Abstract
Phylogenetic analyses of four nuclear genes, namely the large and small subunits of the nuclear ribosomal RNA, transcription elongation factor 1-alpha and the second largest RNA polymerase II subunit, established that the ecological group of marine bitunicate ascomycetes has representatives in the orders Capnodiales, Hysteriales, Jahnulales, Mytilinidiales, Patellariales and Pleosporales. Most of the fungi sequenced were intertidal mangrove taxa and belong to members of 12 families in the Pleosporales: Aigialaceae, Didymellaceae, Leptosphaeriaceae, Lenthitheciaceae, Lophiostomataceae, Massarinaceae, Montagnulaceae, Morosphaeriaceae, Phaeosphaeriaceae, Pleosporaceae, Testudinaceae and Trematosphaeriaceae. Two new families are described: Aigialaceae and Morosphaeriaceae, and three new genera proposed: Halomassarina, Morosphaeria and Rimora. Few marine species are reported from the Dothideomycetidae (e.g. Mycosphaerellaceae, Capnodiales), a group poorly studied at the molecular level. New marine lineages include the Testudinaceae and Manglicola guatemalensis in the Jahnulales. Significantly, most marine Dothideomycetes are intertidal tropical species with only a few from temperate regions on salt marsh plants (Spartina species and Juncus roemerianus), and rarely totally submerged (e.g. Halotthia posidoniae and Pontoporeia biturbinata on the seagrasses Posidonia oceanica and Cymodocea nodosum). Specific attention is given to the adaptation of the Dothideomycetes to the marine milieu, new lineages of marine fungi and their host specificity.
Keywords: Dothideomycetes, ecology, marine fungi, multi-locus, new genera, systematics
INTRODUCTION
Most marine Dothideomycetes are intertidal, primarily from mangrove habitats and rely on the active discharge of their ascospores. They are frequently found as saprobes of decaying woody materials in the marine environment. The species that occur completely submerged in the sea are mostly parasites or symbionts of seagrasses or marine algae. It is not clear how ascospore discharge occurs in these species as their hosts are often submerged for most of the time. Jones et al. (2009) list 64 genera and ca. 108 species of marine Dothideomycetes that fall into three accepted orders (Capnodiales, Dothideales, Pleosporales), three orders incertae sedis (Hysteriales, Patellariales, Jahnulales) and 23 genera not assigned with confidence to any order. Most of these higher order taxa are represented by a single genus or species while most are members of the Pleosporales with 25 genera and 61 species (+ 13 genera, 20 species, incertae sedis). Taxa that can not be assigned with confidence to either an order or family include Aigialus, Halotthia, Lautospora, Manglicola, Mauritiana, Passeriniella, Pontoporeia, and Tirisporella. A notable feature of the marine Dothideomycetes is how few anamorphs are known. Examples include Amarenographium metableticum, Scolecosporiella typhae, Stemphylium triglochinicola and Phialophora cf. olivacea and molecular data indicates that the teleomorphs of Amorosia littoralis, Dendryphiella salina and D. arenaria may be in the Pleosporales (Mantle et al. 2006, Jones et al. 2008). This paucity of marine anamorphic fungi is in marked contrast to freshwater fungi and terrestrial genera of the class (Cai et al. 2006, Shenoy et al. 2007, Shearer et al. 2009; this volume).
Marine Dothideomycetes occur on a wide range of substrata: mangrove wood, twigs and leaves; sea and marsh grasses (especially Spartina spp. and Juncus roemerianus) (Kohlmeyer et al. 1995a, b, c, 1996, 1997a, b). Culms and leaves of sea and marsh grasses are ideal substrata for saprobic fungi because they may remain standing for several years during and after senescence (Christian et al. 1990, Kohlmeyer & Volkmann-Kohlmeyer 2001). Other species are found on brown and red seaweeds, e.g. Lautitia danica and Pleospora gracilariae (Schatz 1984, Simmons & Schatz 1989), on wood associated with sand e.g. Caryospora australiensis and Decaisnella formosa (Abdel-Wahab & Jones 2003) or on the brackish water palm Nypa fruticans, e.g. Carinispora nypae, Herpotrichia nypicola, Tirisporella beccariana and Helicascus nypae (Jones et al. 1996, Hyde & Alias 2000). Few marine Dothideomycetes produce elaborate appendaged ascospores, and most possess gelatinous sheaths that swell in water when released from the asci (Massarina velataspora and Tremateia halophila). Genera with appendaged ascospores, although generally modifications of a gelatinous sheath, include: Carinispora nypae, Decorospora gaudefroyi and Falciformispora lignatilis.
The main objective of this study is to provide information on the taxa that are unique to the marine milieu, e.g. Aigialus spp., Manglicola guatemalensis, Halotthia posidoniae and Pontoporeia biturbinata and confirm the taxonomic assignment of other marine ascomycetes within the context of a well sampled analysis with other related fungi.
MATERIAL AND METHODS
Collection of fungi
Drift and attached wood, culms and leaves of marsh plants, seagrasses and seaweeds were collected from a variety of habitats and geographical locations, placed in clean plastic bags and returned to the laboratory. After washing with freshwater to remove sediments, the samples were examined for fungi. Samples were kept moist by spraying with sterilised distilled water. Sporulating fungi were examined, identified, illustrated and single-spore isolations made. Most of the fungi sequenced in this study were obligate species, but some facultative and halotolerant terrestrial taxa from Juncus roemerianus have also been included so as to increase the sampling diversity.
