Abstract
We present a revised phylogeny of lichenised Dothideomyceta (Arthoniomycetes and Dothideomycetes) based on a combined data set of nuclear large subunit (nuLSU) and mitochondrial small subunit (mtSSU) rDNA data. Dothideomyceta is supported as monophyletic with monophyletic classes Arthoniomycetes and Dothideomycetes; the latter, however, lacking support in this study. The phylogeny of lichenised Arthoniomycetes supports the current division into three families: Chrysothrichaceae (Chrysothrix), Arthoniaceae (Arthonia s. l., Cryptothecia, Herpothallon), and Roccellaceae (Chiodecton, Combea, Dendrographa, Dichosporidium, Enterographa, Erythrodecton, Lecanactis, Opegrapha, Roccella, Roccellographa, Schismatomma, Simonyella). The widespread and common Arthonia caesia is strongly supported as a (non-pigmented) member of Chrysothrix. Monoblastiaceae, Strigulaceae, and Trypetheliaceae are recovered as unrelated, monophyletic clades within Dothideomycetes. Also, the genera Arthopyrenia (Arthopyreniaceae) and Cystocoleus and Racodium (Capnodiales) are confirmed as Dothideomycetes but unrelated to each other. Mycomicrothelia is shown to be unrelated to Arthopyrenia s.str., but is supported as a monophyletic clade sister to Trypetheliaceae, which is supported by hamathecium characters. The generic concept in several groups is in need of revision, as indicated by non-monophyly of genera, such as Arthonia, Astrothelium, Cryptothecia, Cryptothelium, Enterographa, Opegrapha, and Trypethelium in our analyses.
Keywords: Arthoniomycetes, Ascolocularous fungi, bitunicate fungi, Dothideomycetes, lichens, phylogeny, ribosomal DNA
INTRODUCTION
Mutualism is one of the three main modes of nutrition within Ascomycota, besides saprotrophism and parasitism. A large number of mutualistic ascomycetes form symbiotic relationships with algae and/or cyanobacteria, so-called lichens. Of the 64 000 species currently accepted in Ascomycota (Kirk et al. 2008), about almost 30 % (17 600) are lichen-forming fungi (Feuerer & Hawksworth 2007, Kirk et al. 2008). Lichenised fungi differ from all other fungi in the formation of complex, persistent vegetative thalli, which makes them a prime subject for evolutionary studies.
It was long believed that lichens evolved several times independently within Ascomycota (and Basidiomycota), an idea supported by the first molecular study testing this hypothesis (Gargas et al. 1995). Lutzoni et al. (2001, 2004) were unable to conclusively determine whether there were multiple gains of lichenisation or whether an initial lichenisation event occurred deep within Ascomycota, however, Lutzoni et al. (2001) found some Eurotiomycetes to be secondarily de-lichenised. This is particularly intriguing as Eurotiomycetes includes economically important fungi in the genera Aspergillus and Penicillium that feature a complex secondary chemistry similar to that found in lichens produced by homologous polyketide synthase genes (Grube & Blaha 2003, Kroken et al. 2003, Schmitt et al. 2005, Schmitt & Lumbsch 2009).
Since then, the phylogeny and classification of Ascomycota has further advanced (Lindemuth et al. 2001, Lumbsch et al. 2001, 2002a, b, 2004, Grube et al. 2004, Lücking et al. 2004, Lutzoni et al. 2004, Persoh et al. 2004, Wedin et al. 2005, del Prado et al. 2006, Miadlikoswka et al. 2006, Schmitt et al. 2006, Spatafora et al. 2006, Hibbett et al. 2007, Hofstetter et al. 2007, Lumbsch & Huhndorf 2007a, Schoch et al. 2006, 2009a, b, c). Our current understanding suggests that there were several lichenisation events but also some major delichenisation events during the evolution of Ascomycota (Gargas et al. 1995, Lutzoni et al. 2001, Liu & Hall 2004, Gueidan et al. 2008, Schoch et al. 2009a). The largest clade of lichenised fungi, Lecanoromycetes, with 14 000 accepted species, appears to be the result of a single lichenisation event with at least one major delichenisation event in Ostropales and several delichenisation events throughout the class (Lumbsch et al. 2004, Persoh et al. 2004, Wedin et al. 2005, Miadlikoswka et al. 2006, Hofstetter et al. 2007, Schoch et al. 2009a, Baloch et al. in prep.). A similar pattern is suggested within the second largest lichenised clade, Arthoniomycetes, with about 1 500 species (Tehler 1995, Myllys et al. 1998, Sundin 2000, Tehler & Irestedt 2007, Ertz et al. 2008). This class was recently shown to include the mazaediate genus Tylophoron (Lumbsch et al. 2009a), previously considered to be related to pyrenocarpous lichens (Aptroot et al. 2008). Arthoniomycetes is composed primarily of lichenised fungi producing apothecia or apothecioid ascomata with partially ascolocular development and bitunicate asci (Henssen & Jahns 1974, Eriksson & Winka 1997). The base of this clade was reconstructed as lichenised (Schoch et al. 2009a) and it is presumed that non-lichenised and lichenicolous species within the class represent reversions to the unlichenised state. One family that has not yet been confirmed within Arthoniomycetes using molecular data is Chrysothrichaceae, a small family of two genera (Byssocaulon, Chrysothrix) and little over 20 species (Kirk et al. 2008). The third primarily lichenised class is Lichinomycetes (350 species).
The remaining lichenised fungi are primarily restricted to Dothideomycetes and Eurotiomycetes (subclass Chaetothyriomycetidae). Gueidan et al. (2008) demonstrated that lichenisation may have evolved at least twice within Eurotiomycetes (once at base of Verrucariales and once at base of Pyrenulales), though, this is uncertain as the ancestral state of the common ancestor to Pyrenulales, Verrucariales and Chaetothyriales, is not unambiguously resolved (Gueidan et al. 2008, Schoch et al. 2009a). Within both Verrucariales and Pyrenulales, there appears to be at least one loss of lichenisation each. Dothideomycetes and Arthoniomycetes together form the rankless clade Dothideomyceta, a name introduced by Schoch et al. (2009a, b). The ancestral state of Dothideomyceta and Dothideomycetes nodes are not resolved with confidence (Gueidan et al. 2008, Schoch et al. 2009a, b). In this paper we do not aim to resolve this issue but rather attempt to clarify, confirm or reject the placement of lichenised lineages within Dothideomyceta, specifically Dothideomycetes.
The following families have been confirmed or are believed to belong in either Chaeothyriomycetidae or Dothideomycetes: Verrucariaceae (930 species), Pyrenulaceae (280 species), Celotheliaceae (eight species), Microtheliopsidaceae (three species), and Pyrenothrichaceae (three species) in Chaetothyriomycetidae (Herrera-Campos et al. 2005, del Prado et al. 2006, Lücking 2008), and Trypetheliaceae (200 species), Monoblastiaceae (130 species), Strigulaceae (120 species), and Arthopyreniaceae (120 species) in Dothideomycetes (Lutzoni et al. 2004, del Prado et al. 2006, Lumbsch & Huhndorf 2007b). Most of these families have traditionally been placed within Pyrenulales (Poelt 1973, Henssen & Jahns 1974, Hafellner 1986, Kirk et al. 2001, Eriksson et al. 2004, Cannon & Kirk 2007), and much of the confusion regarding previous classifications of these pyrenocarpous lichens stems from the fact that Pyrenulales were at some point considered synonymous with the ascolocular Melanommatales (currently regarded synonymous with Pleosporales; Barr 1980, Harris 1984, 1990, 1991, 1995), whereas other workers considered Pyrenulales to be ascohymenial (Henssen & Jahns 1974). The fact that Trypetheliaceae have no close relative within Dothideomycetes was reflected in the establishment of a separate order, Trypetheliales (Aptroot et al. 2008).
