, M. grandispora, Westerdykella cylindrica and Preussia terricola based on GenBank sequences. The family Hypsostromataceae was recovered as a strongly supported monophyletic group nested within the Pleosporales.
Keywords: Eumycota, evolution, fungi, Hypsostromataceae, phylogeny, taxonomy
INTRODUCTION
Pleosporales is one of the largest orders of loculoascomycetous fungi and includes a complex array of organisms (Schoch et al. 2009, Zhang et al. 2009). Consequently, Barr (1987) considered arrangement of the genera and families to be far from satisfactory and work continues to this day to try to clarify the relationships. Luttrell (1955) included seven families and Barr (1987) recognised 18 families in her revised concept of the group. Presently it contains 20 families encompassing roughly 167 genera (Lumbsch & Huhndorf 2007). In synonymy with Pleosporales is the order Melanommatales, created by Barr (1983) for taxa that had a combination of centrum (peripherally occurring asci) and hamathecium (trabeculate pseudoparaphyses) characters she believed were important at the ordinal level. Recent molecular phylogenetic studies (e.g., Berbee 1996, Liew et al. 2000, Winka 2000, Lumbsch & Lindemuth 2001, del Prado et al. 2005, Schoch et al. 2006, Kruys et al. 2006, Wang et al. 2007) have not supported the separation of Melanommatales from Pleosporales.
Although the concept of Pleosporales has recently attained some consensus (e.g. Winka 2000, Lumbsch & Lindemuth 2001, Kruys et al. 2006, Schoch et al. 2006, Lumbsch & Huhndorf 2007, Kirk et al. 2008, Zhang et al. 2008), many authors have differed on the circumscription of the families therein (e.g. Chesters & Bell 1970, Holm & Holm 1988, Barr 1984, 1987, 1990a, b, Lumbsch & Huhndorf 2007, Kirk et al. 2008). In Melanommataceae, Barr (1990a) accepted five genera, Kirk et al. (2008) accepted 21 genera, while Lumbsch & Huhndorf (2007) accept 18 genera with six of questionable placement. The taxonomy of Lophiostomataceae, another family in Pleosporales, has followed a similar path with Barr (1987) recognising six genera, Holm & Holm (1988) five genera, while Kirk et al., (2008) treated 15 genera and Lumbsch & Huhndorf (2007) 12 genera in the family.
Barr's (1990a) treatment of Melanommataceae included the following genera: Ostropella, Keissleriella, Strickeria, Byssosphaeria and Melanomma united on the basis of similar erumpent to superficial ascomata with walls composed of small, thick-walled cells. Byssosphaeria was re-instated by Barr (1984) for species that are separable from Herpotrichia, where it had been in synonymy for many years. The classification of Byssosphaeria, Herpotrichia and Pseudotrichia has posed major challenges to many authors because the morphological characters used to distinguish between the genera are not necessarily obvious (Samuels & Müller 1978). This has resulted in varied taxonomic treatments of the groups (e.g. Bose 1961, Samuels 1973, Samuels & Müller 1978, Barr 1984). Detailed taxonomic revision on the genera is offered by Bose (1961) and Barr (1984). The two studies provide detailed morphological characters distinguishing the genera. Pseudotrichia differs from Herpotrichia in its rather large ascomata, often with compressed apices, while in Herpotrichia smaller ascomata are often covered with long flexuous hyphae and with subiculum that sometimes overgrows the fruiting bodies (Bose 1961, Barr 1984). Byssosphaeria on the other hand possesses superficial ascomata that are turbinate with a rounded pore and apical area that is usually light coloured (Barr, 1984). Barr (1984) initially segregated Herpotrichia into Massarinaceae but later transferred it to Lophiostomataceae (Barr 1987). Bose's (1961) study on Massarina and related genera concluded that Massarina, Herpotrichia and Keissleriella are distinct but closely related, and he placed them in the Pleosporaceae citing lack of striking characters to justify the creation of a new family. Pseudotrichia was described for ascomycete fungi with immersed-erumpent to superficial ascomata with rounded or laterally compressed apex. On the basis of the shape of the apex, Petrak (1940) placed it in Lophiostomataceae. However, Barr (1990a) accepted the genus in Platystomaceae and noted substantial variability in ascomatal apical morphology. She observed that even in a single collection it could be rounded or compressed to somewhat triangular.
The genus Ostropella was originally named as a subgenus of Ostropa for O. albocincta and was raised to generic status and redescribed by von Höhnel (1918). Müller & von Arx (1962) reduced Schizostoma, Xenolophium and Ostreionella all to synonymy under Ostropella. Barr (1990a) provided the history of Ostropella and its relationship to these other taxa. Holm & Yue (1988) presented work on Schizostoma in which they tried to clarify some misconceptions that have surrounded the genus since its establishment and to resolve the placement of some species. Chesters & Bell (1970) reduced Xenolophium into synonymy under Lophiostoma and treated it in their “L. pachythele group”. Barr (1990a) pointed to the close relationship of Xenolophium to Ostropella and proposed it be accommodated under Ostropella. She also noted the differences between the low apical crest observed in Ostropella (that appears almost ornamental) from the compressed papilla in Lophiostoma and thus placed the genus in Melanommataceae. Xenolophium was established for two species of fungi from Hawaii by Sydow (in Stevens 1925). However, the genus was in synonymy for a long time until resurrected by Huhndorf (1993) who also added two new species to the group. She treated Ostropella and Xenolophium in Melanommataceae and distinguished the two genera based on their ascomatal surface structure, morphology of the ascomatal wall in longitudinal section and ascospore morphology.
Lophiostomataceae was first erected by Nitschke (1869) and in its long existence the group has greatly increased in size. The overall character that has historically been used to distinguish the family is the slit-like ostiolar opening on a laterally compressed papilla (Chesters & Bell 1970, Holm & Holm 1988). Chesters & Bell (1970) considered the family to comprise taxa with laterally compressed apices, hence they included Lophiostoma, and Platystomum in the group. However, the variability in the papillate form in the lophiostomataceous fungi had been noted for quite some time with certain species exhibiting mixed morphologies even within a single collection (Holm 1957, Eriksson 1981, Holm & Holm 1988, Barr 1990a). Holm & Holm (1988) took a broad concept of the family including taxa with laterally compressed and rounded apices in the group. They accepted five genera in the family, Lophiostoma, Lophiotrema, Massariosphaeria, Navicella and Trematosphaeria, while Barr (1987) accepted Dangeardiella, Herpotrichia, Massarina, Lophiostoma, Lophidiopsis, Trichometasphaeria, and Cilioplea. Recent molecular studies seem to support the view that the family is not exclusively composed of taxa with compressed papillae and that some taxa traditionally placed in this group belong elsewhere (e.g. Wang et al. 2007, Zhang et al. 2008).
Lophiostoma was circumscribed by Holm & Holm (1988) to include taxa that have immersed-erumpent ascomata with a distinctly flattened neck and opening by a slit-like ostiole. Asci are mostly clavate and ascospores are 1-septate, multiseptate or muriform, hyaline to dark brown. Recent phylogenetic work carried out on Lophiostoma species bearing these typical characters including the type species, L. macrostomum, indicated that the genus formed a monophyletic group (Tanaka & Hosoya 2008). Lophiotrema on the other hand was erected by Saccardo for “Hyalophragmiae” and has been used in this sense for a long time. Its circumscription is thus highly heterogeneous (Holm & Holm 1988). Chester & Bell (1970) did not recognise Lophiotrema but Holm & Holm (1988) accepted it in the strict sense for the group comprising the type species. Massariosphaeria was revised by Crivelli (1983) and Leuchtmann (1984) and its principal characteristics are the gelatinous ascospore sheath and tendency towards formation of red pigment, especially in mycelial cultures (Holm & Holm 1988). Leuchtmann (1984) transferred Lophiotrema microthecum to the group as M. grandispora. Recent molecular study of Massariosphaeria by Wang et al. (2007) indicated that the genus is highly polyphyletic with only M. grandispora among the species included in the analyses grouping in Lophiostomataceae.
Hypsostromataceae (Huhndorf 1994) was described for two tropical genera, Hypsostroma and Manglicola. In setting up the family Huhndorf (1994) noted its affinities to taxa in the Melanommatales (= Pleosporales) where she suggested it belonged but appeared unrelated to any known families. Characters that united the two genera in the family included superficial, large, elongate ascomata, soft-textured pseudoparenchymatous wall, trabeculate pseudoparaphyses, asci with an apical chamber and fluorescing ring and stipitate, basally arranged and fusiform, septate ascospores (Huhndorf 1994). Hypsostroma was erected by Huhndorf (1992) for two tropical wood-inhabiting species H. saxicola and H. caimitalensis. The two species bear close morphological resemblance, only slightly differing in their ascomatal and ascospore characters, with H. caimitalensis bearing long papillate ascomata and ascospores constricted at septum. Currently the family resides in the Dothideomycetes, family incertae sedis (Lumbsch & Huhndorf 2007).
Many of the recent molecular phylogenetic studies involving the Pleosporales seem to reject the monophyly of the families in the order (e.g. Liew et al. 2000, Lumbsch & Lindemuth 2001, Kruys et al. 2006, Schoch et al. 2006, Wang et al. 2007, Zhang et al. 2008). This polyphyly witnessed in major lineages within Pleosporales indicates that the order is in urgent need for revision. This study contributes to this endeavor and it employs two nuclear gene markers, nuclear ribosomal large subunit DNA (LSU) and translation elongation factor 1-alpha (TEF) in order to: 1) assess the generic constitution and relationships within Melanommataceae and Lophiostomataceae, 2) verify the phylogenetic placement of the genus Hypsostroma, 3) discuss phylogenetic findings with respect to morphological-based classification schemes.
MATERIALS AND METHODS
Taxon sampling and morphological analyses
The taxa used in this study are listed in Table 1 - see online Supplementary Information. Those newly sequenced together with their collection information are indicated in bold while the others were obtained from GenBank. Representative species covering 10 families in the Dothideomycetes were targeted for analyses. A total of 149 taxa were included in the analyses with 75 taxa newly sequenced during this study (Table 1). The microscopy and image capture follow methods outlined in Huhndorf & Fernández (1998). The ascomata were squash-mounted in water and images of anatomical structures captured with a Dage DC-330 video system (Dage-MTI®, U.S.A.) mounted on a Zeiss Axioskop microscope (Carl Zeiss®, U.S.A.). Format of the individual figures for most of the species follow those produced for the pyrenomycete website (Pyrenomycetes of the World: www-s.life.illinois.edu/pyrenos/).
Table 1.
Taxa used in the study with those newly sequenced for LSU and TEF genes shown in bold, the rest obtained from GenBank.