Fungal isolates and culture characteristics
A selection of specimens were isolated by cutting the top of an ascoma with a sterilised razor blade, removing the contents of the centrum by making a spore suspension and then streaking the spores on antibiotic seawater agar (Kohlmeyer & Kohlmeyer 1979, Schoch et al. 2007) and germinating spores picked up. Other single ascospore isolations were made on cornmeal seawater agar (CMA/SW) with added antibiotics (streptomycin sulfate 0.5g/L, penicillin G 0.5 g/L) and allowed to germinate overnight. Germinating spores were transferred to a fresh agar plate and incubated for 2 wk at 25 °C and deposited in relevant culture collections (Table 1 - see online Supplementary Information).
Table 1.
The list of species used in this study.
| Taxon | Substrate | Collector | Location | Source | SSU | LSU | RPB2 | TEF1 |
|---|---|---|---|---|---|---|---|---|
| Acrocordiopsis patilii | Mangrove wood | J. Sakayaroj | Thailand, Hat Khanom Mu Ko Thale Tai National Park | BCC 28166 | GU479736 | GU479772 | GU479811 | — |
| Acrocordiopsis patilii | Mangrove wood | J. Sakayaroj. | Thailand, Hat Khanom Mu Ko Thale Tai National Park | BCC 28167 | GU479737 | GU479773 | GU479812 | — |
| Aigialus grandis | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 18419 | GU479738 | GU479774 | GU479813 | GU479838 |
| Aigialus grandis | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 20000 | GU479739 | GU479775 | GU479814 | GU479839 |
| Aigialus grandis | Mangrove wood | J. Kohlmeyer | Belize, Stewart Island | JK 5244A | GU296131 | GU301793 | GU371762 | — |
| Aigialus grandis | Mangrove wood | J. Kohlmeyer | Bahamas, Mores Island | JK 4770 | GU479740 | — | — | — |
| Aigialus grandis | Mangrove wood | E.B.G. Jones | Malaysia, Morib | CY 2909 | AF441172 | — | — | — |
| Aigialus mangrovei | Mangrove wood | S. Suetrong | Thailand, Kung Krabaen Bay Royal development Study Center | BCC 33563 | GU479741 | GU479776 | GU479815 | GU479840 |
| Aigialus mangrovei | Mangrove wood | S. Suetrong | Thailand, Kung Krabaen Bay Royal development Study Center | BCC 33564 | GU479742 | GU479777 | GU479816 | GU479841 |
| Aigialus parvus | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 18403 | GU479743 | GU479778 | GU479817 | GU479842 |
| Aigialus parvus | Mangrove wood | E.B.G. Jones | Malaysia Morib | BCC 32558 | GU479744 | GU479779 | GU479818 | GU479843 |
| Aigialus parvus | Mangrove wood | E.B.G. Jones | Malaysia Morib | CY 5061 | AF441173 | — | — | — |
| Aigialus rhizophorae | Mangrove wood | S. Suetrong | Thailand, Mu Ko Chang National Park | BCC 33572 | GU479745 | GU479780 | GU479819 | GU479844 |
| Aigialus rhizophorae | Mangrove wood | S. Suetrong | Thailand, Mu Ko Chang National Park | BCC 33573 | GU479746 | GU479781 | GU479820 | GU479845 |
| Allewia eureka | DAOM 195275 | DQ677994 | DQ678044 | DQ677938 | DQ677883 | |||
| Alternaria alternata | CBS 916.96 | DQ678031 | DQ678082 | DQ677980 | DQ677927 | |||
| Alternaria maritima | Ubiquitous | CBS 126.60 | GU456294 | GU456317 | — | — | ||
| Amorosia littoralis | Littoral zone | P.G. Mantle | Bahamas, Crooked Island | NN 6654 | AM292056 | AM292055 | — | — |
| Ascochyta pisi | CBS 126.54 | DQ678018 | DQ678070 | DQ677967 | DQ677913 | |||
| Ascocratera manglicola | K. Tanaka | Japan, Okinawa | HHUF 30032 | GU479748 | GU479783 | GU479822 | GU479847 | |
| Ascocratera manglicola | Mangrove wood | E.B.G. Jones | Thailand, Ranong Mangrove forest | BCC 09270 | GU479747 | GU479782 | GU479821 | GU479846 |
| Ascocratera manglicola | J. Kohlmeyer | Belize, Tobacco Range | JK 5262C, CBS 120023 | GU296136 | GU301799 | GU371763 | — | |
| Aureobasidium pullulans | CBS 584.75 | DQ471004 | DQ470956 | DQ470906 | DQ471075 | |||
| Berkleasmium micronescium | BCC 8141 | DQ280268 | DQ280272 | — | — | |||
| Berkleasmium nigroapicale | BCC 8220 | DQ280269 | DQ280273 | — | — | |||
| Biatriospora marina | Mangrove wood | E.B.G. Jones | Singapore, Singapore mangrove forest | CY 1228 | GQ925835 | GQ925848 | GU479823 | GU479848 |
| Bimuria novae-zelandiae | CBS 107.79 | DQ677998 | DQ678051 | DQ677944 | DQ767637 | |||
| Botryosphaeria dothidea | CBS 115476 | DQ677998 | DQ678051 | DQ677944 | DQ767637 | |||
| Botryosphaeria ribis | CBS 115475 | DQ678000 | DQ678053 | DQ677947 | DQ677893 | |||