In addition to the aforementioned families, there are several genera of uncertain position, such as Cystocoleus and Racodium, both of which belong in Capnodiales/Dothideomycetes (Muggia et al. 2007), as well as Julella, Mycoporum, Collemopsidium (Pyrenocollema), and others, of unconfirmed affinities (Harris 1995). Yet other lineages, such as the recently discovered Eremithallus (Lücking et al. 2008) or the genera Thelocarpon and Vezdaea (Reeb et al. 2004, Lumbsch et al. 2009b) appear to fall outside the currently accepted classes known to contain lichen-forming fungi. The current phylogeny of Chaetothyriomycetidae suggests that the two large lichen-forming families in this subclass may have emerged from distinct lichenisation events, however, this could not be resolved with confidence (see node 18 in fig. 1 and table 1 of Gueidan et al. 2008, Schoch et al. 2009a). It thus appears that Dothideomycetes, the largest class of Ascomycota with an estimated number of 19 000 species (Kirk et al. 2008), a class that has largely been neglected when assessing the phylogeny of lichenised fungi, might be the only class within Ascomycota containing several lineages that evolved through independent lichenisation. In addition to Trypetheliaceae, at least two other families, which exhibit substantial radiation accompanied with morphological variation at the generic and species level (Monoblastiaceae and Strigulaceae) have been suggested to belong to Dothideomycetes. The only sequenced species of Strigula has been suggested to belong to Eurotiomycetes (Schmitt et al. 2005); however, re-examination of the specimen used in this study showed that it belonged in Verrucariaceae. Therefore the phylogenetic position of Strigulaceae remains unresolved. In addition, Anisomeridium polypori (Monoblastiaceae) was suggested to belong to Dothideomycetes (James et al. 2006).
In this paper, we are using nuclear large subunit (nuLSU) and mitochondrial small subunit (mtSSU) rDNA data, to construct a phylogeny of lichenised fungi with bitunicate asci, focusing on Dothideomyceta. We also present novel data that require adjustments in the systematic classification of taxa within both classes. A further objective was to begin to examine generic concepts within the family Trypetheliaceae, which is comprised of 11 genera (Lumbsch & Huhndorf 2007b) and approximately 200 species (Harris 1984, Aptroot 1991b, del Prado et al. 2006).
MATERIAL AND METHODS
Taxon sampling
Representatives of lichenised Dothideomyceta taxa were obtained through recent field work in the U.S.A., Central and South America, Europe, India, Thailand, and Fiji. Newly generated sequences were supplemented with other lichenised and non-lichenised Dothideomyceta from GenBank plus additional taxa in Pezizomycetes, Leotiomycetes, Sordariomycetes, Eurotiomycetes, and Lecanoromycetes, chiefly from a previous alignment published by Schoch et al. (2009a). In total, we analysed 162 operational taxonomic units (OTUs) representing 152 species and 111 genera. All OTUs included in the analyses, along with GenBank accession numbers and collection information for newly sequenced samples, are listed in Table 1 - see online Supplementary Information.
Table 1.
Taxa included in this study with GenBank accession numbers and collection information. Numbers following taxon names are DNA identification numbers used in this study.
| Taxon | Collection | Accession Number | |
|---|---|---|---|
| nuLSU | mtSSU | ||
| Acrocordia subglobosa (HTL940) | Palice s.n., Poland (F) | GU327681 | |
| Amphisphaeria umbrina | FJ176863 | FJ713609 | |
| Anisomeridium ubianum (94) | Lumbsch 19845j, Fiji (F) | GU327709 | GU327682 |
| Aptrootia terricola | DQ328995 | ||
| Arthonia caesia | FJ469668 | FJ469671 | |
| Arthonia didyma | EU704083 | EU704047 | |
| Arthonia dispersa | AY571381 | AY571383 | |
| Arthonia radiate | EU704048 | ||
| Arthonia ruana (79B) | Zimmerman 1117, Germany (F) | GU327683 | |
| Arthonia rubrocincta (129) | Nelsen 4010, U.S.A. (F) | GU327684 | |
| Arthopyrenia salicis | AY538339 | AY538345 | |
| AY607730 | AY607742 | ||
| Ascobolus crenulatus | AY544678 | FJ713607 | |
| Astrothelium cinnamomeum | AY584652 | AY584632 | |
| Astrothelium confusum (98) | Nelsen 4004a, Peru (F) | GU327710 | GU327685 |
| Bacidia schweinitzii | DQ782911 | DQ972998 | |
| Bathelium degenerans | DQ328987 | ||
| DQ328988 | |||
| Bimuria novae-zelandiae | AY016356 | FJ190605 | |
| Bionectria ochroleuca | AY489716 | FJ713619 | |
| Botryosphaeria dothidea | DQ678051 | FJ190612 | |
| Botryosphaeria stevensii | DQ678064 | ||
| Botryosphaeria tsugae | DQ767655 | ||
| Botryotinia fuckeliana | AY544651 | AY544732 | |
| Caliciopsis orientalis | DQ470987 | FJ190654 | |
| Caliciopsis pinea | DQ678097 | FJ190653 | |
| Camarops ustulinoides | DQ470941 | FJ190588 | |
| Capnodium coffeae | DQ247800 | FJ190609 | |
| Capronia pilosella | DQ823099 | FJ225725 | |
| Cercospora beticola | DQ678091 | FJ190647 | |
| Cheilymenia stercorea | AY544661 | AY544733 | |
| Chiodecton natalense | EU704085 | EU704051 | |
| Chlorociboria aeruginosa | AY544669 | AY544734 | |
| Chrysothrix flavovirens (L466) | Perlmutter 786, U.S.A. (NCU) | GU327711 | GU327686 |
| Chrysothrix xanthina (126) | Nelsen 4005, U.S.A. (F) | GU327712 | GU327687 |
| Cladosporium cladosporioides | DQ678057 | FJ190628 | |
| Cochliobolus heterostrophus | AY544645 | AY544737 | |
| Cochliobolus sativus | DQ678045 | FJ190589 | |
| Columnosphaeria fagi | DQ470956 | FJ713608 | |
| Combea mollusca | AY571382 | AY571384 | |
| Coniothyrium palmarum | DQ767653 | FJ190638 | |
| Cordyceps capitata | AY489721 | FJ713628 | |
| Cryptothecia assimilis (86B) | Lumbsch 19815l, Fiji (F) | GU327688 | |
| Cryptothecia candida | EU704052 | ||
| Cryptothelium amazonum (47) | Nelsen 4000a, Peru (F) | GU327713 | GU327689 |
| Cryptothelium cecidiogenum | DQ328991 | ||
| Cryptothelium sepultum (63C) | Nelsen 4001a, Peru (F) | GU327714 | GU327690 |
| Cudoniella cf. clavus | DQ470944 | FJ713604 | |
| Cystocoleus ebeneus | EU048578 | EU048584 | |
| EU048579 | EU048585 | ||
| EU048580 | EU048586 | ||
| EU048587 | |||
| Delitschia winteri | DQ678077 | FJ190644 | |
| Dendrographa alectoroides (100) | Lumbsch 19914g, U.S.A. (F) | GU327715 | GU327691 |
| Dendrographa leucophaea f. minor | AF279382 | AY548811 | |
| Dendryphiella arenaria | DQ470971 | FJ190617 | |
| Dermatocarpon miniatum | AY584644 | AY584616 | |
| Diaporthe eres | AF408350 | FJ190607 | |
| Dichosporidium boschianum (89B) | Lumbsch 19815a, Fiji (F) | GU327716 | GU327692 |
| Dirina catalinariae | EF081387 | ||
| Dothidea insculpta | DQ247802 | FJ190602 | |
| Dothidea sambuci | AY544681 | AY544739 | |
| Dothiora cannabinae | DQ470984 | FJ190636 | |
| Eleutherascus lectardii | DQ470966 | FJ190606 | |
| Elsinoe centrolobi | DQ678094 | FJ190651 | |
| Elsinoe phaseoli | DQ678095 | FJ190652 | |
| Elsinoe veneta | DQ767658 | FJ190650 | |
| Endocarpon pallidulum | DQ823097 | FJ225674 | |
| Enterographa anguinella | EU704086 | EU704054 | |
| Enterographa crassa | EU704088 | EU704056 | |
| Erythrodecton granulatum | EU704090 | EU704058 | |