| Taxon | Collection locality | Collector and accession number | LSU | TEF |
|---|---|---|---|---|
| Alternaria alternata | DQ678082 | DQ677927 | ||
| Arthopyrenia salicis I | AY538339 | |||
| Arthopyrenia salicis II | AY607730 | |||
| Arthopyrenia sp. | Costa Rica, Puntarenas, Monteverde | S.M. Huhndorf, SMH4900 | GU385149 | |
| Bertiella macrospora I | Costa Rica, Puntarenas, Monteverde | I. Lopez, IL5005 | GU385150 | |
| Bertiella macrospora II | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM L122N | GU327743 | |
| Bertiella macrospora III | U.S.A., Michigan, Huron Mt. Club | S.M. Huhndorf, SMH3953 | GU327744 | |
| Bimuria novae-zelandiae | AY016356 | DQ471087 | ||
| Byssosphaeria jamaicana I | U.S.A., Puerto Rico, Luquillo Mts. | S.M. Huhndorf, SMH1403 | GU385152 | GU327746 |
| Byssosphaeria jamaicana II | U.S.A., Puerto Rico, Luquillo Mts. | S.M. Huhndorf, SMH3085 | GU385154 | |
| Byssosphaeria jamaicana III | Panama, Barro Colorado Island | S.M. Huhndorf, SMH3464 | GU385153 | |
| Byssosphaeria rhodomphala I | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM L153N | GU385157 | GU327747 |
| Byssosphaeria rhodomphala II | U.S.A., North Carolina, Smoky Mts. | A.N. Miller, ANM942 | GU385160 | |
| Byssosphaeria rhodomphala III | U.S.A., Puerto Rico, Luquillo Mts. | S.M. Huhndorf, SMH3086 | GU385155 | |
| Byssosphaeria rhodomphala IV | Panama, Barro Colorado Island | S.M. Huhndorf, SMH3402 | GU385170 | |
| Byssosphaeria rhodomphala V | Ecuador, Yasuni | F.A. Fernández, A.N. Miller, SMH4363 | GU385156 | |
| Byssosphaeria salebrosa | Costa Rica, San Jose, San Gerardo de Dota | S.M. Huhndorf SMH2387 | GU385162 | GU327748 |
| Byssosphaeria schiedermayeriana I | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM152N | GU385168 | GU327749 |
| Byssosphaeria schiedermayeriana II | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM1197 | GU385161 | GU327750 |
| Byssosphaeria schiedermayeriana III | U.S.A. Puerto Rico, Luquillo Mts. | S.M. Huhndorf, SMH1296 | GU385158 | |
| Byssosphaeria schiedermayeriana IV | U.S.A. Puerto Rico, Luquillo Mts. | S.M. Huhndorf, SMH1816 | GU385159 | |
| Byssosphaeria schiedermayeriana V | U.S.A. Puerto Rico, Luquillo Mts. | S.M. Huhndorf, SMH3157 | GU385163 | GU327745 |
| Byssosphaeria villosa | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.M. Mugambi, GKM204N | GU385151 | GU327751 |
| Byssothecium circinans | AY016357 | |||
| Cochliobolus eragrostidis | AB288215 | |||
| Cochliobolus geniculatus | AB444670 | |||
| Cochliobolus heterostrophus | AY544645 | DQ497603 | ||
| Cochliobolus lunatus | AB444681 | |||
| Cochliobolus pallescens | AB288225 | |||
| Cochliobolus sativus | DQ678045 | |||
| Cochliobolus verruculosus | AB444680 | |||
| Delitschia cf. anisomera | Kenya, Mt. Kenya Forest, along the track past Sirimon entrance | G.K. Mugambi, GKM1205 | GU385171 | |
| Delitschia chaetomioides I | Kenya, Rift Valley Province, Lembus forest along Eldoret — Elderma Ravine road | G.K. Mugambi, GKM1283 | GU385172 | GU327752 |
| Delitschia chaetomioides II | Costa Rica, Guanacaste, Bosque Encantado | S.M. Huhndorf, SMH3253.2 | GU390656 | GU327753 |
| Delitschia didyma | DQ384090 | |||
| Delitschia winteri I | DQ384091 | |||
| Delitschia winteri II | DQ678077 | DQ677922 | ||
| Dothidea ribesia | AY016360 | |||
| Gloniopsis praelonga I | FJ161193 | |||
| Gloniopsis praelonga II | FJ161195 | FJ161103 | ||
| Glonium stellatum | FJ161179 | FJ161095 | ||
| Helicomyces roseus | DQ678083 | DQ677928 | ||
| Herpotrichia cf. herpotrichoides I | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM212N | GU385169 | |
| Herpotrichia cf. herpotrichoides II | U.S.A., Wisconsin, Upham Woods | S.M. Huhndorf, SMH5167 | GU385175 | |
| Herpotrichia diffusa (= Byssosphaeria) | DQ678071 | |||
| Herpotrichia juniperi I | DQ384093 | |||
| Herpotrichia juniperi II | DQ678080 | DQ677925 | ||
| Herpotrichia macrotricha I | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM196N | GU385176 | GU327755 |
| Herpotrichia macrotricha II | Kenya, Rift Valley Province, Kajiando District, Ngong hills forest | G.K. Mugambi, GKM1128 | GU385178 | |
| Herpotrichia macrotricha III | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM1193 | GU385179 | |
| Herpotrichia macrotricha IV | U.S.A., Puerto Rico, Toro Negro Forest | S.M. Huhndorf, SMH269 | GU385177 | GU327756 |
| Herpotrichia macrotricha V | Costa Rica, Puntarenas, Monteverde | S.M. Huhndorf, SMH4913 | GU385164 | GU327754 |
| Hypsostroma caimetalensis | Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park | G.K. Mugambi, GKM1165 | GU385180 | |
| Hypsostroma saxicola | Costa Rica, San Jose, INBio Parque | S.M. Huhndorf, SMH5005 | GU385181 | |
| Hysterium angustatum | FJ161194 | |||
| Hysterographium mori | FJ161196 | FJ161104 | ||
| Leptosphaeria doliolum I | U43473 | |||
| Leptosphaeria doliolum II | U43474 | |||
| Leptosphaeria doliolum III | U43475 | |||
| Leptosphaeria macrospora | DQ384092 | |||
| Leptosphaeria sp. | Kenya, Rift Valley Province, Kajiando District, Ngong hills forest | G.K. Mugambi, GKM1090 | GU327757 | |
| Letendraea helminthicola | AY016362 | |||
| Lophiostoma alpigenum | Kenya, Rift Valley Province, Kajiando District, Ololua forest | G.K. Mugambi, GKM1091b | GU385193 | GU327758 |
| Lophiostoma arundinis | DQ782384 | DQ782387 | ||
| Lophiostoma caulium | DQ528763 | |||
| Lophiostoma crenatum | DQ678069 | DQ677912 | ||
| Lophiostoma fuckelii I | U.S.A., Puerto Rico, Luquillo Mts. | S.M. Huhndorf SMH1371 | GU385186 | |
| Lophiostoma fuckelii II | Kenya, Nairobi Province, Nairobi Museum Botanic Garden grounds | G.K. Mugambi, GKM1063 | GU385192 | GU327759 |
| Lophiostoma fuckelii III | DQ399531 | |||
| Lophiostoma heterospora | AY016369 | DQ497609 | ||
| Lophiostoma macrostomum I | AB433273 | |||
| Lophiostoma macrostomum II | AB433274 | |||
| Lophiostoma quadrinucleatum | Kenya, Central Province, Nyeri district, Mt. Kenya forest, behind Bantu lodge | G.K. Mugambi, GKM1233 | GU385184 | GU327760 |
| Lophiostoma sagittiforme | AB369267 | |||
| Lophiostoma triseptatum I | U.S.A., Michigan, Huron Mt. Club | S.M. Huhndorf, SMH2591 | GU385183 | |
| Lophiostoma triseptatum II | U.S.A., Michigan, Headland Park | S.M. Huhndorf, SMH5287 | GU385187 | |
| Lophium mytilinum I | EF596819 | |||
| Lophium mytilinum II | DQ678081 | DQ677926 | ||
| Massariosphaeria grandispora | EF165034 | |||
| Massariosphaeria roumeguerei | EF165032 | |||
| Massariosphaeria triseptata | EF165031 | |||
| Massariosphaeria typhicola | EF165033 | |||
| Melanomma pulvis pyrius I | DQ384095 | |||
| Melanomma pulvis pyrius II | FJ201984 | |||
| Melanomma pulvis pyrius III | FJ201986 | |||
| Melanomma pulvis pyrius IV | FJ201988 | |||
| Melanomma pulvis-pyrius V | U.S.A., North Carolina, Highlands Biological Station | S.M. Huhndorf, SMH3291 | GU385197 | |
| Melanomma rhododendri | U.S.A., Tennessee, Smoky Mts | A.N. Miller, ANM73 | GU385198 | |
| Misturatosphaeria aurantonotata I | Kenya, Rift Valley Province, Kajiando District, Ngong hills forest | G.K. Mugambi, GKM1238 | GU385173 | GU327761 |
| Misturatosphaeria aurantonotata II | Kenya, Rift Valley Province, Lembus forest, along Eldoret — Elderma Ravine road | G.K. Mugambi, GKM1280 | GU385174 | GU327762 |
| Misturatosphaeria claviformis | Kenya, Central Province, Nyeri District, Mt. Kenya forest, behind Bantu lodge | G.K. Mugambi, GKM1210 | GU385212 | GU327763 |
| Misturatosphaeria cruciformis | U.S.A., Illinois, Swallow Cliff Woods | S.M. Huhndorf, SMH5151 | GU385211 | |
| Misturatosphaeria kenyensis I | Kenya, Coast Province, Taita District, Taita Hills, Ngango forest | G.K. Mugambi, GKM194N | GU327764 | |
| Misturatosphaeria kenyensis II | Kenya, Coast Province, Taita District, Taita hills, Ngangao forest | G.K. Mugambi, GKM234N | GU385188 | GU327765 |
| Misturatosphaeria kenyensis III | Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park | G.K. Mugambi, GKM L100Na | GU385189 | GU327766 |
| Misturatosphaeria kenyensis IV | Kenya, Coast Province, Taita District, Taita hills, Ngangao forest | G.K. Mugambi, GKM1195 | GU385194 | GU327767 |
| Misturatosphaeria minima I | Kenya, Coast Province, Taita District, Taita hills, Ngangao forest | G.K. Mugambi, GKM169N | GU385165 | GU327768 |
| Misturatosphaeria minima II | U.S.A., North Carolina, Smoky Mts | A.N. Miller, ANM60 | GU385182 | |
| Misturatosphaeria minima III | U.S.A., North Carolina, Smoky Mts | A.N. Miller, ANM933 | GU385195 | |
| Misturatosphaeria minima IV | Costa Rica, San Jose, San Gerardo de Dota | S.M. Huhndorf, SMH2448 | GU385166 | |
| Misturatosphaeria sp. | French Guiana, Saül | S.M. Huhndorf, SMH3747 | GU385196 | |
| Misturatosphaeria tennesseensis | U.S.A., Tennessee, Smoky Mts | A.N. Miller, ANM911 | GU385207 | GU327769 |
| Misturatosphaeria uniseptata | Ecuador, Yasuni | F.A. Fernández, A.N. Miller, SMH4330 | GU385167 | GU327770 |
| Misturatosphaeria uniseriata | U.S.A., Tennessee, Smoky Mts | A.N. Miller, ANM909 | GU385206 | |
| Montagnula opulenta | DQ678086 | |||
| Munkovalsaria appendiculata | AY772016 | |||
| Munkovalsaria sp. I | Kenya, Nairobi Province, Nairobi Museum Botanic Garden grounds | G.K. Mugambi, GKM1286 | GU327771 | |
| Mytilinidion australe | FJ161183 | |||
| Ostropella albocincta I | Panama, Barro Colorado Island | S.M. Huhndorf, SMH3536 | GU385200 | |
| Ostropella striata | Costa Rica, Arenal | SMH1854 | GU385203 | |
| Phaeodothis winteri | DQ678073 | DQ677917 | ||
| Phaeosphaeria eustoma | DQ678063 | DQ677906 | ||
| Platystomum sp. | U.S.A., Wisconsin, Upham Woods | S.M. Huhndorf, SMH5174 | GU385199 | |
| Platystomum compressum | Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park | G.K. Mugambi, GKM1048 | GU385204 | GU327772 |
| Pleomassaria siparia | DQ678078 | DQ677923 | ||
| Pleomassariaceae | New Zealand, Auckland, Wenderholm Regional Park | S.M. Huhndorf, SMH5232 | GU385205 | GU327773 |
| Pleospora herbarum I | DQ247804 | DQ471090 | ||
| Pleospora herbarum II | DQ678049 | |||
| Pleospora herbarum III | DQ677888 | |||
| Pleospora herbarum IV | AF382386 | |||
| Pleospora herbarum V | U43476 | |||
| Pleospora sp. | EF177848 | |||
| Preussia terricola | DQ471137 | |||
| Pseudotrichia guatopoensis I | U.S.A., Puerto Rico, Luquillo Mts | S.M. Huhndorf, SMH1288 | GU385208 | |
| Pseudotrichia guatopoensis II | Costa Rica, San Jose, San Gerardo de Dota | S.M. Huhndorf, SMH2383 | GU327775 | |
| Pseudotrichia guatopoensis III | Costa Rica, Alajuela, Volcan Arenal | S.M. Huhndorf, SMH4535 | GU385202 | GU327774 |
| Pseudotrichia mutabilis I | U.S.A., Wisconsin, New Glarus State Park | S.M. Huhndorf, SMH1541 | GU385209 | |
| Pseudotrichia mutabilis II | U.S.A., Michigan, Headlands Park | S.M. Huhndorf, SMH5288 | GU385210 | |
| Psiloglonium clavisporum II | FJ167526 | FJ161105 | ||
| Pyrenophora phaeocomes | DQ499596 | |||
| Pyrenophora tritici repentis | AY544672 | |||
| Setosphaeria monoceras | AY016368 | |||
| Stylodothis puccinioides | AY004342 | |||
| Thyridaria macrostomoides I | Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park | G.K. Mugambi, GKM1033 | GU385190 | GU327776 |
| Thyridaria macrostomoides II | Kenya, Coast Province, Taita District, Taita Hills, Ngangao forest | G.K. Mugambi, GKM224N | GU385191 | GU327777 |
| Thyridaria macrostomoides III | Kenya, Coast Province, Malindi District, Arabuko-Sokoke National Park | G.K. Mugambi, GKM1159 | GU385185 | GU327778 |
| Trematosphaeria pertusa I | U.S.A., Wisconsin, Madison, Picnic Point Park | S.M. Huhndorf, SMH1448 | GU385213 | |
| Trematosphaeria pertusa II | FJ201990 | |||
| Trematosphaeria pertusa III | FJ201992 | |||
| Tubeufia cerea | DQ470982 | |||
| Tubeufia helicomyces | DQ767638 | |||
| Tubeufia paludosa | AY849966 | |||
| Ulospora bilgramii | DQ678076 | |||
| Verruculina enalia | DQ678079 | |||
| Westerdykella cylindrica | AY004343 | |||
| Xenolophium sp. | Panama, Barro Colorado Island | S.M. Huhndorf, SMH3537 | GU385201 | |
| Xenolophium applanatum | U.S.A., Puerto Rico, Luquillo Mts | S.M. Huhndorf, SMH2055 | GU385214 | |
| Xenolophium guianense | Ecuador, Yasuni | F.A. Fernández, A.N. Miller, SMH4711 | GU385215 | |
| Xenolophium pachythele | French Guiana, Saül | S.M. Huhndorf, SMH996 | GU327779 |
DNA extraction, PCR amplification, sequencing and sequence alignment
Total fungal DNA was extracted from whole fruiting bodies using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer. Phylogenetic analyses were conducted using partial sequences of two genes, the Translation Elongation Factor 1-Alpha (TEF) and nuclear ribosomal large subunit (LSU) DNA. Nuclear LSU was amplified using the primers LR0R, LR6 and LR3 (Vilgalys & Hester 1990) and TEF was amplified using primers EF1-526F, EF1-983F, EF1-1567R, Ef-df and EF-gr obtained from D. Hibbett's website (www.clarku.edu/faculty/dhibbett/Protocols_Folder/Primers/Primers.htm).