| Botryosphaeria stevensii | CBS 431.82 | DQ678012 | DQ678064 | DQ677960 | DQ677907 | |||
| Botryosphaeria tsugae | CBS 418.64 | AF271127 | DQ767655 | DQ767644 | DQ677914 | |||
| Byssothecium circinnans | CBS 675.92 | AY016339 | AY016357 | DQ767646 | - | |||
| Capnodium coffeae | CBS 147.52 | DQ247808 | DQ247800 | DQ247788 | DQ471089 | |||
| Capnodium salicinum | CBS 131.34 | DQ677997 | DQ678050 | — | DQ677889 | |||
| Carinispora nypae | Mangrove wood (Nypa fruticans) | A. Loilong | Thailand, Tambon Bang Pao | BCC 36316 | GU479749 | — | — | GU479849 |
| Caryosporella rhizophorae | Mangrove wood | J. Kohlmeyer | Fiji, Suva | JK 5302A | GU479750 | GU479784 | — | — |
| Cladosporium cladosporioides | CBS 170.54 | DQ678004 | DQ678057 | DQ677952 | DQ677898 | |||
| Columnosphaeria fagi | CBS 171.93 | AY016342 | AY016359 | DQ677966 | — | |||
| Davidiella tassiana | CBS 399.80 | DQ678022 | DQ678074 | DQ677971 | DQ677918 | |||
| Decaisnella formosa | E.B.G. Jones | Australia, The Mornington Peninsula National Park | BCC 25617 | GQ925834 | GQ925847 | GU479824 | GU479850 | |
| Decaisnella formosa | Wood, sand | E.B.G. Jones | Australia, The Mornington Peninsula National Park | BCC 25616 | GQ925833 | GQ925846 | GU479825 | GU479851 |
| Decorospora gaudefroyi | Salt marsh plants | CBS 322.63 | AF394542 | — | — | — | ||
| Delitschia winteri | CBS 225.62 | DQ678026 | DQ678077 | DQ677975 | DQ677922 | |||
| Delphinella strobiligena | CBS 735.71 | DQ471029 | DQ470977 | DQ677951 | DQ471100 | |||
| Dendryphiella arenaria | Algae, sand | J. Nicot | France, Gironde, Arcachon area | CBS 181.58 | DQ471022 | DQ470971 | DQ470924 | DQ677890 |
| Dendryphiella salina | Spartina sp. | E.B.G. Jones | U.K., England; Southampton, Langstone Harbour | CBS 142.60 | — | — | DQ435066 | DQ414251 |
| Didymella cucurbitacearum | IMI 373225 | AY293779 | AY293792 | — | — | |||
| Didymella fucicola | Alga (Fucus vesiculosus) | J. Kohlmeyer | U.K., West Looe | JK 2932 | — | EF177852 | — | — |
| Dothidea hippophaes | DAOM 231303 | U42475 | DQ678048 | DQ677942 | DQ677887 | |||
| Dothidea insculpta | CBS 189.58 | DQ247810 | DQ247802 | AF107800 | DQ471081 | |||
| Dothidea sambuci | DAOM 231303 | AY544722 | AY544681 | DQ522854 | DQ497606 | |||
| Dothiora cannabinae | CBS 737.71 | DQ479933 | DQ470984 | DQ470936 | DQ471107 | |||
| Elsinoë centrolobi | CBS 222.50 | DQ678041 | DQ678094 | — | DQ677934 | |||
| Elsinoë phaseoli | CBS 165.31 | DQ678042 | DQ678095 | — | DQ677935 | |||
| Elsinoë veneta | CBS 150.27 | DQ767651 | DQ767658 | — | DQ767641 | |||
| Falciformispora lignatilis | Mangrove wood (Elaeis guineensis) | U. Pinruan | Thailand, Ban Bang Sak | BCC 21118 | GU371835 | GU371827 | — | GU371820 |
| Falciformispora lignatilis | Mangrove wood (Elaeis guineensis) | U. Pinruan | Thailand, Ban Bang Sak | BCC 21117 | GU371834 | GU371826 | — | GU371819 |
| Farlowiella carmichaeliana | CBS 206.36 | AY541482 | AY541492 | DQ677989 | DQ677931 | |||
| Floricola striata | Juncus roemerianus (Facultative) | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5678I | GU296149 | GU301813 | GU371758 | GU479852 |
| Floricola striata | Juncus roemerianus (Facultative) | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5603K | GU479751 | GU479785 | — | — |
| Gloniopsis praelonga | CBS 112415 | FJ161134 | FJ161173 | FJ161113 | FJ161090 | |||
| Gloniopsis subrugosa | CBS 123346 | FJ161170 | FJ161210 | FJ161131 | — | |||
| Guignardia bidwellii | CBS 237.48 | DQ678034 | DQ678085 | DQ677983 | — | |||
| Guignardia gaultheriae | CBS 444.70 | — | DQ678089 | DQ677987 | DQ677930 | |||
| Halomassarina (Massarina) thalassiae | Mangrove wood | J. Kohlmeyer | Fiji, Viti Levu, Suva | JK 5385B | — | GU479804 | — | GU479853 |
| Halomassarina (Massarina) thalassiae | Mangrove wood | J. Kohlmeyer. | Belize, Tobacco Range | JK 5262D | — | GU301816 | — | GU349011 |
| Halomassarina (Massarina) thalassiae | Mangrove wood | E.B.G. Jones | U.S.A., Florida | BCC 17055 | GQ925843 | GQ925850 | — | — |
| Halomassarina (Massarina) thalassiae | Mangrove wood | E.B.G. Jones | U.S.A., Florida | BCC 17054 | GQ925842 | GQ925849 | — | — |
| Halotthia posidoniae | Seagrasses (Posidoniae oceanica) | E.B.G. Jones | Cyprus | BBH 22481 | GU479752 | GU479786 | — | — |
| Heleiosa barbatula | Juncus roemerianus | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 55481 | GU479753 | GU479787 | — | — |