| Eupenicillium javanicum | EF413621 | FJ225778 | |
| Exophiala salmonis | EF413609 | FJ225745 | |
| Flavobathelium epiphyllum (67) | Lücking s.n. Panama (F) | GU327717 | |
| Glomerella cingulata | AF543786 | FJ190626 | |
| Glyphium elatum | AF346420 | AF346425 | |
| Gnomonia gnomon | AF408361 | FJ190615 | |
| Guignardia gaulteriae | DQ678089 | FJ190646 | |
| Herpothallon rubrocinctum (128) | Nelsen 4006, U.S.A. (F) | GU327693 | |
| Herpotrichia diffusa | DQ678071 | DQ384076 | |
| Hypocrea lutea | AF543791 | FJ713620 | |
| Hysteropatella cf. elliptica | DQ767657 | FJ190649 | |
| Kirschsteiniothelia aethiops | AY016361 | FJ190604 | |
| DQ678046 | FJ190590 | ||
| Lachnum virgineum | AY544646 | AY544745 | |
| Laurera megasperma | FJ267702 | ||
| Lecanactis abietina | AY548812 | AY548813 | |
| Lecanactis sp. | EU704091 | EU704059 | |
| Lecanora hybocarpa | DQ782910 | DQ912273 | |
| Macrophomina phaseolina | DQ678088 | FJ190645 | |
| Megalotremis verrucosa (104) | Lücking 26316, Colombia (F) | GU327718 | GU327694 |
| Monilinia laxa | AY544670 | AY544748 | |
| Mycomicrothelia hemispherica (102) | Lücking 28641, Nicaragua (F) | GU327719 | GU327695 |
| Mycomicrothelia miculiformis (101B) | Lücking 28637, Nicaragua (F) | GU327720 | GU327696 |
| Mycomicrothelia obovata (95) | Nelsen 4007a, Peru (F) | GU327721 | GU327697 |
| Mycosphaerella fijiensis | DQ678098 | FJ190656 | |
| Mycosphaerella punctiformis | DQ470968 | FJ190611 | |
| Myriangium duriaei | DQ678059 | AY571389 | |
| Nectria cinnabarina | U00748 | FJ713622 | |
| Opegrapha celtidicola | EU704094 | EU704066 | |
| Opegrapha filicina | EU704095 | EU704067 | |
| Opegrapha lithyrga | EU704096 | EU704068 | |
| Opegrapha varia | EU704103 | EU704075 | |
| Ophionectria trichospora | AF543790 | FJ713626 | |
| Peltigera degenii | AY584657 | AY584628 | |
| Penicillium freii | AY640958 | AY584712 | |
| Pertusaria dactylina | DQ782907 | DQ972973 | |
| Phaeotrichum benjaminii | AY004340 | AY538349 | |
| Phoma herbarum | DQ678066 | FJ190640 | |
| Phyllobathelium anomalum (242) | Lücking s.n., Panama (F) | GU327722 | GU327698 |
| Phyllobathelium firmum (HTL3175) | Lücking s.n., Panama (F) | GU327723 | |
| Pleospora herbarum var. herbarum | DQ247804 | FJ190610 | |
| Preussia terricola | AY544686 | AY544754 | |
| Pseudopyrenula subgregaria (106) | Lücking 24079, Thailand (F) | GU327724 | GU327699 |
| Pseudopyrenula subnudata | DQ328997 | ||
| Pyrenophora phaeocomes | DQ499596 | FJ190591 | |
| Pyrenophora tritici-repentis | AY544672 | FJ713605 | |
| Pyrenula pseudobufonia | AY640962 | AY584720 | |
| Pyrgillus javanicus | DQ823103 | FJ225774 | |
| Pyxine subcinerea | DQ883802 | DQ912292 | |
| Racodium rupestre | EU048583 | EU048588 | |
| EU048581 | |||
| EU048582 | EU048589 | ||
| Ramichloridium anceps | DQ823102 | FJ225752 | |
| Roccella canariensis | AY779328 | ||
| Roccella fuciformis | AY584654 | EU704082 | |
| Roccella montagnei (109) | Lumbsch 19700a, India (F) | GU327725 | GU327700 |
| Roccella tuberculata | AY779328 | ||
| Roccellographa cretacea | DQ883696 | FJ772240 | |
| Schismatomma decolorans | AY548815 | AY548816 | |
| Schismatomma pericleum | AF279408 | AY571390 | |
| Scorias spongiosa | DQ678075 | FJ190643 | |
| Scutellinia scutellata | DQ247806 | FJ190587 | |
| Simonyella variegate | AY584631 | ||
| Sphinctrina turbinate | EF413632 | FJ713611 | |
| Spiromastix warcupii | DQ782909 | FJ225794 | |
| Sporormiella minima | DQ678056 | FJ190624 | |
| Staurothele frustulenta | DQ823098 | FJ225702 | |
| Strigula nemathora (72) | Lücking s.n., Costa Rica (F) | GU327701 | |
| Strigula schizospora (73) | Lücking s.n., Costa Rica (F) | GU327702 | |
| Stylodothis puccinioides | AY004342 | AF346428 | |
| Sydowia polyspora | DQ678058 | FJ190631 | |
| Syncesia farinacea | EF081452 | ||
| Trematosphaeria heterospora | AY016369 | AF346429 | |
| Trematosphaeria pertusa | DQ678072 | FJ190641 | |
| Trimmatostroma abietis | DQ678092 | FJ190648 | |
| Trypetheliopsis kalbii (243) | Lücking s.n., Panama (F) | GU327703 | |
| Trypethelium eluteriae | DQ328989 | ||
| Trypethelium eluteriae (111) | Lumbsch 19701a, India (F) | GU327726 | GU327704 |
| Trypethelium marcidum | DQ329007 | ||
| Trypethelium marcidum (132) | Nelsen 4008, U.S.A. (F) | GU327727 | GU327705 |
| Trypethelium nitidiusculum (139) | Nelsen 4002a, U.S.A. (F) | GU327728 | GU327706 |
| Trypethelium papulosum (97) | Nelsen 4009a, Peru (F) | GU327729 | GU327707 |
| Trypethelium platystomum | DQ329009 | ||
| Trypethelium tropicum (25) | Nelsen 4003, Thailand (F) | GU327730 | GU327708 |
| Tubeufia cerea | DQ470982 | FJ190634 | |
| Tylophoron crassiusculum | EU670258 | ||
| Tylophoron moderatum | EU670256 | ||
| Tyrannosorus pinicola | DQ470974 | FJ190620 | |
| Vibrissea truncorum | FJ176874 | FJ190635 | |
| Westerdykella cylindrical | AY004343 | AF346430 | |
| Xylaria hypoxylon | AY544648 | AY544760 | |
Molecular methods
The Sigma REDExtract-N-Amp Plant PCR Kit (St. Louis, Missouri, U.S.A.) was used to isolate DNA, following the manufacturer's instructions, except only 10 μL of extraction buffer and 10 μL dilution buffer were used, following Avis et al. (2003). Dilutions of these extractions (rather than the stock DNA solution) were found to work best for PCR (C. Andrew, pers. comm. 2009), and a 20× DNA dilution was then used in subsequent PCR reactions.
Samples were PCR amplified and/or sequenced using the mrSSU1, mrSSU2, mrSSU2r and mrSSU3r primers (Zoller et al. 1999) for the mitochondrial small subunit (mtSSU) and the AL2R (Mangold et al. 2008), LR3R, LR3, LR5, LR6, LR7 (Vilgalys & Hester 1990) primers for the nuclear ribosomal large subunit rDNA (nuLSU). The 10 μL PCR reactions consisted of 5 μM of each PCR primer, 3 mM of each dNTP, 2 μL of 10 mg/mL 100x BSA (New England BioLabs, Ipswich, Massachusetts, U.S.A.), 1.5 μL 10× PCR buffer (Roche Applied Science, Indianapolis, Indiana, U.S.A.), 0.5 μL Taq, approximately 2 μL diluted DNA, and 2 μL water. The PCR cycling conditions were as follows: 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, a locus-specific annealing temperature for 1 min, and 72 °C for 1 min, followed by a single 72 °C final extension for 7 min. An annealing temperature of 53 °C was used for mtSSU, while 57 °C was used for nrLSU.
Samples were visualised on a 1 % ethidium bromide-stained agarose gel under UV light and bands were gel extracted, heated at 70 °C for 5 min, cooled to 45 °C for 10 min, treated with 1 μL GELase (Epicentre Biotechnologies, Madison, WI, U.S.A.) and incubated at 45 °C for at least 24 h. The 10 μL cycle sequencing reactions consisted of 1–1.5 μL of Big Dye v. 3.1 (Perkin-Elmer Applied Biosystems, Foster City, California, U.S.A.), 2.5–3 μL of Big Dye buffer, 6 μM primer, 0.75–2 μL Gelased PCR product and water. The cycle sequencing conditions were as follows: 96 °C for 1 min, followed by 25 cycles of 96 °C for 10 s, 50 °C for 5 s and 60 °C for 4 min. Samples were precipitated and sequenced in an Applied Biosystems 3730 DNA Analyser (Foster City, California, U.S.A.), and sequences assembled in Sequencher 4.9 (Gene Codes Corporation, Ann Arbor, Michigan, U.S.A.).