Polymerase chain reaction (PCR) was carried out using the following protocol: The final volume of the PCR reaction was 25 μL and contained 2.5 μL buffer, 2.5 μL dNTP mix, 1 μL of each primer (10 μM), 5 μL of Bovine Serum Albumin (BSA), 1.5 μL Taq polymerase (Roche®, U.S.A.), 2 μL genomic DNA extract and 9.5 μL deionised water. The reaction was then allowed to run for 34 cycles. The annealing temperature was 50 °C for LSU, and initial 58 °C for TEF and then reduced by 1 °C during each of the first eight cycles and maintained at 50 °C for the remaining cycles. The fragments were sequenced using the Big Dye® Terminator Cycle Sequencing kit v. 3.1 (ABI PRISM, Applied Biosystems, Forster City, U.S.A.). Sequencing was performed using the same set of primers as the PCR. The other sequences used in the analyses were obtained from GenBank. Sequences were aligned using multiple sequence program Muscle® v. 3.6 (Edger 2004). The alignments were further manipulated manually and those regions, which could not be aligned with confidence were excluded from analyses. The final data matrices comprised of LSU data set with 140 taxa with 1179 unambiguously aligned characters, and a TEF data set with 57 taxa and 750 unambiguously aligned characters while the combined data set comprised of 49 taxa for which both genes were available with 1929 unambiguously aligned characters. Voucher specimens are deposited in the Field Museum Herbarium (F), while Kenyan specimens are deposited at the East Africa Herbarium (EA).
Phylogenetic analyses
MODELTEST v. 3.7 (Posada & Crandall, 1998) following Akaike Information Criterion was used to determine the best-fit model of evolution for each data set for Bayesian and Maximum Likelihood analyses. Bayesian analyses employing Markov Chain Monte Carlo (MCMC) were carried out using MrBayes v. 3.1 (Huelsenbeck & Ronquist, 2001). Four MCMC chains were ran simultaneously for 5–7 million generations for single-gene and combined gene analyses, the temperature of the heated chains was set at 0.05 for LSU and at 0.2 for TEF and combined gene analyses. Trees were sampled every 100th generation. The TEF gene matrix was partitioned into three parts to take into account the codon positions, while combined gene matrix had four partitions. Independent models of evolution were applied on the partitions for Bayesian analyses. AWTY was used to check the stationarity of the Bayesian tree sampling procedure (Nylander et al. 2007). All the trees obtained before the MCMC chains attained stationarity in each analysis were discarded and posterior clade probabilities were determined from the consensus tree generated from the rest. The majority rule consensus tree was obtained by executing the MrBayes sumt command. Maximum likelihood (ML) analyses were carried out for each of the three data sets using RAxML (Stamatakis et al. 2008) employing mixed models of evolution settings of the program and Bootstrap support obtained by running 1000 pseudo replicates. Five independent ML tree searches were done in RAxML (Stamatakis et al. 2008) each one starting from randomised tree.
Test of conflict was based on single gene analyses and doing comparison based on Bootstrap and Bayesian posterior probabilities support. Clades with greater than or equal to 70 % bootstrap support (BS) and 95 % posterior probabilities (PP) were considered strongly supported. There were no major conflicts in the phylogenies obtained from single-gene analyses. The differences observed were mainly the family relationships in the Pleosporales, which nonetheless received low support. As a result the data sets were combined for Maximum Likelihood (ML) and Bayesian analyses.
RESULTS
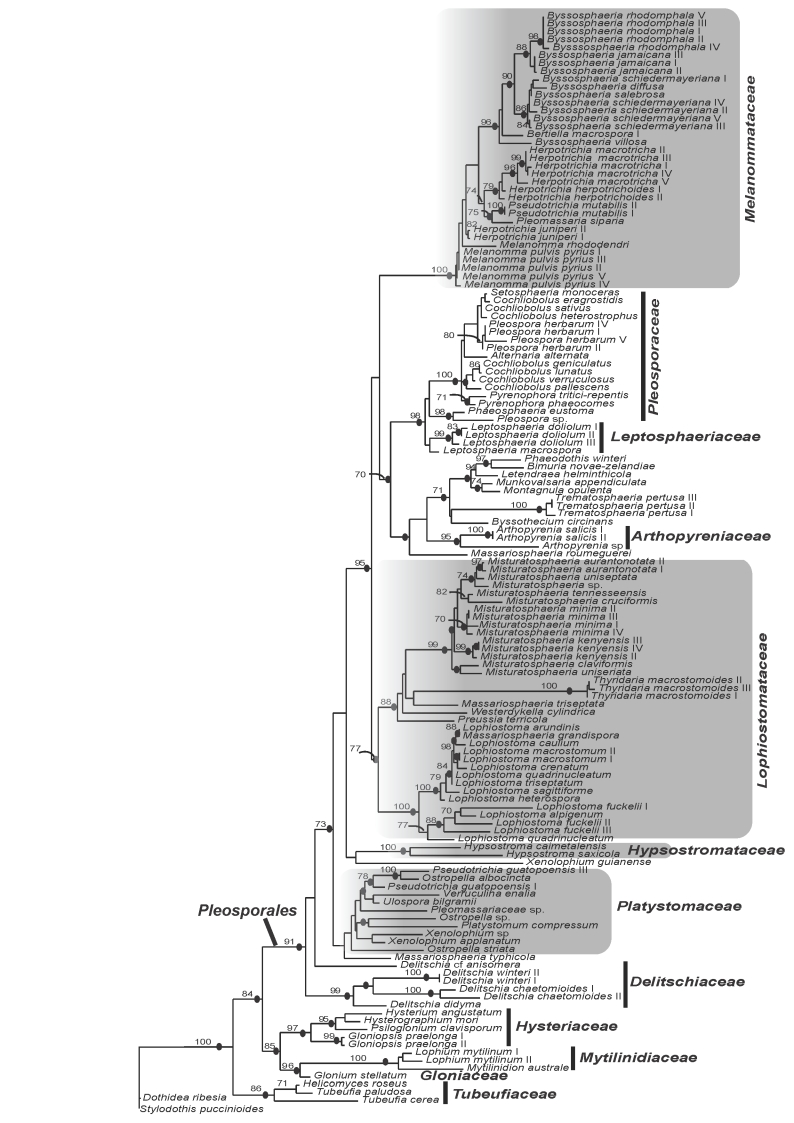
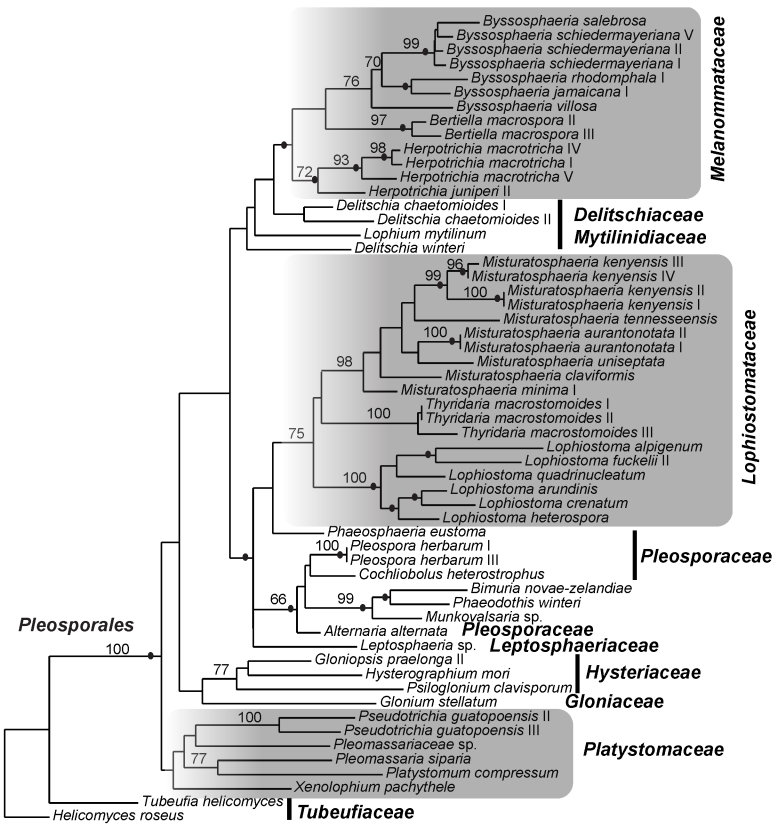
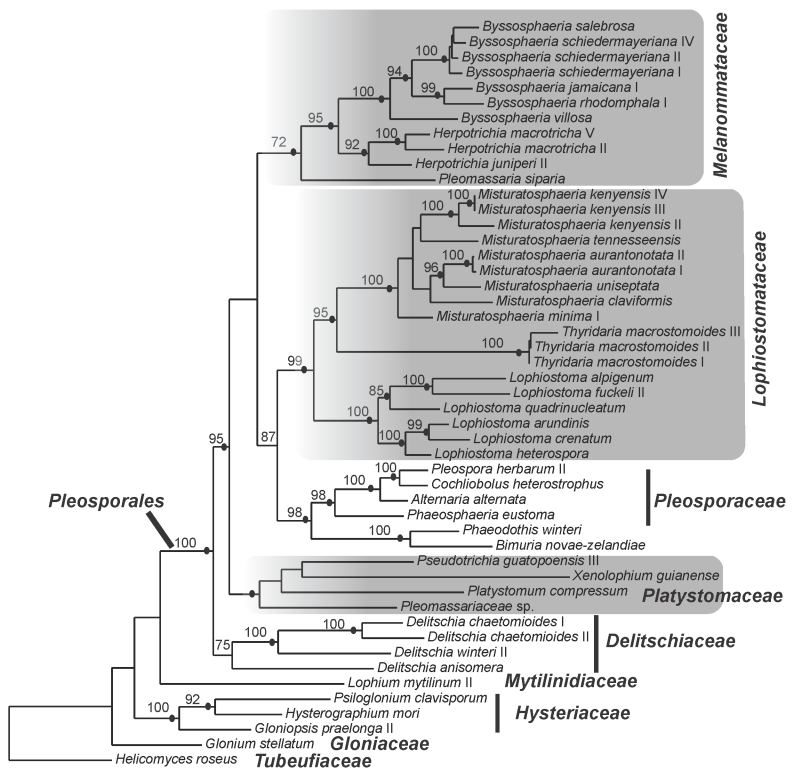
The best-fit model of evolution for LSU, TEF and combined gene data sets was GTR+I+G (Rodriguez et al. 1990) following Akaike Information Criterion implemented by ModelTest v. 3.7 (Posada & Crandall, 1998). Analyses using Maximum Likelihood and Bayesian methods resulted in phylogenies with similar topologies. Consequently only results of ML phylograms of single-gene analyses (Figs 1, 2) and combined gene matrix (Fig. 3) are presented. The gene genealogies recovered a strongly supported Pleosporales that is composed of strongly supported clades for Melanommataceae, Pleosporaceae, Lophiostomataceae, Delitschiaceae, Arthopyreniaceae and Hypsostromataceae in at least one of the trees (Figs 1, 2, 3). The LSU tree showed clades with stronger BS support than the TEF tree. Melanommataceae includes Melanomma, Byssosphaeria, Herpotrichia, and Pseudotrichia. Nested in the family are collections representing Bertiella macrospora and Pleomassaria siparia. Taxa in Ostropella and Xenolophium that were previously placed in Melanommataceae, group in an unsupported clade distant from the family (Figs 1, 2, 3). The genus Herpotrichia was recovered as polyphyletic using LSU, with H. juniperi grouping separate from a well-supported clade that includes H. macrotricha and H. herpotrichoides (Fig. 1), but the genus resolves as monophyletic in the TEF and combined gene trees (Figs 2, 3). A well-supported clade for Byssosphaeria was recovered in all three trees. Only LSU sequences were obtained for taxa in Melanomma and they did not resolve in a monophyletic clade (Fig. 1). Pseudotrichia is polyphyletic with the type species, P. mutabilis occurring in the Melanommataceae and P. guatopoensis grouping with Ostropella spp. (Figs 1, 2). A strong BS and PP supported clade composed of a new genus with nine species was recovered nested within Lophiostomataceae (Figs 1, 3) and the taxa are described below. The clade was obtained in TEF analyses but was not strongly supported (Fig. 2). Sister to the new genus are three collections of Thyridaria macrostomoides (Figs 1, 2, 3). A strongly supported clade for Lophiostoma was recovered (Figs 1, 2, 3) including two collections of Lophiostoma macrostomum. These grouped together in a strongly supported clade that included nine other species in the genus (Fig. 1). Massariosphaeria grandispora, a sequence obtained from GenBank, was found nested within Lophiostoma (Fig. 1). Single collections of Preussia terricola and Westerdykella cylindrica whose sequences were obtained from GenBank were found nested within Lophiostomataceae (Fig. 1).