| Helicascus kanaloanus | A 237 | AF053729 | — | — | — | |||
| Helicascus nypae | Mangrove wood (Nypa fruticans) | A. Loilong | Thailand, Tambon Bang Pao | BCC 36751 | GU479754 | GU479788 | GU479826 | GU479854 |
| Helicascus nypae | Mangrove wood (Nypa fruticans) | A. Loilong | Thailand, Tambon Bang Pao | BCC 36752 | GU479755 | GU479789 | GU479827 | GU479855 |
| Helicascus nypae | Mangrove wood (Nypa fruticans) | E.B.G. Jones | Malaysia, Kuala Selangor | PP 6066 | AF441174 | — | — | — |
| Helminthosporium solani | HSWS 04 | AF120253 | — | — | — | |||
| Helminthosporium velutinum | ATCC 38969 | AF120254 | — | — | — | |||
| Herpotrichia diffusa | CBS 250.62 | DQ678019 | DQ678071 | DQ677968 | DQ677915 | |||
| Herpotrichia juniperi | CBS 200.31 | DDQ678029 | DQ678080 | DQ677978 | DQ677925 | |||
| Hysterium andinense | CBS 123562 | FJ161159 | FJ161199 | FJ161125 | FJ161107 | |||
| Hysterium angustatum | CBS 236.34 | — | FJ161180 | FJ161117 | FJ161096 | |||
| Hysterium pulicare | CBS 123377 | FJ161161 | FJ161201 | FJ161127 | FJ161109 | |||
| Hysterobrevium mori | CBS 123564 | FJ161158 | FJ161198 | — | FJ161106 | |||
| Hysterobrevium smilacis | CBS 114601 | FJ161135 | FJ161174 | FJ161114 | FJ161091 | |||
| Hysteropatella clavispora | CBS 247.34 | DQ678006 | AY541493 | DQ677955 | DQ677901 | |||
| Hysteropatella elliptica | CBS 935.97 | EF495114 | DQ767657 | DQ767647 | DQ767640 | |||
| Julella avicenniae | Mangrove wood | E.B.G. Jones | Thailand, Mu Ko Chang National Park | BCC 18422 | GU371831 | GU371823 | GU371787 | GU371816 |
| Julella avicenniae | Mangrove wood | E.B.G. Jones | Thailand, Mu Ko Chang National Park | BCC 20173 | GU371830 | GU371822 | GU371786 | GU371815 |
| Julella avicenniae | Mangrove wood | J. Kohlmeyer | JK 5326A | GU479756 | GU479790 | — | — | |
| Julella avicenniae | Mangrove wood | E.B.G. Jones | Hong Kong Tingkok | CY 2462 | AF441175 | — | — | — |
| Keissleriella cladophila | CBS 104.55 | GU296155 | GU301822 | GU371735 | GU349043 | |||
| Keissleriella rara | Juncus roemerianus | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | CBS 118429 | GU479757 | GU479791 | — | — |
| Kirschsteiniothelia elaterascus | HKUCC 7769 & A22-5A | AF053727 | AY787934 | — | — | |||
| Kirschsteiniothelia maritima | Driftwood | J. Kohlmeyer, B. Kohlmeyer | U.S.A., Washington, Friday Harbor Laboratories | CBS 221.60 | — | GU323203 | — | GU349001 |
| Lentithecium (Massarina) phragmiticola | Phragmites, grass | C. Tsui | Hong KongTai, O Lantau Island | CBS 110446 | DQ813512 | DQ813510 | — | — |
| Lentithecium arundinaceum (Massarina arundinacea) | CBS 619.86 | DQ813513 | DQ813509 | — | — | |||
| Leptosphaeria biglobosa | CBS 303.51 | — | GU301826 | — | GU349010 | |||
| Leptosphaeria doliolum | CBS 505.75 | U43447 | U43474 | — | — | |||
| Leptosphaeria maculans | DAOM 2229267 | DQ470993 | DQ470946 | DQ471062 | DQ471062 | |||
| Leptosphaerulina australis | CBS 939.69 | EU754068 | EU754167 | — | — | |||
| Lewia infectoria | IMI 303186 | U43465 | U43482 | — | — | |||
| Lineolata rhizophorae | Mangrove wood | J. Kohlmeyer | U.S.A., Florida | CBS 641.66 | GU479758 | GU479792 | GU479828 | — |
| Lineolata rhizophorae | Mangrove wood | J. Kohlmeyer | Australia, Queensland | CBS 118422 | — | GU479805 | — | — |
| Lineolata rhizophorae | Mangrove wood | J. Kohlmeyer | Belize, Blue Ground Range | JK 5248A | — | GU479806 | — | — |
| Lophiostoma (Platystomum) scabridisporum | Wood, sand | E.B.G. Jones | Australia, The Mornington Peninsula National Park | BCC 22836 | GQ925832 | GQ925845 | GU479829 | GU479856 |
| Lophiostoma (Platystomum) scabridisporum | Wood, sand | E.B.G. Jones | Australia, The Mornington Peninsula National Park | BCC 22835 | GQ925831 | GQ925844 | GU479830 | GU479857 |
| Lophiostoma arundinis | CBS 621.86 | DQ782383 | DQ782384 | DQ782386 | DQ782387 | |||
| Lophiostoma bipolarae (Massarina bipolaris) | HKUCC 1053 | AF164365 | — | — | — | |||
| Lophiostoma crenatum | CBS 629.86 | DQ678017 | DQ678069 | DQ677965 | DQ677912 | |||
| Lophiostoma fuckelii | CBS 113432 | — | EU552139 | — | — | |||
| Lophiostoma fuckelii | CBS 101952 | — | DQ399531 | — | — | |||
| Lophiostoma macrostomum | KT 709 | AB521732 | AB433274 | — | — | |||
| Lophiostoma macrostomum | KT 635 | AB521731 | AB433273 | — | — | |||