Phylogenetic analysis
The alignment of Schoch et al. (2009a) was used as a starting point, from which a large number of sequences were removed. Newly generated sequences were added and manually aligned (nuLSU), or were separately aligned, added to the Schoch et al. (2009a) alignment, and manually adjusted (mtSSU). In addition to a representative set of dothideomycetous fungi, members of several Ascomycota classes were retained and Pezizomycetes taxa were used as the outgroup. The entire set of sequences generated in the present study plus those from GenBank were aligned in Se-Al v. 2.0a11 (Rambaut 1996) and BioEdit 7.0.9 (Hall 1999). An iterative procedure was used for the nuLSU in which ambiguous regions were aligned with Muscle 3.6 (Edgar 2004) through Mesquite 2.71 (Maddison & Maddison 2009); the alignment was again manually refined and other portions realigned with Muscle. After a final manual refinement, ambiguous regions and introns were removed and the alignment was deposited in TreeBase.
Alignments for each gene were concatenated in Mesquite 2.71 (Maddison & Maddison 2009) and analysed under the maximum likelihood (ML) optimality criterion in RAxML 7.0.4 (Stamatakis 2006). The data set was partitioned by locus and the GTRMIXI model with twenty-five rate parameter categories (default) was used for each partition. In addition, support was estimated by performing 1000 bootstrap replicates, and clades with bootstrap support of 70 % or greater were considered strongly supported. Additionally, the data sets were analyzed in GARLI 0.96 (Zwickl 2006) using the GTR-gamma-invariant model which is similar to the model used in RAxML.
RESULTS
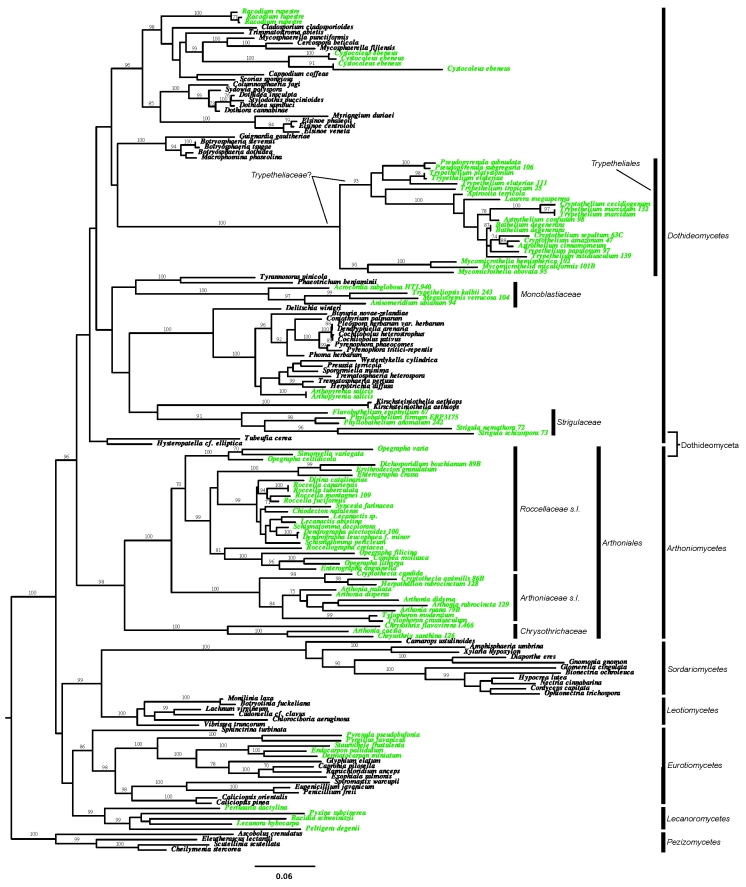
The final alignment consisted of 1 915 unambiguously aligned characters (1 199: nuLSU; 716: mtSSU). Both ML analyses recovered the major class-level ingroup nodes (Fig. 1) corresponding to other recent studies (Leotiomycetes, Sordariomycetes, Eurotiomycetes, Lecanoromycetes, Arthoniomycetes, Dothideomycetes). Arthoniomycetes and Dothideomycetes form a strongly supported sister-group relationship, corresponding to Dothideomyceta. Individual gene phylogenies suggested some incongruence between loci (unpubl. data), however, the topology in the combined analysis is in agreement with previously reported phylogenies and we did not exclude taxa.
Fig.1.
The ML tree from RAxML maximum likelihood analysis with bootstrap percentages equal to or greater than 70 are plotted above or below branches. Lichenised taxa are in green, while non-lichenised taxa are in black.
The phylogeny of Arthoniomycetes (Arthoniales) largely confirmed previous analyses, with Chrysothrichaceae forming an additional family within this clade (Fig. 1). Arthoniaceae s. l. and Roccellaceae s. l. are both monophyletic and well separated. However, several smaller lineages that eventually could be reinstated at the family level show strong support: Arthoniaceae s. str., Cryptotheciaceae (Cryptothecia-Herpothallon), the Tylophoron clade, Roccellaceae s. str., Opegraphaceae s. str., and possibly Chiodectonaceae (as Chiodecton sphaerale is closely related to Erythrodecton and Dichosporidium whereas the sequenced C. natalense is apparently not a Chiodecton s. str.). Surprisingly, Arthonia caesia clustered with Chrysothrichaceae and not Arthoniaceae. Herpothallon rubrocinctum is nested within Cryptothecia s. l.
Six distinct, lichenised lineages were confirmed as belonging to Dothideomycetes (Fig. 1): the order Trypetheliales, the families Arthopyreniaceae, Monoblastiaceae, and Strigulaceae, and the genera Cystocoleus and Racodium. The latter two (Cystocoleus and Racodium) are members of the order Capnodiales, whereas Arthopyreniaceae, represented by the species Arthopyrenia salicis, was confirmed as clustering within Pleosporales. However, Arthopyreniaceae as currently defined, including the genera Julella (not sequenced) and Mycomicrothelia, is not monophyletic, as the sequenced species of Mycomicrothelia appeared outside Pleosporales and form a sister-group to Trypetheliaceae.
Strigulaceae is represented by five samples of the three genera Flavobathelium, Phyllobathelium, and Strigula, which formed a supported monophyletic clade sister to Kirschsteiniothelia aethiops, but without support. Monoblastiaceae was strongly supported and included four genera with one species each in this analysis: Acrocordia subglobosa, Anisomeridium ubianum, Megalotremis verrucosa, and Trypetheliopsis (syn. Musaespora) kalbii. Initially we also included a GenBank sequence of Anisomeridium polypori in the data set, but the nuLSU sequence was recovered in Eurotiomycetes and the taxon was excluded from the final analysis. It is possible that this sequence is derived from a contaminant or that it was confused with a similar species in an unrelated lineage.
Trypetheliaceae was strongly supported as monophyletic, being sister to the genus Mycomicrothelia. There was no support for the traditional separation into the perithecial and ascospore core genera Astrothelium, Laurera, and Trypethelium, as species of these genera were found scattered over the Trypetheliaceae clade.
DISCUSSION
This is the first molecular phylogenetic study that includes presumably all major lichenised lineages within Dothideomyceta. This rankless taxon was informally introduced by Schoch et al. (2009a, b) for the clade including Arthoniomycetes and Dothideomycetes. The sister group of Dothideomyceta is not yet resolved but Ruibal et al. (2009; this volume) demonstrated an unnamed lineage of melanised rock-inhabiting fungi to be basal to Arthoniomycetes (not included in our sampling).
Arthoniomycetes is the second largest class of primarily lichenised Ascomycota and exhibits considerable morphoanatomical variation (Fig. 2). The molecular phylogeny presented here confirms the current classification of lichenised Arthoniomycetes in three families: Arthoniaceae, Chrysothrichaceae, and Roccellaceae (Tehler 1995, Grube 1998, Tehler & Irestedt 2007). The morphological concept used to classify the single order included few large genera, with Arthonia and Opegrapha having the highest number of species (500 and 300, respectively). The infrageneric relationships of these species were repeatedly discussed and there was common agreement that these genera were not monophyletic and include morphologically distinct groups. Similarly the relationships of other genera with fewer species or of monospecific genera in the family Roccellaceae was unclear. Along with previous data (Tehler 1995, Myllys et al. 1998, Tehler & Irestedt 2007) and recent results by Ertz et al. (2009), the present tree is a further step to resolve these questions based on molecular data.