Fig. 1.
Phylogram of the maximum likelihood analyses generated from LSU sequences. Bootstrap support values ≥ 70 % are shown above or below the branches. Black circles indicate branches with Bayesian posterior probabilities ≥ 95 %. The families treated in this study are indicated (shaded).
Fig. 2.
Phylogram of the maximum likelihood analyses generated from TEF sequences. Bootstrap support values ≥ 70 % are shown above or below the branches. Black circles indicate branches with Bayesian posterior probabilities ≥ 95 %. The families treated in this study are indicated (shaded).
Fig. 3.
Phylogram of the maximum likelihood analyses generated from the combined genes (LSU and TEF). Bootstrap support values ≥ 70 % are shown above or below the branches. Black circles indicate branches with Bayesian posterior probabilities ≥ 95 %. The families treated in this study are indicated (shaded).
Hypsostromataceae was recovered as a well-supported clade within Pleosporales comprising two species accepted in the family (Fig. 1). Species of Ostropella, Xenolophium (except Xenolophium pachythele that groups separate in TEF tree) and other taxa assemble in a mostly unsupported clade together with a collection of Platystomum compressum, possibly representing the Platystomaceae. The taxa group together in all three trees but only obtain PP support in the combined gene tree (Fig. 3). A monophyletic clade for Delitschiaceae was recovered within Pleosporales; Hysteriaceae, Mytilinidiaceae, Gloniaceae and Tubeufiaceae were recovered as strongly supported monophyletic groups outside the Pleosporales (Figs 1, 2, 3).
TAXONOMY
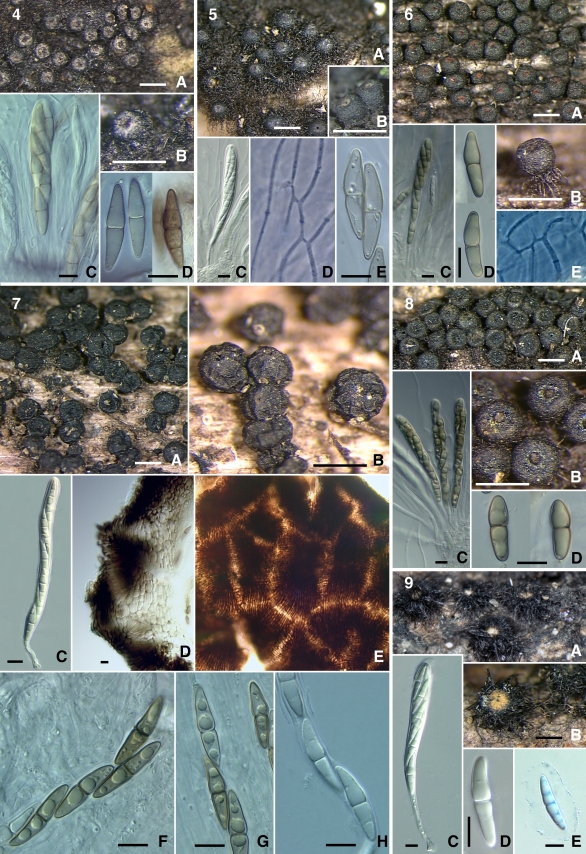
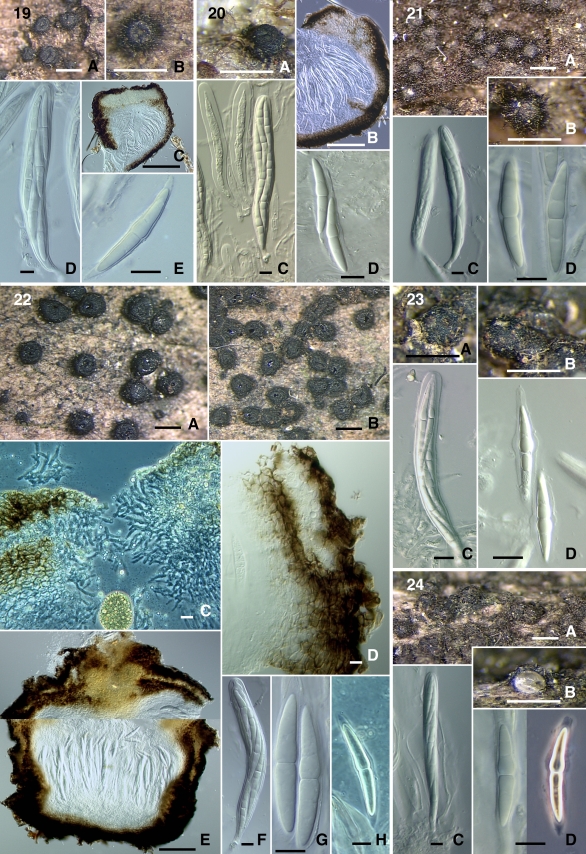
Images of sequenced taxa are included and grouped together to facilitate comparison of morphological characteristics. The species of Byssosphaeria and Bertiella are arranged together in the first plate: Byssosphaeria jamaicana (Figs 4–5), B. rhodomphala (Figs 6, 8), Bertiella macrospora (Fig. 7), and Byssosphaeria villosa (Fig. 9). The second plate contains species of Byssosphaeria, Melanomma and Pseudotrichia: Byssosphaeria schiedermayeriana (Figs 10–13, 15), B. salebrosa (Fig. 14), Melanomma pulvis-pyrius (Fig. 16), M. rhododendri (Fig. 17), and Pseudotrichia mutabilis (Fig. 18). The third plate contains Herpotrichia macrotricha (Figs 19–22) and H. cf. herpotrichoides (Figs 23–24).
Figs 4–9.
4. Byssosphaeria jamaicana (SMH1403) A–B. Ascomata. C. Ascus. D. Ascospores. 5. B. jamaicana (SMH3464/3085) A–B. Ascomata. C. Ascus. D. Pseudoparaphyses. E. Ascospores. 6. B. rhodomphala (ANM942) A, B. Ascomata. C. Ascus. D. Ascospores. E. Pseudoparaphyses. 7. Bertiella macrospora (IL5005) A–B. Ascomata. C. Ascus. D. Ascomatal wall section. E. Ascomatal wall surface. F–H. Ascospores. 8. B. rhodomphala (SMH3402) A. Ascomata. B. Asci. C. Ascospores. 9. B. villosa (GKM204N) A–B. Ascomata. C. Ascus. D–E. Ascospores. Scale bars: Ascomata = 500 μm. Wall = 10 μm. Ascus = 10 μm. Ascospore = 10 μm.
Figs 10–18.
10. Byssosphaeria schiedermayeriana (SMH1296) A. Ascomata. B. Pseudoparaphyses. C. Ascus. D. Ascospores. 11. B. schiedermayeriana (SMH3157) A. Ascomata. B. Ascus. C. Ascospores. 12. B. schiedermayeriana (SMH1816) A. Ascomata. B. Ascus. C. Ascospores. 13. B. schiedermayeriana (GKM1197) A–B. Ascomata. C. Ascus. D, E. Ascospores. 14. B. salebrosa (SMH2387) A, C. Ascomata. B. Ascomatal wall surface. D. Ascus. E. Ascospore. 15. B. schiedermayeriana (GKM152N) A. Ascomata. B–C. Asci. D. Ascospores. 16. Melanomma pulvis-pyrius (SMH3291) A–B. Ascomata. C. Ascus. D. Ascospores. 17. M. rhododendri (ANM73) A–B. Ascomata. C. Asci. D. Ascospores. 18. Pseudotrichia mutabilis (SMH5288) A. Ascomata. B. Ascus. C. Ascospores. Scale bars: Ascomata = 500 μm. Ascus = 10 μm. Ascospore = 10 μm.
Figs 19–24.
19. Herpotrichia macrotricha (SMH269) A–B. Ascomata. C. Ascomatal section. D. Ascus. E. Ascospore. 20. H. macrotricha (GKM196N) A. Ascoma. B. Ascomatal section. C. Ascus. D. Ascospores. 21. H. macrotricha (GKM1128) A–B. Ascomata. C. Ascus. D. Ascospores. 22. H. macrotricha (SMH4913) A–B. Ascomata. C. Ascomatal neck section. D. Ascomatal wall section. E. Ascomatal section. F. Ascus. G, H. Ascospores. 23. H. cf. herpotrichoides (GKM212N) A–B. Ascomata. C. Ascus. D. Ascospores. 24. H. cf. herpotrichoides (SMH5167) A–B. Ascomata. C. Ascus. D. Ascospores. Scale bars: Ascomata = 500 μm. Section = 100 μm. Wall = 10 μm. Ascus = 10 μm. Ascospore = 10 μm.
Misturatosphaeria Mugambi & Huhndorf, gen. nov. MycoBank MB515583.
Etymology: Misturatus (L.) = mixed, refers to the mixed ascospore morphology in the group.
Ascomata erumpentia ad superficialia, solitaria vel aggregata, cum subiculum vel sine subicolo, apicibus rotundatis pallide coloratis vel incoloratis. Asci claviti vel cylindrici, breve stipitati, octospori, pseudoparaphysibus numerosis, hyalinis et septatis, in matrice mucosa. Ascosporae hyalinae vel brunneae, septatae, cum vagina mucosa vel sine vagina.
Typus: Misturatosphaeria aurantonotata Mugambi & Huhndorf, sp. nov.
Ascomata erumpent to superficial, occurring singly or aggregated in clusters, subiculum present or lacking, apex rounded, with raised papilla or not, ostiole area light coloured or not and ostiole opening appearing plugged by gelatinous tissue. Asci cylindrical or clavate, short stipitate, 8-spored, held in gelatinous matrix, pseudoparaphyses numerous, septate, branching and anastomosing between and above the asci. Ascospores brown or hyaline, phragmosporous or dictyosporous, external walls roughened or smooth, with or without a gelatinous sheath covering.
Misturatosphaeria aurantonotata Mugambi & Huhndorf, sp. nov. MycoBank MB516007. Fig. 25.
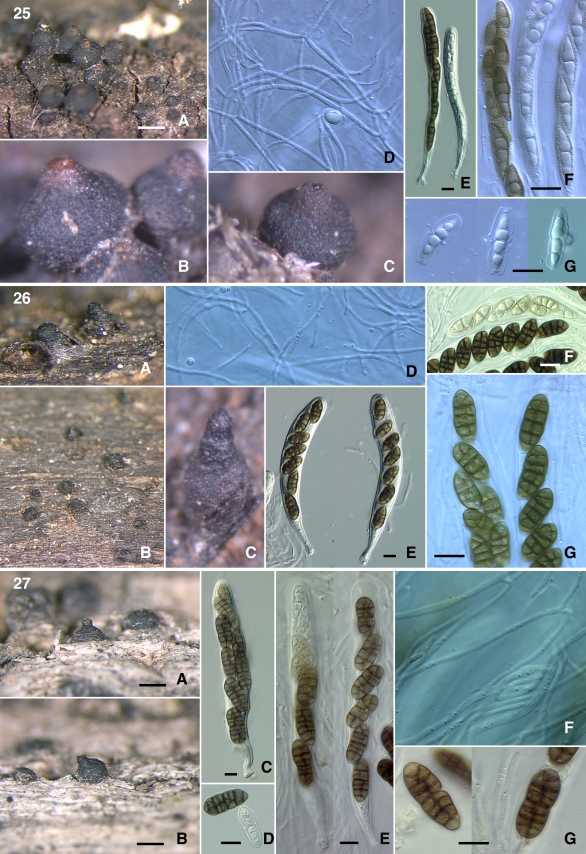
Figs 25–27.
25. Misturatosphaeria aurantonotata (GKM1238) A–C. Ascomata. D. pseudoparaphyses. E. Asci. F–G. Ascospores. 26. Misturatosphaeria claviformis (GKM1210) A–C. Ascomata. D. Pseudoparaphyses. E. Asci. F–G. Ascospores. 27. Misturatosphaeria cruciformis (SMH5151) A–B. Ascomata. C, E. Asci. D, G. Ascospores. F. Pseudoparaphyses. Scale bars: Ascomata = 500 μm. Ascus = 10 μm. Ascospore = 10 μm.