| Lophiostoma sagittiforme | HHUF 29754 | — | AB369267 | — | — | |||
| Lophium mytilinum | CBS 269.34 | DQ678030 | DQ678081 | DQ677979 | DQ677926 | |||
| Loratospora aestuarii | Juncus roemerianus | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5535D | GU296168 | GU301838 | GU371760 | |
| Macrophomina phaseolina | CBS 277.33 | DQ678037 | DQ678088 | DQ677986 | DQ677929 | |||
| Massaria platani | CBS 221.37 | DQ678013 | DQ678065 | DQ677961 | DQ677908 | |||
| Massarina eburnea | CBS 473.64 | AF164367 | — | — | — | |||
| Massarina eburnea | HKUCC 4054 | AF164366 | — | — | — | |||
| Massarina igniaria | CBS 845.96 | DQ813511 | DQ810223 | — | — | |||
| Massarina ricifera | Juncus roemerianus | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5535F | GU479759 | GU479793 | — | — |
| Mauritiana rhizophorae | Mangrove wood | S. Suetrong | Thailand, Kung Krabaen Bay Royal development Study Center | BCC 28866 | GU371832 | GU371824 | GU371796 | GU371817 |
| Mauritiana rhizophorae | Mangrove wood | S. Suetrong | Thailand, Kung Krabaen Bay Royal development Study Center | BCC 28867 | GU371833 | GU371825 | GU371797 | GU371818 |
| Melanomma pulvis-pyrius | CBS 109.77 | AF164369 | DQ384095 | — | — | |||
| Melanomma radicans | ATCC 42522 | U43461 | U43479 | AY485625 | — | |||
| Montagnula opulenta | CBS 168.34 | AF164370 | DQ678086 | DQ677984 | — | |||
| Morosphaeria (Massarina) ramunculicola | Mangrove wood | J. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5304B | GU479760 | GU479794 | GU479831 | — |
| Morosphaeria (Massarina) ramunculicola | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 18405 | GQ925839 | GQ925854 | — | — |
| Morosphaeria (Massarina) ramunculicola | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 18404 | GQ925838 | GQ925853 | — | — |
| Morosphaeria (Massarina) ramunculicola | Mangrove wood | HKUCC 7649 | — | DQ528762 | — | — | ||
| Morosphaeria (Massarina) velataspora | Mangrove wood | E.B.G. Jones | U.S.A., Florida | BCC 17059 | GQ925841 | GQ925852 | — | — |
| Morosphaeria (Massarina) velataspora | Mangrove wood | E.B.G. Jones | U.S.A., Florida | BCC 17058 | GQ925840 | GQ925851 | — | — |
| Mycosphaerella eurypotami | Juncus roemerianus | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5586J | GU479761 | GU301852 | GU371722 | GU371722 |
| Mycosphaerella fijiensis | OSC 100622 | DQ767652 | DQ678098 | DQ677993 | — | |||
| Mycosphaerella graminicola | CBS 292.38 | DQ678033 | DQ678084 | DQ677982 | — | |||
| Mycosphaerella punctiformis | CBS 113265 | DQ471017 | DQ470968 | DQ470920 | — | |||
| Myrangium duriaei | CBS 260.36 | AY016347 | DQ678059 | DQ677954 | DQ677900 | |||
| Myriangium hispanicum | CBS 247.33 | GU296180 | GU301854 | GU371744 | GU349055 | |||
| Mytilinidimytilinellum | CBS 303.34 | FJ161144 | FJ161184 | FJ161119 | FJ161100 | |||
| Neotestudina rosatii | CBS 690.82 | DQ384069 | DQ384107 | — | — | |||
| Oedohysterium insidens | CBS 238.34 | FJ161142 | FJ161182 | FJ161118 | FJ161097 | |||
| Oedohysterium sinense | EB 0333 | FJ161169 | FJ161209 | FJ161130 | — | |||
| Opegrapha dolomitica | — | DQ883706 | — | DQ883714 | DQ883732 | |||
| Ophiosphaerella herpotrichus | ATCC 12279 | U43453 | U43471 | — | — | |||
| Ostreichnicurtisii | CBS 19834 | FJ161137 | FJ161176 | — | FJ161093 | |||
| Ostreichnisassafras | CBS 322.34 | FJ161148 | FJ161188 | FJ161122 | — | |||
| Paraliomyces lentiferus | Mangrove wood | E.B.G. Jones | Hong Kong, North Lantau | CY 3525 | AF441176 | — | — | — |
| Passeriniella savoryellopsis | Mangrove wood | J. Kohlmeyer | Belize, Tobacco Range | JK 5167C | GU479762 | GU479795 | — | GU479858 |
| Patellaria atrata | CBS 958.97 | GU296181 | GU301855 | — | GU349038 | |||
| Patellaria cf. atrata 1 | Mangrove wood | S. Suetrong | Thailand, Kung Krabaen Bay Royal development Study Center | BCC 28877 | GU371837 | GU371829 | — | — |
| Patellaria cf. atrata 2 | Mangrove wood | S. Suetrong | Thailand, Kung Krabaen Bay Royal development Study Center | BCC 28876 | GU371836 | GU371828 | — | — |
| Phaeodothis winteri | CBS 182.58 | DQ678021 | DQ678073 | DQ677970 | DQ677917 | |||
| Phaeosphaeria albopunctata (Leptosphaeria albopunctata) | Spartina alterniflora | J. Kohlmeyer | U.S.A., North Carolina, Beaufort | CBS 254.64 | — | GU45631 | — | — |