Fig. 2.
Select lichenised Arthoniomycetes. A. Chrysothrix xanthina; B. C. septemseptata; C. Arthonia caesia; D. A. cyanea; E. A. pulcherrima; F. A. rubrocincta; G. Cryptothecia candida; H. Herpothallon rubrocinctum; I. Tylophoron crassiusculum (teleomorph); J. T. crassiusculum (anamorph); K. Opegrapha filicina; L. O. astraea; M. Enterographa anguinella; N. Syncesia glyphysoides; O. S. byssina; P. Lecanactis epileuca; Q. Chiodecton sphaerale; R–S. Erythrodecton granulatum; T. Dichosporidium boschianum; U. D. nigrocinctum (ascomata); V. D. nigrocinctum (isidia); W. Mazosia rotula; X. Roccella spec. Photo credits: R. Lücking.
Little can be said regarding generic concepts of most genera, as the taxon sampling is still far too incomplete for this group, but it appears that some of the traditional concepts based on fruit body structure are not supported, which suggests some degree of parallel evolution. An example is the Chiodecton-Enterographa complex: while the sequenced Chiodecton natalense appears to be unrelated to the morphologically and anatomically similar Dichosporidium and Erythrodecton (Thor 1990), Enterographa and the similar Schismatomma (Sparrius 2004) were found in three different clades related to either Chiodecton natalense (Schismatomma), Dichosporidium (Enterographa crassa), and Opegrapha (Enterographa anguinella), respectively. This is in agreement with Ertz et al. (2009), who showed that Enterographa is not monophyletic and groups either with the core Opegrapha clade (here represented by O. lithyrgica), or with Chiodecton-like species (Dichosporidium and Erythrodecton). Consequently, Ertz et al. (2009) tranferred Enterographa anguinella to Opegrapha. Not surprisingly, neither Arthonia nor Opegrapha are monophyletic. Ertz et al. (2009) showed convincingly that despite different ascomatal structure, Opegrapha atra and O. calcarea (with distinct excipulum) are closely related to Arthonia radiata (lacking an excipulum), which is confirmed by similarities of ascus structure and pigment type. Subsequently, Ertz et al. (2009) suggested these two Opegrapha species be recognised as belonging to Arthonia. Opegrapha varia and O. celtidicola form another monophyletic lineage together with Simonyella variegata. Most likely this branch also includes other Opegrapha species, according to the results of Ertz et al. (2009). Opegrapha s. str. forms a further lineage including O. lithyrgica, which is closely related to the type species O. vulgata (Ertz et al. 2009), the foliicolous O. filicina, as well as Combea mollusca and Roccellographa cretacea.
Herpothallon rubrocinctum is now confirmed as an ascomycete in Arthoniomycetes. This seems trivial as the species also morphologically shows clear affinities with Cryptothecia (Aptroot et al. 2008), but the position of this taxon was questioned long ago and was even considered a basidiomycete (see discussion in Withrow & Ahmadjian 1983, Aptroot et al. 2008). Our analysis shows Herpothallon nested within Cryptothecia, supporting the previous hypothesis that byssoid-isidiate species within this complex are indeed members of Cryptothecia rather than forming a separate genus, as proposed by Aptroot et al. (2008). However, a larger taxon sampling is needed to resolve the Cryptothecia-Herpothallon complex, especially considering that there are other genera such as Stirtonia involved and even further new genera have been segregated recently (Aptroot et al. 2009, Frisch & Thor 2010). The fruticose Roccella species form a clearly monophyletic branch together with several crustose species representing various genera; this assemblage of core Roccellaceae has already been recognised previously (Tehler 1995, Myllys et al. 1998, Tehler & Irestedt 2007). The placement of Tylophoron, a genus that has passive spore dispersal and was previously assigned to Caliciales, is here confirmed as a member of Arthoniaceae s. l., in agreement with Lumbsch et al. (2009a).
The strongly supported placement of Arthonia caesia within Chrysothrix is unexpected; however, fertile species of Chrysothrix are very similar to Arthonia in ascoma morphology and anatomy, and particularly A. caesia and allies can be easily perceived as non-pigmented species of Chrysothrix in apothecial anatomy and morphology and thallus structure (including the chlorococcoid photobiont). Similar Arthonia species include A. cupressina, which is closely related to A. caesia. Further studies are needed to elucidate which additional Arthonia taxa need to be placed in Chrysothrix. The latter genus was variously placed in its own family Chrysothrichaceae mainly due to the presence of pulvinic acids as secondary metabolites but also in Arthoniaceae due to similarities in ascus characters (Grube 1998). The present data strongly support Chrysothrichaceae as a separate family, especially as it is sister to all remaining Arthoniales and not to Arthoniaceae. It is therefore necessary to transfer Arthonia caesia (which lacks pulvinic acids) and related species to this family. The other Arthonia species sampled group form a fairly well supported monophyletic group, which includes a species formerly assigned to Arthothelium, i.e. Arthonia ruana, because of its muriform ascospores; however, it has been known for some time that most species with muriform ascospores are more closely related to Arthonia than to the type of Arthothelium, A. spectabile (Tehler 1990, Sundin & Tehler 1998, Cáceres 2007, Grube 2007), which has not yet been sequenced. Notably, Arthonia didyma and A. rubrocincta, two species with reddish pigments, form a weakly supported group. If future efforts confirm this grouping, the name Coniocarpon could be used for this clade (Cáceres 2007).
In contrast to Arthoniomycetes, the overwhelming majority of Dothideomycetes species are non-lichenised. In addition to Arthopyreniaceae, Trypetheliaceae and Cystocoleus and Racodium (Muggia et al. 2007), this study confirms the placement of Monoblastiaceae and Strigulaceae within Dothideomycetes. Although our support for the Dothideomycetes node is weak, the included non-lichenised taxa are well supported within this class in other studies (Schoch et al. 2006, 2009a, b); in addition, placement within Dothideomyceta is strongly supported. Both, Monoblastiaceae and Strigulaceae are comparatively large with over 100 accepted species each and show substantial morphological and ecological radiation (Fig. 3); both are chiefly tropical. The mostly corticolous Monoblastiaceae range from barely lichenised forms with exposed perithecia (many species of Anisomeridium) to taxa with well-developed, corticate thalli (Anisomeridium p.p., Megalotremis, Trypetheliopsis). Ascospores vary from small to large and thick-walled but are always simple or transversely septate only (Harris 1995). Substantial variation is found in the conidiomata, and many species, particularly in the genera Caprettia, Megalotremis, and Trypetheliopsis (= Musaespora) have developed unique pycnidia that in part are similar to campylidia or hyphophores found in certain Lecanoromycetes (Aptroot & Sipman 1993, Lücking et al. 1998, Aptroot et al. 2008, Lücking 2008). Secondary substances are few, including lichexanthone and anthraquinones. All species of Monoblastiaceae in which conidiomata are known share a particular synapomorphy: the conidia are always embedded in a strongly coherent, gelatinous matrix. Thus, besides the uniform hamathecium and ascus anatomy, there is substantial phenotypic evidence for monophyly of this family, now confirmed by molecular data.
Fig. 3.
Select lichenised Dothideomycetes; A. Arthopyrenia cinchonae; B. Mycomicrothelia modesta; C. Anisomeridium subprostans; D. Anisomeridium spec. (pycnidia); E. A. foliicola (pycnidia); F. Caprettia amazonensis (pycnidia); G. Megalotremis cauliflora (pycnidia); H. Trypetheliopsis (= Musaespora) coccinea (campylidia); I. Strigula viridiseda; J. S. laureriformis (pycnidia); K. S. smaragdula; L. Flavobathelium epiphyllum; M. Phyllobathelium firmum; N. P. leguminosae (pycnidia); O. Pseudopyrenula subnudata; P. Trypethelium tropicum; Q. T. platystomum; R. Bathelium degenerans; S. Laurera purpurina; T. Astrothelium cinnamomeum; U. A. eustomum; V. Trypethelium nitidiusculum; W. Laurera megasperma; X. Campylothelium spec. Photo credits: R. Lücking.