Etymology: Aurantiacum (L.) = orange, notatus (L.) = marked, refers to the orange colour markings at the ascomata apices.
Ascomata superficialia, atrobrunnea, solitaria vel dense aggregata, in subiculo sparso ex hyphis brunneis, pyriformia, 441–710 μm alta, 461–573 μm diam, apicibus rotundatis, saepe aurantiacis. Asci cylindrici-clavati, breve stipitati, octospori, 103–122 x 8–12 μm. Pseudoparaphyses numerosae, septatae, in matrice mucosa, 1–2 μm. Ascosporae fusoides, primo hyalinae, deinde atrobrunnae, 3-septatae, cum vagina mucosa, 17–22 x 5–6 μm.
Ascomata superficial, occurring singly or aggregated into large clusters, occasionally even growing on old ascomatal tissue, often sited on sparse brown subiculum, pyriform in shape, dark brown, ascomatal wall smooth, 441–710 μm high, 461–573 μm wide. Apices rounded, usually with raised papillae, ostiole area orange in colour or the colouring lacking all together. Asci are cylindrical–clavate with short stipes, 8-spored partially biseriate in arrangement, 103–122 x 8–12 μm. Pseudoparaphyses are numerous, septate, branching and anastomosing between and above the asci, held in gelatinous matrix, 1–2 μm diam. Ascospores fusiform, often slightly curved, first hyaline later becoming brown to dark brown, the outer walls are thick and roughened, 3-septate at maturity, one of the middle cells is often larger than the rest, only slightly constricted at the middle septum. Mucilaginous sheath present when the spores are young and falls off upon attaining maturity, 17–22 x 5–6 μm.
Substratum: Found on decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Presently only known from two tropical forests in the central highlands of Kenya.
Specimens examined: Kenya, Rift Valley Province, Kajiando District, Ngong hills forest res., 1°23'934”S, 36°38'287”E, 12 July 2006, on woody branch, G.K. Mugambi, GKM1238, holotype EA, isotype F; Koibatek District, Lembus forest along Eldoret-Eldama Ravine Rd, 0°04'N, 35°35'E, 19 Jan. 2007, on branch on the ground, G.K. Mugambi, GKM1280, EA, F.
Misturatosphaeria claviformis Mugambi & Huhndorf, sp. nov. MycoBank MB516008. Fig. 26.
Etymology: Clavatus (L.) = club, refers to the club-shaped ascomata usually witnessed in the species.
Ascomata erumpentia ad superficialia, atrobrunnea, solitaria vel sparse aggregata, sine subiculo, pyriformia ad obclavata, 235–520 μm alta, 195–322 μm diam, apicibus rotundatis, ostiolata. Asci cylindrici-clavati, breve stipitati, octospori, 85–134 x 12–18 μm. Pseudoparaphyses numerosae, septatae, in matrice mucosa, 1–2 μm diam. Ascosporae ellipticae, brunneae ad atrobrunnae, muriformes, since vagina mucosa, 12–20 x 7–9 μm.
Ascomata erumpent to superficial, occurring singly or aggregated into small groups, subiculum absent, pyriform to obclavate in shape, dark brown, ascomatal wall smooth, 235–520 μm high and 195–322 μm wide. Apices rounded, usually possess raised, broad rounded papillae also with rounded openings. Asci cylindrical-clavate, with short stipes, 8-spored partially biseriate or uniseriate, oblique or sometimes irregularly arranged in an oblique fashion or irregular, 85–134 x 12–18 μm. Pseudoparaphyses numerous, septate, branching and anastomosing between and above the asci, held in gelatinous matrix, 1–2 μm diam. Ascospores elliptical, straight or inequilateral, brown to dark brown in colour, outer wall smooth, dictyosporous, with no mucilaginous sheath, 12–20 x 7–9 μm.
Substratum: Found on decorticated woody branches on the ground in forested areas.
Anamorph: Unknown.
Distribution: Currently known only from a tropical highland forest in central Kenya.
Specimen examined: Kenya, Central Province, Nyeri District, Mt Kenya forest, behind Bantu lodge, 0°6'907”S, 37°2'699”E, 30 Nov. 2006, on woody branch, G.K. Mugambi, GKM1210, holotype EA, isotype F.
Misturatosphaeria cruciformis Mugambi & Huhndorf, sp. nov. MycoBank MB516009. Fig. 27.
Etymology: Cruciatus (L.) = cross-wise, refers to the ascospore septation, transverse and longitudinal septa.
Ascomata erumpentia, atrobrunnea, solitaria vel sparse aggregata, pyriformia, globosa, 500–535 μm alta, 545–649 μm diam, apicibus rotundatis, ostiolata, since subiculo. Asci cylindrici-clavati, breve stipitati, octospori, 127–154 x 14–17 μm. Pseudoparaphyses numerosae, septatae, in matrice mucosa, 1–2 μm diam. Ascosporae oblongae ad ellipticae, brunneae ad atrobrunnae, muriformes, saepe constrictae ad septum medium, sine vagina mucosa, 19–26 x 8–13 μm.
Ascomata erumpent, usually occurring singly rarely clustered into small groups, subiculum lacking, pyriform to globose in shape, dark brown, ascomatal wall smooth, 500–535 μm high and 545–649 μm wide. Apices are rounded with papillae that are usually raised, ostiole opening rounded. Asci cylindrical-clavate in shape and bearing short stipes, 8-spored partially biseriate to sometimes overlapping uniseriate, 127–154 x 14–17 μm. Pseudoparaphyses are numerous, septate, branching and anastomosing between and above the asci, held in a gelatinous matrix, 1–2 μm diam. Ascospores oblong to elliptical, brown becoming dark brown with age, outer wall smooth, dictyosporous, usually constricted at the middle transverse septum, possess no mucilaginous sheath, 19–26 x 8–13 μm.
Substratum: Found on decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Currently only known from a forest reserve in Illinois, U.S.A.
Specimen examined: u.s.A., Illinois, Cook Co., Swallow Cliff Woods Forest Preserve, 21 May 2004, on woody branch, G.K. Mugambi, SMH5151, holotype, F.
Misturatosphaeria kenyensis Mugambi & Huhndorf, sp. nov. MycoBank MB516010. Figs 28–29.
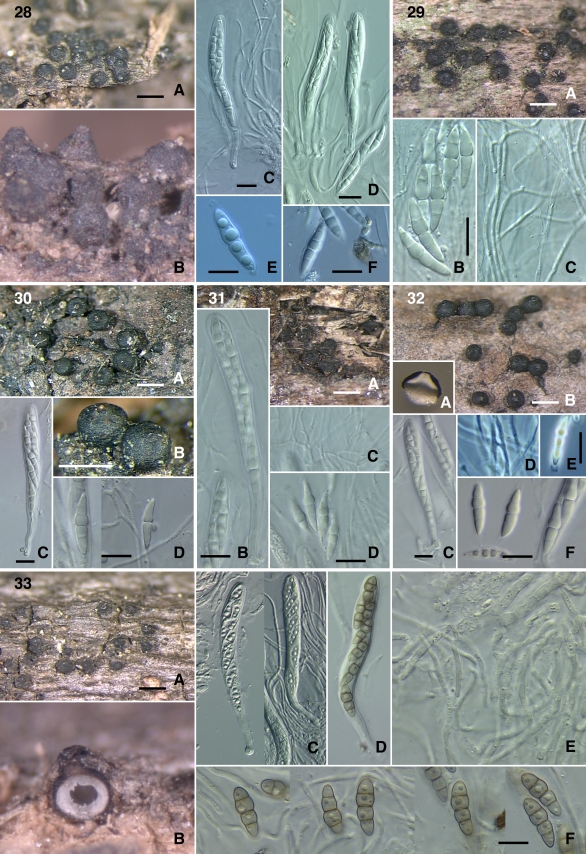
Figs 28–33.
28. Misturatosphaeria kenyensis (GKM1195) A–B. Ascomata. C–D. Asci. E, F. Ascospores. 29. M. kenyensis (GKM L100Na) A. Ascomata. B. Ascospores. C. Pseudoparaphyses. 30. Misturatosphaeria minima (SMH2448) A, B. Ascomata. C. Ascus. D. Ascospores. 31. M. minima (GKM169N) A. Ascomata. B. Ascus. C. Pseudoparaphyses. D. Ascospores. 32. M. minima (ANM60) A, B. Ascomata. C. Ascus. D. Pseudoparaphyses. E–F. Ascospores. 33. Misturatosphaeria tennesseensis (ANM911) A–B. Ascomata. C–D. Asci. E. Pseudoparaphyses. F. Ascospores. Scale bars: Ascomata = 500 μm. Ascus = 10 μm. Ascospore = 10 μm.
Etymology: Refers to the country the species was collected, Kenya.
Ascomata erumpentia ad superficialia, solitaria vel dense aggregata, atrobrunnea, pyriformia ad globosa, 185–305 μm alta, 245–334 μm diam, apicibus rotundatis pallide coloratis, ostiolo rotundato. Asci cylindrici-clavati, breve stipitati, octospori, 71–79 x 8–9 μm. Pseudoparaphyses, numerosae, septatae, in matrice mucosa, 1–2 μm diam. Ascosporae fusoides, hyalinae, 1–3-septatae vulgo 1–septatae, cum vagina mucosa parva, 15–24 x 4–6 μm.
Ascomata erumpent to superficial, occurring singly or aggregated into large clusters, without subiculum, pyriform to globose in shape, dark brown, ascomatal wall smooth, 185–305 μm high and 245–334 μm wide. Apices rounded, usually with raised papillae, opening rounded and the ostiole area often of lighter colour. Asci cylindrical-clavate in shape, short stipitate, 8-spored, partially biseriate in arrangement, 71–79 x 8–9 μm. Pseudoparaphyses numerous and septate, branching and anastomosing between and above the asci, held in gelatinous matrix, 1–2 μm diam. Ascospores fusiform in shape, hyaline, outer wall smooth, usually 1–3-septate but commonly 1-septate, occasionally one of the middle cells broader than others, possess small mucilaginous sheaths that extends at the tip of spores, 15–24 x 4–6 μm.
Substratum: Found on decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Presently only known from a tropical cloud forest in Kenya.
Specimen examined: Kenya, Coast Province, Taita Taveta District, Taita hills, Ngangao forest res., 3°22'30”S, 38°20'45”E, 30 Oct. 2006, on woody branch, G.K. Mugambi, GKM1195, holotype EA, isotype F.
Misturatosphaeria minima Mugambi, A.N. Mill. & Huhndorf, sp. nov. MycoBank MB516011. Figs 30–32.
Etymology: Minus (L.) = less, refers to the relatively smaller size ascomata observed in this species.
Ascomata erumpentia ad superficialia, solitaria vel sparse aggregata, atrobrunnea, pyriformia ad globosa, 194–389 μm alta, 244–355 μm diam, since subiculo, apicibus rotundatis saepe aurantiacis, ostiolo rotundato. Asci cylindrici-clavati, breve stipitati, octospori, 72–112 x 8–11 μm. Pseudoparaphyses numerosae, septatae, in matrice mucosae, 1–2 μm diam. Ascosporae fusoides, hyalinae, 1–3-septatae, constrictae ad septum medium, cum vagina mucosa parva, 18–22 x 3–4 μm.
Ascomata erumpent through the bark or sometimes appearing superficial after the breakdown of the surrounding plant tissue, occurring singly or aggregated into small clusters usually less than five individuals, possesses no subiculum, pyriform to subglobose in shape, dark brown, ascomatal wall smooth, 194–389 μm high and 244–355 μm wide. Apices rounded, raised with rounded openings, occassionally the pore area appear orange in colour or the colouring is lacking. Asci cylindrical-clavate in shape bearing short stipes, 8-spored partially biseriate in arrangement, 72–112 x 8–11 μm. Pseudoparaphyses are numerous, septate, branching and anastomosing between and above the asci, held in a gelatinous matrix, 1–2 μm diam. Ascospores are fusiform, hyaline, the outer wall smooth, 1–3-septate and constricted at the middle septum, one of the middle cells is broader than the rest, possesses a small mucilaginous sheath that extends at the tips of the spore, 18–22 x 3–4 μm.
Substratum: Found on decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Currently known from forested areas in Kenya, Costa Rica and U.S.A.
Specimens examined: Kenya, Coast Province, Taita Taveta District, Taita hills, Ngangao forest res., 3° 22' 30” S, 38° 20' 45” E, 1800 m elev., 13 Apr. 2005, on woody branch, G.K. Mugambi, GKM169N, holotype EA, isotype F. costa rica, San Jose, Albergue de Montagne, Savegre, Sendero la Cataracta, 9° 32' 38” N, 83° 48' 51” W, 13 May 1996, on woody branch on the ground, S.M. Huhndorf, F. Fernández, SMH2448, F. u.s.A., North Carolina, Smoky Mountains National Park, Cataloochee, Rough Fork Trail, 35° 61' N, 83° 12' W, 868 m elev., 23 May 2006, 1 cm diam woody branch on ground, A.N. Miller, A.Y. Rossman, M. Sogonov, L. Vasilyeva, G.K. Mugambi, ANM933, ILLS; Tennessee: Smoky Mountains National Park, vic. of Gatlinburg, Grotto Falls Trailhead, 35° 68' N, 83° 46' W, 944 m elev., 12 July 2004, 1 cm diam woody branch on ground, A.N. Miller, S.M. Huhndorf, G.K. Mugambi, L. Ruiz-Sanchez, ANM60, ILLS.