| Phaeosphaeria avenaria | DAOM 226215 | AY544725 | AY544684 | DQ677941 | DQ677885 | |||
| Phaeosphaeria eustoma | CBS 576.86 | DQ678011 | DQ678063 | DQ677959 | DQ677906 | |||
| Phaeosphaeria olivacea | Juncus roemeriaus | J. Kohlmeyer, B. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5540Q | — | GU479807 | — | — |
| Phaeosphaeria spartinicola | Spartina sp. | J.Kohlmeyer | U.S.A., Maryland, Solomons | JK 5177A | — | GU479808 | — | — |
| Phoma herbarum | CBS 615.75 | EU754087 | EU754186 | — | — | |||
| Platychora ulmi | CBS 361.52 | EF114726 | EF114702 | — | — | |||
| Pleospora herbarum | CBS 191.86 | DQ247812 | DQ247804 | DQ247794 | DQ471090 | |||
| Pleospora sedicola | CBS 109843 | — | AY849958 | — | — | |||
| Pleosporaceae sp. 1 | OSC 100706 | — | GU479809 | — | — | |||
| Pontoporeia biturbinata | Seagrasses | E.B.G. Jones | Cyprus | BBH 23338 | GU479763 | GU479796 | GU479837 | — |
| Preussia minima | CBS 524.50 | DQ678003 | DQ678056 | DQ677950 | DQ677897 | |||
| Preussia terricola | DAOM 230091 | AY544726 | AY544686 | DQ470895 | DQ471063 | |||
| Pseudorobillarda phragmitis | CBS 842.84 | EU754103 | EU754202 | — | — | |||
| Pseudorobillarda siamensis | BCC 12531 | FJ825365 | FJ825375 | — | — | |||
| Pseudorobillarda texana | BCC 12535 | FJ825367 | FJ825377 | — | — | |||
| Psiloglonium araucanum | CBS 112412 | FJ161133 | FJ161172 | FJ161112 | FJ161089 | |||
| Psiloglonium clavisporum | CBS 123339 | FJ161157 | FJ167526 | FJ161124 | FJ161105 | |||
| Psiloglonium simulans | CBS 206.34 | FJ161139 | FJ161178 | FJ161116 | FJ161094 | |||
| Pyrenophora phaeocomes | DAOM 222769 | DQ499595 | DQ499596 | DQ497614 | DQ497607 | |||
| Pyrenophora tritici-repentis | OSC 100066 | AY544716 | AY544672 | — | DQ677882 | |||
| Quintaria lignatilis | Mangrove wood | J. Kohlmeyer, B. Kohlmeyer | French Polynesia, Moorea | JK 5390A, CBS 117700 | GU296188 | GU301865 | GU371761 | — |
| Quintaria lignatilis | Mangrove wood | E.B.G. Jones | U.S.A., Florida | BCC 17444 | GU479764 | GU479797 | GU479832 | GU479859 |
| Quintaria submersa | CBS 115553 | — | GU479810 | — | — | |||
| Repetophragma ontariense | HKUCC 10830 | — | DQ408575 | DQ435077 | — | |||
| Rimora (Lophiostoma) mangrovei | Mangrove wood | J. Kohlmeyer | Belize, Blue Ground Range | JK 5246A | GU296193 | GU301868 | GU371759 | — |
| Rimora (Lophiostoma) mangrovei | Mangrove wood | J. Kohlmeyer | India, Goa | JK 5437B | GU479765 | GU479798 | — | — |
| Roccella fuciformis | DUKE 15572 | AY584678 | AY584654 | DQ782866 | — | |||
| Saccardoella rhizophorae | Mangrove wood | J. Kohlmeyer, B. Kohlmeyer | Hawaii, Oahu | JK 5456A | GU479766 | GU479799 | — | GU479860 |
| Salsuginea ramicola | Mangrove wood | K. Tanaka | Japan, Okinawa | KT 2597.1 | GU479767 | GU479800 | GU479833 | GU479861 |
| Salsuginea ramicola | K. Tanaka | Japan, Okinawa | KT 2597.2 | GU479768 | GU479801 | GU479834 | GU479862 | |
| Scirrhia annulata | Juncus roemerianus | S. Newell | U.S.A., Georgia, Sapelo Island | JK 5546G | GU479769 | — | — | — |
| Scorias spongiosa | CBS 325.33 | DQ678024 | DQ678075 | DQ677973 | DQ677920 | |||
| Stylodothis puccinioides | CBS 193.58 | AY016353 | AY004342 | — | DQ677886 | |||
| Sydowia polyspora | CBS 116.29 | DQ678005 | DQ678058 | DQ677953 | DQ677899 | |||
| Tremateia halophila | Juncus roemeriaus | J. Kohlmeyer | U.S.A., North Carolina, Carteret County | JK 5517J | GU296201 | — | GU371721 | — |
| Trematosphaeria (Lophiostoma) heterospora | CBS 644.86 | AY016354 | AY016369 | DQ497615 | DQ471049 | |||
| Trematosphaeria pertusa | CBS 122371 | FJ201993 | FJ201992 | — | — | |||
| Trematosphaeria pertusa | CBS 122368 | FJ201991 | FJ201990 | — | — | |||
| Ulospora bilgramii | CBS 110020 | DQ678025 | DQ678076 | DQ677974 | DQ677921 | |||
| Verruculina enalia | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 18401 | GU479770 | GU479802 | GU479835 | GU479863 |
| Verruculina enalia | Mangrove wood | E.B.G. Jones | Malaysia, Morib | BCC 18402 | GU479771 | GU479803 | GU479836 | GU479864 |
| Verruculina enalia | Mangrove wood | J. Kohlmeyer, B. Kohlmeyer | Belize, Blue Ground Range | JK 5253A | DQ678028 | DQ678079 | DQ677977 | — |
| Westerdykella (Eremodothis) angulata | CBS 610.74 | DQ384067 | DQ384105 | — | — | |||
| Westerdykella cylindrica | CBS 454.72 | AY016355 | AY004343 | DQ470925 | DQ497610 | |||