Strigulaceae share many characteristics with Monoblastiaceae, specifically the ascus type and the mostly 1- or 3-septate ascospores, although some species have muriform ascospores (Harris 1995, Aptroot et al. 2008, Lücking 2008). Species in this family are found on a variety of substrata, including rocks, bark, and living leaves. Poorly developed thalli are found in corticolous species with barely lichenised thalli and exposed perithecia (Strigula p.p.), whereas the genera Flavobathelium, Phyllobathelium, and Phyllocratera include taxa with well-developed, corticate thalli. Also in this family, the most characteristic synapomorphy are the conidia, which feature terminal gelatinous appendices (Harris 1995, Lücking 2008). Unfortunately, our taxon sampling of this family is poor but sufficient to confirm its monophyly and its placement in Dothideomycetes. This is the first molecule-based support for the inclusion of Phyllobatheliaceae within Strigulaceae, a concept first presented by Harris (1995).
The largest lichenised family within Dothideomycetes, Trypetheliaceae, contains members that are typically lichen-forming and tropical to subtropical in distribution, with some taxa extending into temperate regions (Aptroot 1991, Harris 1995, Brodo et al. 2001, Aptroot et al. 2008). The species are almost exclusively corticolous, forming a crustose, endo- or epiperidermal thallus with algae belonging to Trentepohliaceae; however, Anisomeridium is often found lignicolous and Aptrootia grows on bryophytes. Detailed studies in Costa Rica suggest Trypetheliaceae to occur primarily on trunks and branches of trees in exposed habitats of lowland to lower montane (200–1000 m) rain and dry forests and savannas with rather distinct dry season (Aptroot et al. 2008, Rivas-Plata et al. 2008). Trypetheliaceae species are quite variable in perithecial morphology (Fig. 3) but have a rather uniform hamathecium composed of thin, anastomosing pseudoparaphyses embedded in a stiff gelatinous matrix. The most characteristic synapomorphy are the usually hyaline ascospores with internal wall thickenings that cause more or less diamond-shaped septa, but these wall thickenings are often reduced or absent in species with multiseptate or muriform ascospores (Harris 1984, 1990, 1995, Aptroot 1991b, Aptroot et al. 2008). The secondary chemistry is equally simple, with lichexanthone and pigments as most common substances, i.e. polyketide derived aromatic compounds produced through the acetyl-polymalonyl pathway (Elix & Stocker-Wörgötter 2008). However, the number of species with substances present is much higher in Trypetheliaceae than any other lineage within Dothideomycetes: more than 70 species are known to produce secondary substances in this family. The core genera Astrothelium, Campylothelium, Cryptothelium, Laurera, and Trypethelium, are separated primarily on the basis of perithecial arrangement and ostiolar orientation (solitary vs. aggregate, apical vs. excentric) and ascospore septation (transverse vs. muriform; Harris 1990, 1995, del Prado et al. 2006). Because of the schematic classification, Harris (1995) suggested that these genera may be polyphyletic, and del Prado et al. (2006) subsequently illustrated the non-monophyly of Trypethelium. Aptroot et al. (2008) echoed Harris's (1995) sentiment and stated that generic concepts in Trypetheliaceae are in need of revision.
Surprisingly, Mycomicrothelia was recovered as sister to Trypetheliaceae. Mycomicrothelia has traditionally been considered a sister genus to Arthopyrenia with brown ascospores (Harris 1995). However, the hamathecium at least of the sequenced species is identical to that found in Trypetheliaceae, whereas Arthopyrenia has thicker and less branched and anastomosing pseudoparaphyses. Moreover, the ascospores are of a different type, often with internal wall thickenings. It remains to be tested whether Arthopyrenia and Mycomicrothelia in their current circumscriptions are monophyletic genera or whether at least some species currently assigned to these genera perhaps represent further lichenised lineages within Dothideomycetes. Whether Mycomicrothelia should be included within Trypetheliaceae or receive its own family rank is open to question. Mycomicrothelia has primarily thin-walled, dark brown ascospores, whereas in Trypetheliaceae they are primarily thick-walled with diamond-shaped lumina and hyaline (brown only in Aptrootia and Architrypethelium). Understanding the phylogenetic position of Polymeridium, which also has thin-walled ascospores, will hopefully help clarify this.
In spite of the many characters in parallel with Monoblastiaceae and Strigulaceae, also the Trypetheliaceae plus Mycomicrothelia (Trypetheliales) are quite unique genetically and there is no evidence that the three families would be related to each other or with Arthopyreniaceae. This supports the notion of several shifts in lichenisation within the Dothideomycetes (Aptroot 1991a, 1998). However, the often barely lichenised thalli in certain species of Anisomeridium, Arthopyrenia, Julella, Mycomicrothelia, Mycoporum, Pseudopyrenula, and Strigula (Aptroot 1991a, Aptroot 1998, Harris 1995) suggest that these species can possibly switch between being (almost) non-lichenised to distinctly lichenised, a situation also found in the unrelated genus Stictis within Lecanoromycetes (Wedin et al. 2004).
The present study clarifies the systematic position of further pyrenocarpous lichenised lineages within the Ascomycota and shows that previous concepts in part diverged widely from our present understanding but also came suprisingly close even without molecular evidence (Table 2). This study emphasises that pyrenocarpous lichens with bitunicate asci are not only not monophyletic, but belong to at least two different classes (Dothideomycetes and Eurotiomycetes) and several different orders and families; the data at hand also suggest that these represent several independent lineages of lichenisation. Although we consider this study a contribution to clarify the systematic position of pyrenocarpous lichens and the evolution of lichenisation within Dothideomycetes, much remains to be done, considering that at present only a fraction of the presumably 600 species of lichens belonging in this class have been studied using DNA sequences. In particular, clarifying the generic and species concepts within Monoblastiaceae, Strigulaceae, and Trypetheliaceae, speciose families that are important elements of crustose lichen communities especially in the tropics, will be a major challenge in the near future.
Table 2.
Systematic placement of selected pyrenocarpous lichens according to different concepts.
| Genus | Zahlbruckner 1926 | Barr 1987 | Harris 1995 | current |
|---|---|---|---|---|
| Celothelium | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Eurotiomycetes |
| (as Leptorhaphis) | Pleosporales | Melanommatales | Pyrenulales | |
| Pyrenulaceae | Pleosporaceae | Thelenellaceae | Celotheliaceae | |
| Lithothelium | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Eurotiomycetes |
| Astrotheliaceae | Melanommatales | Melanommatales | Pyrenulales | |
| Pyrenula | Pyrenocarpeae | Pyrenulaceae | Pyrenulaceae | Pyrenulaceae |
| Pyrenulaceae | ||||
| Arthopyrenia | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Dothideomycetes |
| Pyrenulaceae | Pleosporales | Pleosporales | Pleosporales | |
| Arthopyreniaceae | Pleosporaceae | Arthopyreniaceae | ||
| Acrocordia | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Dothideomycetes |
| Anisomeridium | (as Arthopyrenia) | Melanommatales | Melanommatales | incertae sedis |
| Pyrenulaceae | Acrocordiaceae | Monoblastiaceae | Monoblastiaceae | |
| Phyllobathelium | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Dothideomycetes |
| Strigula | Strigulaceae | Chaetothyriales | Melanommatales | incertae sedis |
| Strigulaceae | Strigulaceae | Strigulaceae | ||
| Astrothelium | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Dothideomycetes |
| Astrotheliaceae | Melanommatales | Melanommatales | Trypetheliales | |
| Campylothelium | Pyrenocarpeae | Trypetheliaceae | Trypetheliaceae | Trypetheliaceae |
| Paratheliaceae | ||||
| Laurera | Pyrenocarpeae | |||
| Trypetheliaceae | ||||
| Pseudopyrenula | Pyrenocarpeae | |||
| Pyrenulaceae | ||||
| Trypethelium | Pyrenocarpeae | |||
| Trypetheliaceae | ||||
| Mycomicrothelia | Pyrenocarpeae | Loculoascomycetes | Loculoascomycetes | Dothideomycetes |
| (as Microthelia) | Pleosporales | Pleosporales | Trypetheliales | |
| Strigulaceae | Arthopyreniaceae | Arthopyreniaceae | Trypetheliaceae? | |
| Porina | Pyrenocarpeae | Hymenoascomycetes | Lecanoromycetes | |
| Pyrenulaceae | Trichotheliales | Ostropales | ||
| Trichothelium | Pyrenocarpeae | Trichotheliaceae | Porinaceae | |
| Strigulaceae | — |
Acknowledgments
Material used in this study was collecte in the framework of three NSF grants to The Field Museum: DEB 0206125 “TICOLICHEN” (PI Robert Lücking), DEB 0516116 “Phylogeny and Taxonomy of Ostropalean Fungi, with Emphasis on the Lichen-forming Thelotremataceae” (PI Thorsten Lumbsch), and DEB 0715660 “Neotropical Epiphytic Microlichens – An Innovative Inventory of a Highly Diverse yet Little Known Group of Symbiotic Organisms” (PI Robert Lücking). We also thank Z. Palice, G. Perlmutter & D.G. Zimmerman for collections used in this study and K. Feldheim for discussions on laboratory techniques. Most work was performed in the Pritzker Laboratory at the Field Museum of Natural History.