Misturatosphaeria tennesseensis Mugambi, A.N. Mill. & Huhndorf, sp. nov. MycoBank MB516012. Fig. 33.
Etymology: Refers to the locality where the species was collected.
Ascomata erumpentia, solitaria vel dense aggregata, atrobrunnea, pyriformia, 265–398 μm alta, 201–401 μm diam, apicibus rotundatis. Asci cylindrici-clavati, breve stipitati, octospori, 84–118 x 10–11 μm. Pseudoparaphyses numerosae, septatae, in matrice mucosa, 1–2 μm diam. Ascosporae fusoides, brunneae, 3-septatae, constrictae ad septum medium, sine vagina mucosae vagina, 14–19 x 5–6 μm.
Ascomata erumpent through the bark, usually occurring singly or aggregated into small to large clusters, subiculum lacking, pyriform in shape, dark brown, ascomatal wall smooth, 265–398 μm high and 201–401 μm wide. Apices rounded, papillae are usually raised and often slightly sulcate with rounded opening. Asci cylindrical-clavate in shape, bearing short stipes, 8-spored partially biseriate, 84–118 x 10–11 μm. Pseudoparaphyses are numerous, septate, branching and anastomosing between and above the asci, held in gelatinous matrix, 1–2 μm diam. Ascospores fusiform, sometimes slightly curved, pale brown in colour, outer wall smooth, 3-septate with the septal area often of darker colour than rest of the spore, slightly constricted at the middle septum, one half of the spore composed cells that are slightly broader, possess no mucilaginous sheath, 14–19 x 5–6 μm.
Substratum: On decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Presently only known from a forested area in Tennessee, U.S.A.
Specimen examined: u.s.A., Tennessee, Cocke Co., Great Smoky Mountains National Park, Lower Mount Cammerer Trail, 35° 45' 256” N, 83°12' 329” W, 686 m elev., 19 May 2006, on woody branch, A.N. Miller, G.K. Mugambi, ANM911, holotype F, isotype ILLS.
Misturatosphaeria uniseptata Mugambi, A.N. Mill. & Huhndorf, sp. nov. MycoBank MB516013. Fig. 34.
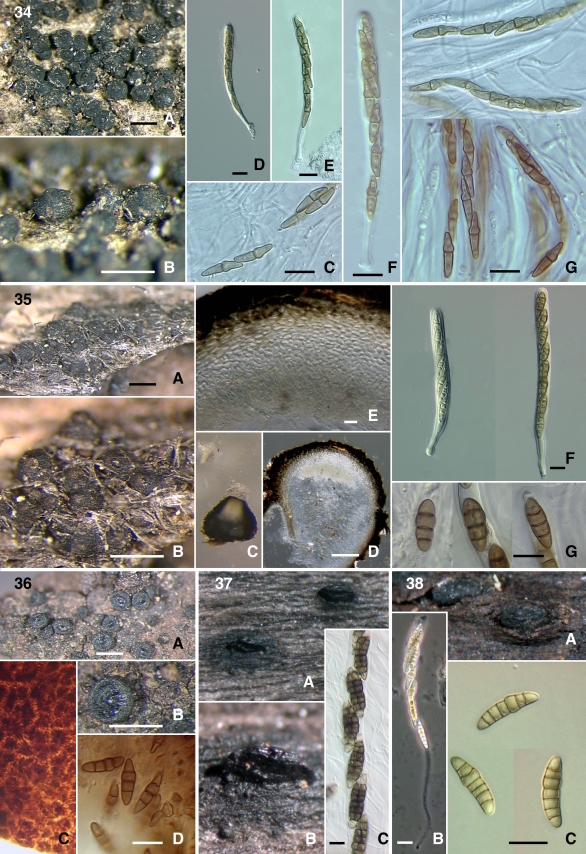
Figs 34–38.
34. Misturatosphaeria uniseptata (SMH4330) A–B. Ascomata. C, G. Ascospores. D–F Asci. 35. Misturatosphaeria uniseriata (ANM909) A–C. Ascomata. D. Ascomatal section. E. Ascomatal wall section. F. Asci. G. Ascospores. 36. Misturatosphaeria sp. (SMH3747) A–B. Ascomata. C. Ascomatal wall surface. D. Ascospores. 37. Platystomum compressum (GKM1048) A–B. Ascomata. C. Ascospores. 38. Thyridaria macrostomoides (GKM1033) A. Ascomata. B. Ascus. C. Ascospores. Scale bars: Ascomata = 500 μm. Section = 100 μm. Wall = 10 μm. Ascus = 10 μm. Ascospore = 10 μm.
Etymology: Unicus (L.) = one, refering to one septate nature of the ascospores in the species.
Ascomata superficialia, solitaria vel sparse ad dense aggregata, atrobrunnea, pyriformia ad globosa, 230–293 μm alta, 318–385 μm diam, sine subiculo, apicibus rotundatis pallide coloratis. Asci cylindrici-clavati, breve stipitati, octospori, 70–82 x 5–6 μm. Pseudoparaphyses numerosae, septatae, in matrice mucosa, 1–2 μm diam. Ascosporae fusoides, brunneae, 1-septatae, constrictae ad septum medium, since vagina mucosa, 12–14 x 3–4 μm.
Ascomata superficial, occurring singly or aggregated into small to large clusters, subiculum absent, pyriform to globose in shape, dark brown, ascomatal wall smooth, 230–293 μm high and 318–385 μm wide. Apices rounded, often with slightly raised papillae, ostiole area often of lighter colour, ostiole openings often quite prominent but appear plugged by centrum tissue. Asci cylindrical-clavate in shape and bearing short stipes, 8-spored partially biseriate or sometimes overlapping uniseriate in arrangement, 70–82 x 5–6 μm. Pseudoparaphyses numerous, septate, branching and anastomosing between and above asci, held in gelatinous matrix, 1–2 μm diam. Ascospores fusiform, sometimes slightly curved or straight, brown, outer wall smooth, 1-septate with the septal area of darker colour, upper cell usually shorter and broader than the basal cell, slightly constricted at septum, possess no mucilaginous sheath, 12–14 x 3–4 μm.
Substratum: Found on decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Currently only known from a tropical forest in Ecuador.
Specimen examined: ecuador, Orellana Province, Yasuni National Park, Botanico trail, 5 Mar. 2001, on woody branch, F.A. Fernández, A.N. Miller, R. Briones, SMH4330, holotype F.
Misturatosphaeria uniseriata Mugambi, A.N. Mill. & Huhndorf, sp. nov. MycoBank MB516014. Fig. 35.
Etymology: Unicus (L.) = one, Serialis (L.) = row, refers to single-row arrangement of the ascospores in the asci.
Ascomata erumpentia, solitaria vel dense aggregata, atrobrunnea, pyriformia ad globosa, 332–343 μm alta, 309–379 μm diam, sine subiculo, apicibus rotundatis, pallide coloratis. Asci cylindrici ad cylindrici-clavati, breve stipitati, octospori, 100–130 x 8–10 μm. Pseudoparaphyses numerosae, septatae in matrice mucosa, 1–2 μm diam. Ascosporae fusoides ad ellipticae, brunneae ad atrobrunnae, 1–3-septatae, vulgo 3-septatae, constrictae ad septa omnia, since vagina mucosa, 14–19 x 4–7 μm.
Ascomata erumpent, occurring aggregated into large clusters, subiculum lacking, pyriform to subglobose in shape, dark brown, ascomatal wall smooth, 332–343 μm high and 309–379 μm wide. Apices rounded, may possess slightly raised papillae, the ostiole area usually of lighter colour, possess prominent pore opening that appears plugged by centrum tissue. Asci cylindrical to cylindrical-clavate, bearing short stipes, 8-spored, overlapping uniseriate in arrangement, 100–130 x 8–10 μm. Pseudoparaphyses are numerous and septate, branching and anastomosing between and above the asci, held in gelatinous matrix, 1–2 μm diam. Ascospores fusiform to ellptical, brown to dark brown in colour, outer wall smooth, 1–3-septate but commonly 3-septate, occasionally constricted at all three septa, with no mucilaginous sheath, 14–19 x 4–7 μm.
Substratum: Found on decorticated woody branches on the ground.
Anamorph: Unknown.
Distribution: Currently only known from forested area in the Great Smoky Mountains National Park in Tennessee, U.S.A.
Specimen examined: u.s.A., Tennessee, Cocke Co., Great Smoky Mountains National Park, Lower Mount Cammerer Trail, 35° 45' 256” N, 83° 12' 329” W, 686 m elev., 19 May 2006, on woody branch, A.N. Miller, G.K. Mugambi, ANM909, holotype F.
Notes: The nine species newly described in Misturatosphaeria (Figs 25–27, 28–33, 34–35) were collected from wide geographic localities from Africa, North, Central and South America. An additional collection was included in the analyses (Misturatosphaeria sp., Fig. 1) that probably represents another species but asci were not seen so the specimen is not described at this time (Fig. 36). Misturatosphaeria aurantonotata bears some similarity with M. tennesseensis and M. uniseriata but differs from both in its much larger superficial ascomata and quite verruculose ascospores. It also differs in its phylogenetic placement (Figs 1, 2, 3). Misturatosphaeria tennesseensis differs from M. uniseriata in the shape and loose aggregation of the ascomata, asci that are predominantly partially biseriate in arrangement and ascospores that are paler brown and strongly constricted at the mid-septum. In contrast M. uniseriata ascomata tend to occur in large clusters, asci have predominantly overlapping uniseriate ascospore arrangement, ascospores are dark brown at maturity and are only rarely slightly constricted. Molecular data also support the separation of these two species (Figs 1, 2, 3). Misturatosphaeria uniseptata differs from all the other species in the group in its pale brown, 1-septate ascospores. Misturatosphaeria minima and M. kenyensis are quite similar in their morphologies but the former differs in its ascomata that are solitary or in small groups usually less than 5 individuals. Misturatosphaeria kenyensis ascomata are aggregated in larger clusters and ascospores are slightly broader. Misturatosphaeria cruciformis shares ascospore morphology with M. claviformis, but the former differs by having much larger ascomata that are predominantly pyriform and ascospores that are often constricted at the middle septum. Misturatosphaeria claviformis has smaller pyriform to obclavate ascomata, and ascospores that are very rarely slightly constricted. Molecular data also indicate the two species are distantly related (Figs 1, 2, 3). In addition to species of Misturatosphaeria, the sixth plate of figures contains two taxa illustrated for morphological comparison: Platystomum compressum (Fig. 37), and Thyridaria macrostomoides (Fig. 38). Both species share features with members of the Lophiostomataceae but only T. macrostomoides finds placement in the family based on molecular data (Figs 1, 2, 3).
DISCUSSION
Melanommataceae
Phylogenetic analyses recovered Melanommataceae, Lophiostomataceae, Hypsostromataceae, and a few others as strongly supported clades within the Pleosporales (Figs 1, 2, 3). Although some genera currently accepted in the Melanommataceae, such as Melanomma, Pseudotrichia and Byssosphaeria, were recovered within the family, others such as Ostropella and Xenolophium are nested outside the family (Figs 1, 2, 3). Herpotrichia and Bertiella currently reside in other families (Kirk et al. 2008) but they, along with a single representative of Pleomassaria siparia, find their placement within the Melanommataceae based on our data. Byssosphaeria, Herpotrichia, and Pseudotrichia are taxa that have been united in the past, mostly under Herpotrichia and all share the distinctive characteristic that at least some species bear subiculate ascomata (Bose 1961, Sivanesan 1971, Barr 1984). Previous analyses of the Pleosporales have included the GenBank sequences Lophiostoma macrostomum (DQ384094) and / or Trematosphaeria pertusa (DQ678020) that nested within Melanommataceae. Although voucher specimens were not obtained to verify their identity, we strongly believe that these collections were misidentified. Lophiostoma macrostomum, the type species of the Lophiostoma, has already been confirmed to reside outside Melanommataceae (Tanaka & Hosoya 2008), which was corroborated by the results of this study (Figs 1, 2, 3). Recently, Zhang et al. (2008) using the epitype strain of Trematosphaeria pertusa have also demonstrated that this species is not closely related to Melanomma and belongs outside Melanommataceae s. str. This is corroborated here by our own collection of T. pertusa (Fig. 1).