| Westerdykella dispersa | CBS 508.75 | U42488 | DQ468050 | — | — | |||
| Wettsteinina lacustris | CBS 618.86 | DQ678023 | — | DQ677972 | DQ677919 |
DNA extraction, amplification and sequencing
Fungal genomic DNA from a selection of cultures was isolated by filtering mycelia grown in seawater broth at 22 °C with subsequent lyophilisation (Spatafora et al. 1998). DNA was then extracted using the FastDNA kit and cells were ground on the Fast-Prep instrument from MPI Biochemicals (Irvine, CA, U.S.A.) following manufacturer recommendations. Fungal biomass was harvested for a different set of isolates by filtering through cheesecloth, and washed several times with sterile distilled water. The harvested mycelium was stored at -20 °C and ground to a fine powder with a mortar and pestle. Fifty to 100 mg ground fungal mycelium was placed into 400 mL lysis buffer (O'Donnell et al. 1997) and DNA extracted as follows: the tube was incubated at 70 °C for 30 min, and an equal volume of phenol-chloroform (PIERCE) added. The upper liquid phase was transferred to a new microtube containing chilled absolute ethanol and 7.5 M ammonium acetate. The mixture was kept at -20 °C for 30 min, or until the DNA had precipitated, and then centrifuged at 14 000 rpm, 4 °C, for 15 min. The DNA pellet was washed twice with chilled 75 % ethanol and air dried. The DNA was resuspended in 50 mL TE buffer and checked for quantity and quality by 1 % agarose gel electrophoresis.
The following four genes were chosen for this study: small (18S) and large subunit (28S) of the nuclear ribosomal DNA (SSU, LSU) plus the gene fragments from the second largest subunit of RNA polymerase (RPB2) and the translation elongation factor 1-alpha (TEF1) gene. The rDNA was amplified with Taq DNA polymerase from FERMENTAS (Cat.No. MBDOEPO402) using PCR Model MJ Research DYAD ALD ALD 1244 thermocycler (MJ Research, Waltham, MA). Primers used for amplification include the SSU, LSU, RPB2 and TEF1 (White et al. 1990, Bunyard et al. 1994, Liu et al. 1999, Rehner 2001, respectively). The PCR products were purified using a NucleoSpin Extraction Kit (Macherey-Nagel, Germany), following the manufacturer's instructions. The characterisation of PCR products was performed via agarose gel electrophoresis on 1 % agarose gel containing ethidium bromide as the staining agent. PCR products were directly sequenced by Macrogen Inc., Korea. The sequencing primers used for as the different regions are SSU: NS1, NS3, NS4, NS6 (White et al. 1990); LSU: JS1, JS8, LROR and LR7 (Bunyard et al. 1994); TEF1: 983F, 2218R, CEFF2 and CEFR2 (Rehner 2001); RPB2: 5F1, 5F2, 7cR and 7R (Liu et al. 1999). Each sequence was checked for ambiguous bases and assembled using BioEdit v. 6.0.7 (Hall 2004) and SeqMerge, forming part of the GCG v. 10 software suite (Accelrys, San Diego, U.S.A.).
Sequence alignment and phylogenetic analyses
A total of 51 species (90 new sequences – Table 1) from the Dothideomycetes, representing 46 teleomorphic genera and five anamorphic genera were analysed along with reference fungal sequences from fungal families that were downloaded from the GenBank (listed in Table 1).
The consensus sequences for each DNA region were initially aligned with ClustalW v. 1.6 (Thompson et al. 1994) and improved in MUSCLE (Edgar 2004) (as part of Geneious Pro v. 4.7.4 (Biomatters, Auckland, N.Z.). When necessary new sequences were added to a core set of seed sequences using MAFFT v. 6.708b (Katoh & Toh 2008) using the e-insi option. Sequence homologies were also analysed using BLAST (Altschul et al. 1990) to facilitate the selection of other fungal sequences to be used in the analyses. Alignments were checked and manually optimised along with other sequences obtained from the GenBank nucleotide database. The dataset was refined visually in BioEdit v. 7.0.1 (Hall 2004). Incomplete data at the 5'- and 3'-end of partial sequences were coded as missing. Following Wiens (2006), we included taxa in our multi-locus matrix even if they did not have all genes present. All absent genes were coded as missing data, forming at least 30 % of the total characters. Two members of the Arthoniomycetes, namely Roccella fuciformis and Opegrapha dolomitica, were chosen as outgroup sequences based on their placement as sister to the Dothideomycetes (Schoch et al. 2009).