References
- Aptroot A (1991a). Tropical pyrenocarpous lichens. A phylogenetic approach. In: Tropical lichens: their systematics, conservation and ecology (Galloway DJ, ed.). Clarendon Press, U.K.: 253–273.
- Aptroot A (1991b). A monograph of the Pyrenulaceae (excluding Anthracothecium and Pyrenula) and the Requienellaceae, with notes on the Pleomassariaceae, the Trypetheliaceae and Mycomicrothelia (lichenized and non-lichenized Ascomycetes). Bibliotheca Lichenologica 44: 1–178. [Google Scholar]
- Aptroot A (1998): Aspects of the integration of the taxonomy of lichenized and non-lichenized pyrenocarpous ascomycetes. Lichenologist 30: 501–514. [Google Scholar]
- Aptroot A, Sipman H (1993): Musaespora, a genus of pyrenocarpous lichens with campylidia, and other additions to the foliicolous lichen flora of New Guinea. Lichenologist 25: 121–135. [Google Scholar]
- Aptroot A, Lücking R, Sipman HJM, Umaña L, Chaves JL (2008). Pyrenocarpous lichens with bitunicate asci: a first assessment of the lichen biodiversity inventory of Costa Rica. Bibliotheca Lichenologica 97: 1–162. [Google Scholar]
- Aptroot A, Thor G, Lücking R, Elix JA, Chaves JL (2009). The lichen genus Herpothallon re-instated. Bibliotheca Lichenologica 99: 19–66. [Google Scholar]
- Avis P, McLaughlin DJ, Dentinger BC, Reich PB (2003). Long-term increase in nitrogen supply alters above- and below-ground ectomycorrhizal communities and increases the dominance of Russula spp. in a temperate oak savanna. New Phytologist 160: 239–253. [DOI] [PubMed] [Google Scholar]
- Barr ME (1983): The ascomycete connection. Mycologia 75: 1–13. [Google Scholar]
- Barr ME (1987). Prodromus to class Loculoascomycetes. Hamilton I. Newell, Inc., Amherst, Massachusetts, published by the author.
- Brodo IM, Sharnoff SD, Sharnoff S (2001). Lichens of North America. Yale University Press, U.S.A.
- Cannon PF, Kirk PM (2007). Fungal families of the world. CABI Publishing, U.K.
- del Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch HT (2006). Molecular data place Trypetheliaceae in Dothideomycetes. Mycological Research 110: 511–520. [DOI] [PubMed] [Google Scholar]
- Edgar RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32(5): 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elix JA, Stocker-Wörgötter E (2008). Biochemistry and secondary metabolites. In: Lichen biology, 2nd edn (Nash III TH, ed.). Cambridge University Press, U.K.: 104–133.
- Eriksson OE, Barah H-O, Curra RS, Hansen K, Kurtzman CP, et al. (2004). Outline of Ascomycota. Myconet 10: 1–99. [Google Scholar]
- Eriksson OE, Winka K (1997). Supraordinal taxa of Ascomycota. Myconet 1: 1–16. [Google Scholar]
- Ertz D, Miadlikowska J, Lutzoni F, Dessein S, Raspé O, et al. (2009). Towards a new classification of the Arthoniales (Ascomycota) based on a three-gene phylogeny focussing on the genus Opegrapha. Mycological Research 113: 141–152. [DOI] [PubMed] [Google Scholar]
- Feuerer T, Hawksworth DL (2007). Biodiversity of lichens, including a world-wide analysis of checklist data based on Takhtajan's floristic regions. Biodiversity and Conservation 16: 85–98. [Google Scholar]
- Frisch A, Thor G (2010). Crypthonia, a new genus of byssoid Arthoniaceae (lichenised Ascomycota). Mycological Progress: In press.
- Gargas A, DePriest PT, Grube M, Tehler A (1995). Multiple origins of lichen symbioses in fungi suggested by SSU rDNA phylogeny. Science 268: 1492–1495. [DOI] [PubMed] [Google Scholar]
- Grube M (1998). Classification and phylogeny in the Arthoniales (lichenized Ascomycetes). Bryologist 101: 377–391. [Google Scholar]
- Grube M (2007). Arthonia. In: Lichen Flora of the Greater Sonoran Desert Region. Volume 3. (TH Nash III, C Gries, F Bungartz, eds). Lichens Unlimited, Arizona State University, Tempe, U.S.A.: 39–61.
- Grube M, Blaha J (2003). On the phylogeny of some polyketide synthase genes in the lichenized genus Lecanora. Mycological Research 107: 1419–1426. [DOI] [PubMed] [Google Scholar]
- Grube M, Baloch E, Lumbsch HT (2004). The phylogeny of Porinaceae (Ostropomycetidae) suggests a neotenic origin of perithecia in Lecanoromycetes. Mycological Research 108: 1111–1118. [DOI] [PubMed] [Google Scholar]
- Gueidan C, Ruibal Villaseñor C, Hoog GS de, Gorbushina AA, et al. (2008). A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Studies in Mycology 61: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafellner J (1986). Systematics of lichenized fungi. Progress in Botany 48: 316-333. [Google Scholar]
- Hall TA (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symposium Series 41: 95–98. [Google Scholar]
- Harris RC (1984). The family Trypetheliceae (Loculoascomycetes: lichenized Melanommatales) in Amazonian Brazil. Acta Amazonica 14 (Supplement): 55–80. [Google Scholar]
- Harris RC (1990). Some Florida lichens. Published by the author, U.S.A.
- Harris RC (1991). A revision of Polymeridium (Muell. Arg.) R.C. Harris (Trypetheliaceae). Boletim do Museu Paraense Emílio Goeldi. Série Botânica 7: 619–644. [Google Scholar]
- Harris RC (1995). More Florida lichens. Including the 10¢ tour of the pyrenolichens. Published by the author, U.S.A.
- Henssen A, Jahns HM (1974). Lichenes. Georg Thieme Verlag,Stuttgart, Germany.
- Herrera-Campos M, Huhndorf S, Lücking R (2005). The foliicolous lichen flora of Mexico IV: a new, foliicolous species of Pyrenothrix (Chaetothyriales: Pyrenothrichaceae). Mycologia 97: 356–361. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. (2007). A higher-level phylogenetic classification of the Fungi. Mycological Research 111: 509–547. [DOI] [PubMed] [Google Scholar]
- Hofstetter V, Miadlikowska J, Kauff F, Lutzoni F (2007). Phylogenetic comparison of protein-coding versus ribosomal RNA-coding sequence data: a case study of the Lecanoromycetes (Ascomycota). Molecular Phylogenetics and Evolution 44: 412–426. [DOI] [PubMed] [Google Scholar]
- James TY, Kauff F, Schoch C, Matheny PB, Hofstetter V, et al. (2006). Reconstructing the early evolution of the fungi using a six-gene phylogeny. Nature 443: 818–822. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Mintner DW, Stalpers JA (2008). Ainsworth & Bisby's dictionary of the Fungi, 10th edn. CAB International, U.K.