The genus Melanomma formed the weakest structure in the clade. While the family was strongly supported, the genus did not unite in a strong clade. All five collections of Melanomma pulvis-pyrius clustered at the base of the family clade in the LSU tree, separated by very short branches (two collections appeared to be identical). The other represented species, M. rhododendri was on a long branch near M. pulvis-pyrius. Morphologically our collections fit within the genus, both species having the clustered, superficial ascomata and small 3-septate brown ascospores in a uniseriate arrangement in the asci (Figs 16–17). It appears that additional collections and other genes might be necessary to understand how the specimens and species relate to each other.
In contrast, a strongly supported, monophyletic Byssosphaeria was recovered, which in this study was represented by six species, three of them with multiple collections (Figs 1, 2, 3). Byssosphaeria was described for taxa bearing superficial ascomata, separate or usually gregarious, turbinate, globose to ovoid and with rounded or minute papilla and opening by rounded pore. The pore and surrounding cells are pallid, whitish, or grey, or bright yellow, orange or red pigmented and the pore region appearing sulcate or plicate at times. Ascomatal wall surfaces are often irregular or slightly roughened, with protruding cells and often bear hyphal hairs (described as appendages in Barr 1984). Asci are clavate to cylindric, peripherally arranged and 8-spored. Ascospores are at first hyaline becoming light reddish brown or clear brown, ellipsoid or fusoid (Barr, 1990a). Byssosphaeria schiedermayeriana, the type species of the genus, is represented by five collections that form a strongly supported clade. The collections are neither morphologically nor molecularly identical. Morphologically the main differences seen are in the ascomatal structure. The collections differ in their amount of subiculum or hyphal hairs, amount of vertical collapsing (tendency toward becoming collabent, see Fig. 11A) and in the amount of colouration around the pore area (Figs 10A, 11A, 12A, 13A, 14A, 15A). The ascospores of these collections have measurements within the size range for the species given by Barr (1984), 25–42 x 5–9 μm and a sheath is seen in some of the collections (Figs 10D, 13D). Nested within the clade is the sequence of B. diffusa from GenBank (as Herpotrichia in GenBank) and one collection representing B. salebrosa. Byssosphaeria diffusa is reported to differ from B. schiedermayeriana in having a pallid or whitish pore area and smaller ascospores (Sivanesan 1971, Barr 1984). Byssosphaeria salebrosa is distinguished by non-subiculate ascomata with a surface that is roughened by projecting masses of cells, a feature present in our collection (Fig. 14B, C). Additional collections with the characteristics of these two species should be added to see if these morphological characters are phylogenetically informative.
Byssosphaeria rhodomphala and B. jamaicana, each represented by several collections, occur in a strongly supported sister relationship (Fig. 1). The collections of Byssosphaeria jamaicana fit well within the decription of the species (Figs 4–5). The subiculate, clustered ascomata, varying in the amount of hyphal hairs, have a pale-coloured pore area, the ascospores appear to lack appendages and in at least one of our collections, the ascospores turn somewhat darker brown and become 3-septate (Fig. 4D) (as was illustrated for the species by Sivanesan 1971). Five collections represent B. rhodomphala and morphologically and molecularly they are remarkably consistent given that they are geographically distant (Figs 1, 6, 8). Among the collections, the pore region of the ascomata can show variation from red to orange to yellowish granular deposits and can macroscopically appear quite dark. The most distinct characteristic of this species and the most useful aid in identification is the oblong-ellipsoid ascospore with obtuse ends. Byssosphaeria rhodomphala differs from B. jamaicana by the colouration of the ascomatal pore region. Hyphal appendages are lacking in B. rhodomphala and the ascospores are smaller than in B. jamaicana (Barr 1984).
On a basal branch in the Byssosphaeria clade is a single collection of B. villosa. The species is distinguished by ascomata bearing a dense covering of outwardly-projecting, long villose hairs and a pale coloured apex (Samuels & Müller 1978). Its general appearance was described as being “similar to Lasiosphaeria phyllophila” (= Iodosphaeria) (Samuels & Müller 1978), an observation that aided us in correctly identifying our own collection. Our collection differs from the description by having a distinctly yellow pore area (Fig. 9). However, Samuels & Müller's (1978) description of a bright yellow colour in the colonies they obtained in pure culture allowed us to conclude that the colour of the pore area might vary among collections of the species.
Bertiella also found its placement in the Melanommataceae in contrast to the Teichosporaceae where it was previously placed (Lumbsch & Huhndorf 2007). Bertiella macrospora is represented by three collections (Costa Rica, Kenya, U.S.A.) and slightly different placements in the LSU and TEF trees. It was unfortunate that we were not able to sequence one of the same collections for both genes. The collections show the distinctive arrangement of thick-walled and highly melanised ascomatal wall cells bearing bands of less pigmented, thin-walled cells (Fig. 7) that was described by Eriksson & Yue (1986) as “resembling cephalothecioid”. As reported for the type specimen (Eriksson & Yue 1986), all three recent collections have ascomata that are superficial, gregarious and non-papillate (Fig. 7) with a roughened surface and lacking appendages or subiculum. The main difference occurs in the size of the ascospores. Whereas Eriksson & Yue (1986) describe the type specimen as having ascospores in the size range of 37–43 x 8–9 μm, our collections have smaller ascospores, ranging 22–30 x 5–7 μm in size. Based on the description given by Barr (1984), another taxon that may be similar to these collections is Byssosphaeria semen. This species differs from other Byssosphaerias in having a roughened ascomatal surface that lacks hairs or subiculum and has ascospores in the same range as our collections. The main difference is that no cephalothecioid cell arrangement is reported for this species and this is quite a prominent feature of our collections.
Barr (1984) revised the genus Herpotrichia and in the process re-established several genera, including Byssosphaeria and Pseudotrichia that had been in synonymy. She suggested that Herpotrichia belonged in Massarinaceae, a family later synonymised with Lophiostomataceae (Barr 1987). Herpotrichia is characterised by immersed, erumpent or superficial ascomata, with tomentum, rounded apex opening by broad pore. The asci are clavate or cylindrical, basal and pseudoparaphyses are narrowly cellular. The ascospores are fusoid, ellipsoid or oblong, usually surrounded by a mucilaginous sheath, and are hyaline becoming light yellow to reddish brown, mostly 1-septate but developing more septa with age. Herpotrichia is represented in our analyses by H. herpotrichoides, H. juniperi and H. macrotricha, and these taxa collectively do not form a monophyletic group in the LSU analyses. Subsets of these taxa group together in the TEF and combined gene trees. Two collections are tentatively identified as H. herpotrichoides, the type species of the genus. Morphologically the collections match this species and they do not differ significantly from each other (Figs 23–24); however in the LSU tree they occur in the same clade but separate from each other. The collections are geographically distant (one from the U.S.A. and one from Kenya) so a sequenced European collection would be useful to aid in the placement of the species. Herpotrichia juniperi, represented by two strains obtained from GenBank grouped consistently separate from the other two species. They also did not appear to closely align with the other taxa in the clade but occupied a position near Melanomma (Fig. 1). Assuming these collections were correctly identified, the results presented suggested that H. juniperi may belong outside the genus and thus may bear affinities to Melanomma. However, this will only become clear with broader taxon sampling in the groups. The described morphologies of Melanomma and Herpotrichia overlap in many ways, with both sharing immersed, erumpent to superficial ascomata that are tomentose and usually sit on an ample subiculum. Asci and ascospore morphologies are also similar, although in Herpotrichia ascospores tend to initially start hyaline and 1-septate later become brown and more septate. Since the Melanomma clade did not receive strong support the true placement of H. juniperi or its relationships to the taxa in the genus remain unclear. Herpotrichia macrotricha is reported to have a wide distribution (Barr 1984) and the five collections of H. macrotricha (representatives from Costa Rica, Kenya and Puerto Rico) form a strongly supported clade (Fig. 1). The species is easily recognised by its distinctive broad cap-like ascomatal apex with a thick inner layer of hyaline pseudoparenchymatous cells (Figs 19–22). One collection stands out from the rest both in sequence data and morphology. The Costa Rican collection (SMH4913, V on Fig. 1) differs from the others in having an upper, ascomal wall with a central hyaline layer that gives the ascomata a wrinkled, collapsed appearance in the dried collections (Fig. 22). It was thought perhaps to be a separate species but other known species should be checked before making that decision.
Pseudotrichia was considered to be in the family Platystomaceae by Barr (1990b), but is correctly placed in the Melanommataceae as in the most recent outline of the Ascomycota in Myconet (Lumbsch & Huhndorf 2007). The type species, P. mutabilis, is represented by two U.S.A. collections and in our LSU tree occurs as a sister taxon to the single collection of Pleomassaria. Pseudotrichia mutabilis is distinctive and easily identified with its gregarious, yellow-green tomentose ascomata and its hyaline, fusiform, septate ascospores (Fig. 18). It is a common entity on decaying wood in temperate forests. As currently circumscribed however, the genus is not monophyletic as our other included species, P. guatopoensis, finds a placement among the taxa in the unsupported Platystomaceae clade.
Pleomassaria siparia (lectotype species of the genus) is represented by two LSU sequences in GenBank (one used here), both coming from the same CBS 279.74 culture. In our analyses the species is nested within the Melanommataceae in the clade with Pseudotrichia mutabilis. According to Barr (1982: 370) Pleomassaria siparia is characterised by immersed, depressed globose ascomata containing oblong asci with simple muriform ascospores. The ascospores are dark brown with a verruculose surface, with 5–7 transverse septa and one longitudinal septum in several cells. The characteristics this species shares with others in the clade are not obvious. Our other collection identified as belonging in the family (Pleomassariaceae; SMH5232) does not cluster with P. siparia but rather finds a placement among the taxa in the unsupported Platystomaceae clade. Additionally, our other unpublished phylogenetic analyses using sequences from putative Asteromassaria and Splanchnonema species (other taxa that are arranged in the morphologically defined Pleomassariaceae) found that these species also do not cluster with P. siparia. It will be necessary to have additional sequences from other collections of the species to confirm its placement.
In the LSU tree, Ostropella and Xenolophium occur distantly related to Melanommataceae in a clade lacking significant support that also includes Pseudotrichia guatopoensis, Platystomum compressum (Fig. 37) and an unnamed Pleomassariaceae (Fig. 1). The clade, however, received significant PP support in the combined-gene analysis (Fig. 3). Ostropella and Xenolophium share a combination of morphological characters that appears distinct from that observed in Melanommataceae s. str. They possess relatively large ascomata, bearing raised apices with slit-like ostiolar openings. The asci in these two genera are clavate with quite long stipes, which is unlike taxa that are here treated in Melanommataceae. The mostly tropical collections of Ostropella, Xenolophium, and P. guatopoensis all share the distinctive, extensive network of trabeculate pseudoparaphyses that Barr (1983, 1990a) emphasised as an important diagnostic character that separates Melanommatales from Pleosporales. Subsequent studies using DNA sequence data have established that pseudoparaphysis type is not a phylogenetically informative character at the ordinal level and hence separation of Melanommatales from Pleosporales based on this character is not supported (Liew et al. 2000, Lumbsch & Lindemuth 2001, Kruys et al. 2006, Schoch et al. 2006, Wang et al. 2007). The results of the phylogenetic analyses presented in this study corroborate these findings. Additional genera in this clade are Ulospora bilgramii and Verruculina enalia whose sequences were obtained from GenBank. Since the clade for the most part did not receive significant support, further studies involving more collections and more markers are needed to confirm the observed relationships. Furthermore, the occurrence of P. guatopoensis and P. compressum in this clade, which were previously not thought to be related to Ostropella and Xenolophium, underscores the need for more work to establish with confidence the nature of the relationships.
Lophiostomataceae
Our analyses recovered a strongly supported Lophiostomataceae comprised of Lophiostoma and some species currently placed in Thyridaria. Sister to the Lophiostoma clade is the strongly supported clade of the genus Misturatosphaeria comprising nine new species (Figs 1, 2, 3). The genus Lophiostoma was established by Fries (1849) to accommodate taxa that possess mostly erumpent ascomata, bearing apices that are raised and laterally compressed with phragmosporous hyaline or brown ascospores. Since its inception, many more species have been added to the genus but the taxonomy of the group remains uncertain, requiring urgent revision. Chesters & Bell (1970) and Holm & Holm (1988) provide comprehensive information on the taxonomic history and morphology of the genus. Barr (1990a) transferred several species of Lophiostoma into Thyridaria, which she placed in the Platystomaceae that included T. macrostomoides. We had among our specimens, collections that bear the morphology of the species and in analyses T. macrostomoides groups separate from taxa in Lophiostoma (Figs 1, 2, 3). Thyridaria macrostomoides (Fig. 38) collections occur in an unsupported sister relationship with Misturatosphaeria in single-gene trees but receives significant PP in the combined-gene tree (Figs 1, 2, 3). Barr (1990a) described Thyridaria to include taxa with the following morphology: ascomata that are immersed or erumpent, in valsoid groups or separate or gregarious; ascomata globose with a well developed papilla or short beak that is rounded or compressed; ostioles that are rounded or slit-like and periphysate or filled with pallid or brightly pigmented hyphal ends. Asci were described as clavate or cylindrical with trabeculate pseudoparaphyses held in gelatinous matrix. Ascospores are brown, symmetric or asymmetric, phragmosporous, three or more septate, smooth, verruculose or striate. The results presented here demonstrate that at least one of the species of Thyridaria resides in Lophiostomataceae. However, since we did not include the type species of Thyridaria in our analyses the generic placement remains unclear.