- Kroken S, Glass NL, Taylor JW, Yoder OC, Turgeon BG (2003). Phylogenomic analysis of type I polyketide synthase genes in pathogenic and saprobic ascomycetes. Proceedings of the National Academy of Sciences (U.S.A.) 100: 15670–15675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemuth R, Wirtz N, Lumbsch HT (2001). Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that loculoascomycetes (Ascomycota) are not monophyletic. Mycological Research 105: 1176–1181. [Google Scholar]
- Liu YJ, Hall BD (2004). Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proceedings of the National Academy of Sciences (U.S.A.) 101: 4507–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücking R (2008) Foliicolous lichenized fungi. Flora Neotropica Monograph 103: 1–873. [Google Scholar]
- Lücking R, Becker U, Follmann G (1998): Foliikole Flechten aus dem Taï-Nationalpark, Elfenbeinküste (Tropisches Afrika). II. Ökologie und Biogeografie [Foliicolous lichens from the Taï National Park, Ivory Coast (Tropical Africa). II. Ecology and biogeography]. Herzogia 13: 207–228. [Google Scholar]
- Lücking R, Stuart BL, Lumbsch HT (2004). Phylogenetic relationships of Gomphillaceae and Asterothyriaceae: evidence from a combined Bayesian analysis of nuclear and mitochondrial sequences. Mycologia 96: 283–294. [DOI] [PubMed] [Google Scholar]
- Lücking R, Lumbsch HT, Di Stéfano JF, Lizano D, Carranza J, et al. (2008). Eremithallus costaricensis (Ascomycota: Lichinomycetes: Eremithallales), a new fungal lineage with a novel lichen symbiotic lifestyle discovered in an urban relict forest in Costa Rica. Symbiosis 46: 161–170. [Google Scholar]
- Lumbsch HT, Lindemuth R (2001). Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycological Research 105: 901–908. [Google Scholar]
- Lumbsch HT, Schmitt I, Döring H, Wedin M (2001). Molecular systematics supports the recognition of an additional order of Ascomycota: the Agyriales. Mycological Research 105: 16–23. [Google Scholar]
- Lumbsch HT, Schmitt I (2002). Molecular data shake the Pertusariaceae tree into order. Lichenology 1: 37–43. [Google Scholar]
- Lumbsch HT, Wirtz N, Lindemuth R, Schmitt I (2002). Higher level phylogenetic relationships of euascomycetes (Pezizomycotina) inferred from a combined analysis of nuclear and mitochondrial sequence data. Mycological Progress 1: 57–70. [Google Scholar]
- Lumbsch HT, Schmitt I, Palice Z, Wiklund E, Ekman S, Wedin M (2004). Supraordinal phylogenetic relationships of Lecanoromycetes based on a Bayesian analysis of combined nuclear and mitochondrial sequences. Molecular Phylogenetics and Evolution 31: 822–832. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Huhndorf SM (2007a). Whatever happened to the pyrenomycetes and loculoascomycetes? Mycological Research 111: 1064–1074. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Huhndorf SM (2007b). Outline of Ascomycota – 2007. Myconet 13: 1–58. [Google Scholar]
- Lumbsch HT, Lücking R, L. Tibbell (2009a). Molecular data place Tylophoron as an additional calicioid genus in the Arthoniales (Ascomycota). Bibliotheca Lichenologica 99: 285–296. [Google Scholar]
- Lumbsch HT, Zimmermann DG, Schmitt I (2009b). Phylogenetic position of ephemeral lichens in Thelocarpaceae and Vezdaeaceae (Ascomycota). Bibliotheca Lichenologica 100: 389–398. [Google Scholar]
- Lutzoni F, Pagel M, Reeb V (2001). Major fungal lineages are derived from lichen symbiotic ancestors. Nature 411: 937–940. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, et al. (2004). Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. American Journal of Botany 91: 1446–1480. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2009). Mesquite: a modular system for evolutionary analysis. Version 2.71 http://mesquiteproject.org
- Mangold A, Martín MP, Lücking R, Lumbsch HT (2008). Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota: Ostropales). Taxon 57: 476–486. [Google Scholar]
- Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, et al. (2006). New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 98: 1090–1103. [PubMed] [Google Scholar]
- Myllys L, Källersjö M, Tehler A (1998), A comparison of SSU rDNA data and morphological data in Arthoniales (Euascomycetes) phylogeny. The Bryologist 101: 70–85. [Google Scholar]
- Persoh D, Beck A, Rambold G (2004). The distribution of ascus types and photobiontal selection in Lecanoromycetes (Ascomycota) against the background of a revised SSU nrDNA phylogeny. Mycological Progress 3: 103–121. [Google Scholar]
- Poelt J (1973). Classification. - In: The Lichens. (V. Ahmadjian & M.E. Hale, eds). Academic Press, New York and London: 599–632.
- Rambaut A (1996). Se-Al: Sequence Alignment Editor. Available at http://evolve.zoo.ox.ac.uk/
- Reeb V, Lutzoni F, Roux C (2004). Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Molecular Phylogenetics and Evolution 32: 1036–1060. [DOI] [PubMed] [Google Scholar]
- Rivas-Plata E, Lücking R, Lumbsch HT (2008). When family matters: an analysis of Thelotremataceae (lichenized Ascomycota: Ostropales) as bioindicators of ecological continuity in tropical forests. Biodiversity and Conservation 17: 1319–1351. [Google Scholar]
- Ruibal C, Gueidan C, Selbmann L, Gorbushina AA, Crous PW, et al. (2009). Phylogeny of rock-inhabiting fungi related to Dothideomycetes. Studies in Mycology 64: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt I, Yamamoto Y, Lumbsch HT (2006). Phylogeny of Pertusariales (Ascomycotina): resurrection of Ochrolechiaceae and new circumscription of Megasporaceae. Journal of the Hattori Botanical Laboratory 100: 753–764. [Google Scholar]
- Schmitt I, Lumbsch HT (2009). Ancient horizontal gene transfer from bacteria enhances biosynthetic capabilities of fungi. PLoS ONE 4: e4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt I, Martin MP, Kautz S, Lumbsch HT (2005). Diversity of non-reducing polyketide synthase genes in the Pertusariales (lichenized Ascomycota): A phylogenetic perspective. Phytochemistry 66: 1241–1253. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Sung G-H, López-Giráldez F, Townsend JP, Miadlikowska J, et al. (2009a). The Ascomycota tree of Life: a phylum wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Wang Z, Townsend JP, Spatafora JW (2009b). Geoglossomycetes cl. nov., Geoglossales ord. nov. and taxa above class rank in the Ascomycota Tree of Life. Persoonia 22: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. (2009c). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrius L (2004). A monograph of Enterographa and Sclerophyton. Bibliotheca Lichenologica 89: 1-141. [Google Scholar]
- Spatafora JW, Johnson D, Sung GH, Hosaka K, O'Rourke B, et al. (2006). A five-gene phylogenetic analysis of the Pezizomycotina. Mycologia 98: 1020–1030. [DOI] [PubMed] [Google Scholar]
- Stamatakis A (2006). RAxML-VI-HPC: Maximum Likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Strimmer K, Rambaut A (2002). Inferring confidence sets of possibly misspecified gene trees. Proceedings of the Royal Society of London, Biological Sciences 269: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin R (2000). Phylogeny and taxonomy within Arthonia Ach. In: The Fourth IAL Symposium, Progress and Problems in Lichenology at the Turn of the Millennium. Universitat de Barcelona, Barcelona, Spain.
- Sundin R, Tehler A (1998). Phylogenetic studies of the genus Arthonia. Lichenologist 30: 381–413. [Google Scholar]
- Tehler A. (1990). A new approach to the phylogeny of euascomycetes with a cladistic outline of Arthoniales focussing on Roccellaceae. Canadian Journal of Botany 68: 2458–2492. [Google Scholar]
- Tehler A (1995). Arthoniales phylogeny as indicated by morphological and rDNA sequence data. Cryptogamic Botany 5: 82–97. [Google Scholar]
- Tehler A, Irestedt M (2007). Parallel evolution of lichen growth forms in the family Roccellaceae (Arthoniales, Ascomycota). Cladistics 23: 432–454. [Google Scholar]
- Vilgalys R, Hester M (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedin M, Döring H, Gilenstam G (2004). Saprotrophy and lichenization as options for the same fungal species on different substrata: environmental plasticity and fungal lifestyles in the Stictis-Conotrema complex. New Phytologist 164: 459–465. [Google Scholar]
- Wedin M, Wiklund E, Crewe A, Döring H, Ekman S, et al. (2005). Phylogenetic relationships of Lecanoromycetes (Ascomycota) as revealed by analyses of mtSSU and nLSU rDNA seqaence data. Mycological Research 109: 159-172. [DOI] [PubMed] [Google Scholar]
- Withrow K, Ahmadjian V (1983). The ultrastructure of lichens. VII. Chiodecton sanguineum. Mycologia 75: 337–339. [Google Scholar]
- Zahlbruckner A (1926). Catalogus Lichenum Universalis. - Borntraeger\Leipzig, Germany.
- Zoller S, Scheidegger C, Sperisen C (1999). PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 31: 511–516. [Google Scholar]
- Zwickl DJ (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Ph.D. dissertation - The University of Texas at Austin, U.S.A.