Misturatosphaeria differs from other genera in Lophiostomataceae by possessing ascomata that are erumpent to superficial, with rounded apices that are often raised. Ostiolar openings are rounded and plugged by gelatinous tissue and occasionally lighter coloured. Asci are cylindrical to clavate with phragmosporous or dictyosporous ascospores (Figs 25–27, 28–33, 34–36). Despite morphological differences of Misturatosphaeria from other lophiostomataceous fungi, we feel justified in placing it in Lophiostomataceae at this point due to the strong support the clade received in our analyses (Figs 1, 2, 3). The only other genus with dictyospores accepted by Barr (1990b) in this family is Cilioplea. This genus was not included in our analyses but it differs markedly from the dictyosporous members of Misturatosphaeria and others in the group in general. It possesses ascomata with thick walled brown or dark brown setae around the apex and a narrow peridium of few internal rows of compressed pallid cells, surrounded by brown hyphae into the substrate. It also differs in the ascospore shape and septation, with the ascospore longitudinal septum in this genus primarily limited to the central cells (Barr 1990b).
Hypsostromataceae
Huhndorf (1992) described the genus Hypsostroma for two tropical species, H. saxicola and H. caimitalensis. She did not prefer familial placement at the time but suggested they bear affinities in the Melanommatales (= Pleosporales). Later, Huhndorf (1994) erected family Hypsostromataceae for the genera Hypsostroma and Manglicola. The two species of Hypsostroma were included in our analyses and they grouped together in a strongly supported clade within Pleosporales distinct from other families in the order (Fig 1). This study is only the second time the two specimens used for sequencing have been collected and this expands their range outside of the Caribbean and South America. Hypsostroma is distinctive in having asci with extremely long stipes, similar to those found in Ostropella and Xenolophium but much longer (Huhndorf 1992, 1994). Although the clade shows no supported relationship with the Platystomaceae taxa, X. guianense tentatively joins the clade in the LSU tree and the morphological similarities of these long-stipitate taxa suggests that they may in some way be related.
CONCLUSION
The Melanommataceae is found to contain taxa that have gregarious, superficial ascomata and the ascomata may be smooth (Byssosphaeria p.p.), roughened (Melanomma, Bertiella macrospora), or clothed in hyphal hairs (Herpotrichia, Byssosphaeria p.p.) or coloured tomentum (P. mutabilis). Subicular hyphae may be present in some taxa in the family (Herpotrichia, Byssosphaeria) or absent (Melanomma, Bertiella). Cephalothecioid-like ascomatal wall structure (Bertiella macrospora), versicolourous-layered walls (H. macrotricha p.p.) or uniformly brown-pigmented walls (Byssosphaeria, Melanomma) occur in the family. The ascospores that occur in the group are hyaline or brown, mostly fusiform in shape and 1–3-septate. Anomalous in the family is the presence of Pleomassaria siparia, differing from the other sequenced taxa by having immersed ascomata and muriform ascospores. Former family members, Ostropella and Xenolophium are found to occur outside the Melanommataceae in a weakly supported group along with Platystomum compressum and Pseudotrichia guatopoensis, that may correspond to the family Platystomaceae.
Lophiostomataceae occurs as a strongly supported monophyletic group but its concept is here expanded to include a new genus Misturatosphaeria that bears morphology traditionally not known to occur in the family. The gregarious ascomata of Misturatosphaeria, especially M. minima, suggests a resemblance and potential relationship to Byssosphaeria species or other taxa in the Melanommataceae. The molecular data shows this not to be the case. The ascomata differ with papillate apices present in Misturatosphaeria versus plane to collapsed apices in Byssosphaeria, along with the lack of hyphal hairs or subiculum in the former. Distinctive characteristics of Misturatosphaeria are the tendencies towards lighter coloured apices (M. aurantonotata) and plugged ostioles (M. minima, M. uniseriata). Additionally the ascomata in the group are actually mostly erumpent from the substrate and not superficial as in Byssosphaeria. The mixture of didymosporous, phragmosporous to dictyosporous ascospore morphologies that gives the genus its name was problematic and for awhile we thought that more than one generic clade would resolve but the molecular data did not provide that satisfaction. Some of the phragmosporous species have ascospores that resemble those in Lophiostoma s. l.
Acknowledgments
This work was supported in part by NSF PEET Grant (Partnerships for Enhancing Expertise in Taxonomy) DEB–0118695. The authors are grateful to A.N. Miller for providing access to specimens and to A.N. Miller, INBio, P. Johnston and D.J. Lodge for fieldwork assistance. The National Museums of Kenya provided logistical support for the fieldwork carried out in Kenya. Sequences were generated in the Pritzker Laboratory for Molecular Systematics and Evolution at The Field Museum of Natural History.
Taxonomic novelties: Misturatosphaeria Mugambi & Huhndorf, gen. nov., M. aurantonotata Mugambi & Huhndorf, sp. nov., M. claviformis Mugambi & Huhndorf, sp. nov., M. cruciformis Mugambi & Huhndorf, sp. nov., M. kenyensis Mugambi & Huhndorf, sp. nov., M. minima Mugambi, A.N. Mill. & Huhndorf, sp. nov., M. tennesseensis Mugambi, A.N. Mill. & Huhndorf, sp. nov., M. uniseptata Mugambi, A.N. Mill. & Huhndorf, sp. nov., M. uniseriata Mugambi, A.N. Mill. & Huhndorf, sp. nov.
References
- Barr ME (1982). On the Pleomassariaceae (Pleosporales) in North America. Mycotaxon 15: 349–383. [Google Scholar]
- Barr ME (1983). The ascomycete connection. Mycologia 75: 1–13. [Google Scholar]
- Barr ME (1984). Herpotrichia and its segregates. Mycotaxon 20: 1–38. [Google Scholar]
- Barr ME (1987). Prodomus to class Loculoascomycetes. Amherst, MA. Published by the author.
- Barr ME (1990a). Melanommatales (Loculoascomycetes). North American Flora, Series II, 13: 1–129. [Google Scholar]
- Barr ME (1990b). Some dictyosporous genera and species of Pleosporales. Memoirs of New York Botanic Garden 62: 1–92. [Google Scholar]
- Berbee ML (1996). Loculoascomycetes origins and evolution of filamentous ascomycete morpholology based on 18A rRNA gene sequence data. Molecular Biology and Evolution 13: 462–470. [DOI] [PubMed] [Google Scholar]
- Bose SK (1961). Studies in Massarina Sacc. and related genera. Phytopathologische Zeitschrift 41: 151–213. [Google Scholar]
- Chesters CGC, Bell A (1970). Studies in the Lophiostomataceae Sacc. Mycological Papers 120: 1–55. [Google Scholar]
- Crivelli PG (1983). Über die heterogene Ascomycetengattung Pleospora Rabh.; Vorschlag für eine Aufteilung. Diss. ETH Zürich 7318: 1–213. [Google Scholar]
- del Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch HT (2005). Molecular data place Trypetheliaceae in Dothideomycetes. Mycological Research 110: 511–520. [DOI] [PubMed] [Google Scholar]
- Edgar RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O (1981). The families of bitunicate ascomycetes. Opera Botanica 60: 1–220. [Google Scholar]
- Eriksson O, Yue J-Z (1986). Bertiella (Sacc.) Sacc. & Sydow a synonym of Massarina Sacc. Mycotaxon 27: 247–253. [Google Scholar]
- Fries E (1849). Summa vegetabilium Scandinaviae 2. Uppsaliae.
- Höhnel F von (1918). Mycologische Fragmente. Annales Mycologici 16: 35–174. [Google Scholar]
- Holm L (1957). Etudes taxonomique sur les Pléosporacées. Symbolae Botanicae Uppsaliensis 14(3): 1–188. [Google Scholar]
- Holm L, Holm K (1988). Studies in the Lophiostomataceae, with emphasis on the Swedish species. Acta Universitatis Uppsaliensis, Symbolae Botanicae Uppsaliensis 28(2): 1–50. [Google Scholar]
- Holm L, Yue J-Z (1988). Notes on some fungi referred to Schizostoma Ces. De Not. Ex Sacc. Acta Mycologica. Sinica Supplement 1: 82–89. [Google Scholar]
- Huelsenbeck JP, Ronquist F (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Huhndorf SM (1992). Neotropical Ascomycetes 2. Hypsostroma, a new genus from the Dominican Republic and Venezuela. Mycologia 84: 750–758. [Google Scholar]
- Huhndorf SM (1993). Neotropical Ascomycetes 3. Reinstatement of the genus Xenolophium and two new species from French Guiana. Mycologia 85: 490–502. [Google Scholar]
- Huhndorf SM (1994). Neotropical Ascomycetes 5. Hypsostromataceae, a new family of Loculoascomycetes and Manglicola samuelsii, a new species from Guyana. Mycologia 86: 266–269. [Google Scholar]
- Huhndorf SM, Fernández FA (1998). Neotropical Ascomycetes 7. Caudatispora biapiculatis sp. nov. from Puerto Rico. Sydowia 50: 200–204. [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA (2008). Dictionary of the Fungi. 10th edn. CABI International, U.K.
- Kruys A, Eriksson OE, Wedin M (2006). Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycological Research 110: 527–536. [DOI] [PubMed] [Google Scholar]
- Leuchtmann A (1984). Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia 37: 75–194. [Google Scholar]
- Liew EC, Aptroot A, Hyde KD (2000). Phylogenetic significance of the pseudoparaphyses in loculoascomycetes taxonomy. Molecular Phylogenetics and Evolution 16: 392–402. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Lindemuth R (2001). Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycological Research 105: 901–908. [Google Scholar]
- Lumbsch HT, Huhndorf SM (2007). Outline of Ascomycota – 2007. Myconet 13: 1–58. [Google Scholar]
- Luttrell ES (1955). The ascostromatic ascomycetes. Mycologia 47: 511–532. [Google Scholar]
- Müller E, Arx JA von (1962). Die Gattungen der didymosporen Pyrenomyceten. Beiträge zur Kryptogamen-Flora der Schweiz 11: 1–922. [Google Scholar]
- Nitschke T (1869). Grundzüge eines Systems der Pyrenomyceten. Verhandlungen des Naturalhistorischen Vereines der Preussischen Rheinlande und Westphalens 26(2): 70–77. [Google Scholar]
- Nylander JA, Wilgenbusch JC, Warren DL, Swofford DL (2007). AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583. [DOI] [PubMed] [Google Scholar]
- Petrak F (1940). Mykologische Notizen. 13. Annales Mycologici 38: 181–267. [Google Scholar]
- Posada D, Crandall KA (1998). ModelTest: testing the model of DNA substitution. Bioinformatics 49: 817–818. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Oliver JF, Marin A, Medina JR (1990). The general stochastic model of nucleotide substitutions. Journal of Theoretical Biology 142: 485–501. [DOI] [PubMed] [Google Scholar]
- Samuels GJ (1973). The genus Macbridella with notes on Calostilbe, Herpotrichia, Phaeonectria, and Letendraea. Canadian Journal of Botany 51: 1275–1283. [Google Scholar]
- Samuels GJ, Müller E (1978). Life-history studied of Brazilian Ascomycetes 4. Three species of Herpotrichia and their Pyrenochaeta-like anamorphs. Sydowia 31: 157–168. [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. (2009). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Sivanesan A (1971). The genus Herpotrichia Fuckel. Mycological Papers 127: 1–37. [Google Scholar]
- Stamatakis A (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stevens FL (1925). Hawaiian fungi. Bernice P. Bishop Museum Bulletin 19: 97–98. [Google Scholar]
- Tanaka K, Hosoya T (2008). Lophiostoma sagittiforme sp. nov., a new ascomycete (Pleosporales, Dothideomycetes) from Island Yakushima in Japan. Sydowia 60: 131–145. [Google Scholar]
- Vilgalys R, Hester M (1990). Rapid identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Aproot A, Crous PW, Hyde KD, Jeewon R (2007). The polyphyletic nature of Pleosporales: an example of Massariosphaeria based on rDNA and RPB2 gene phylogenies. Mycological Research 111: 1268–1276. [DOI] [PubMed] [Google Scholar]
- Winka K (2000). Phylogenetic relationship within the Ascomycota based on 18S rDNA sequences. Ph.D. Thesis. Umeå University, Umeå.
- Zhang Y, Fournier J, Pointing SB, Hyde KD (2008). Are Melanomma pulvi-pyrius and Trematosphaeria pertusa congeneric? Fungal Diversity 33: 47–60. [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de, et al. (2009). Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary reevaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]