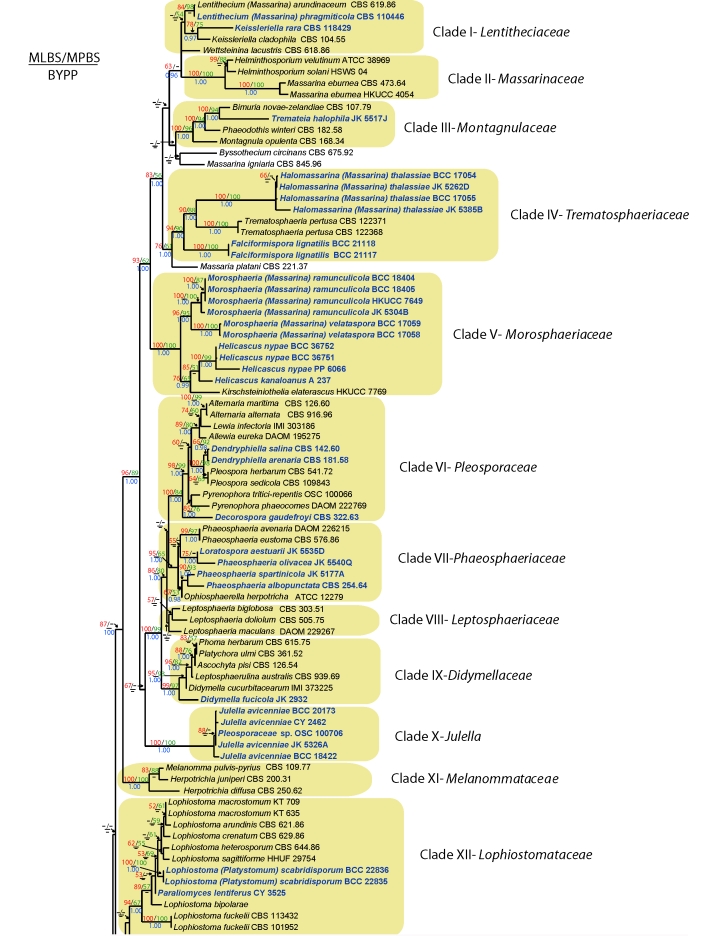
Phylogenetic trees based on individual SSU, individual LSU, combined SSU and LSU and combined SSU, LSU and TEF datasets (data not shown) were congruent with the combined SSU, LSU, RPB2 and TEF1 data sets. However the position of the taxa Biatriospora marina and Quintaria lignatilis (in Clades XIV and XVI, respectively) and Saccardoella rhizophorae (unresolved taxon) were not constant. The phylogenetic analyses of the combined SSU, LSU, RPB2 and TEF1 data were performed using parsimony, Bayesian and maximum likelihood algorithms.
Maximum parsimony (MP) analyses: MP analyses were performed using PAUP v. 4.0b10 (Swofford 2003). Gaps were treated as missing data with 100 replicates of random stepwise addition of sequences and tree-bisection reconnection (TBR) branch-swapping. All characters were given equal weight. The consistency indices (CI; Kluge & Farris 1969), retention indices (RI; Farris 1989) and rescaled consistency indices (RC; Farris 1989) were calculated for each tree generated. Bootstrap support values (Felsenstein 1985) were calculated for all parsimony analyses by 1000 bootstrap replicates (full heuristic searches, 10 replicates of random stepwise addition of sequences). Maximum parsimony bootstrap values (MPBP) equal or greater than 50 % are given above each node (Fig. 1).
Bayesian analyses (Larget & Simon 1999): The model of substitution used for Bayesian analyses was chosen with MrModeltest v. 2.2 (Nylander 2004). Independent Bayesian phylogenetic analyses were performed in MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001) using a uniform [GTR+I+G] model, lset nst = 6 rates = invgamma; prset statefreqpr = dirichlet (1,1,1,1). The Metropolis-Coupled Markov Chain Monte Carlo (MCMC) sampling approach was used to calculate posterior probabilities (PP). Four Markov chains were run from a random starting tree for 5 000 000 generations and trees sampled every 100 generations. The first 5 000 trees were discarded as burn-in prior to convergence of the four chains. The remaining trees were used to construct a 50 % majority rule consensus tree and to calculate Bayesian Posterior Probabilities (BYPP) with those equal or greater than 0.95 given below each node (Fig. 1).
Maximum likelihood analyses (ML) were conducted in RAxML v. 7.2.2 (Stamatakis 2006). The dataset was partitioned according to each gene and separate codons (eight partitions) as previously done in Schoch et al. (2009). A general time reversible model (GTR) with a discrete gamma distribution and four rate classes was applied to each partition. A tree was obtained by simultaneously running a fast bootstrap search of 1 000 pseudoreplicates followed by a search for the most likely tree under functional setting “a”. We also did 100 successive searches in RAxML under the GTR model with gamma rate distribution and starting each search from a randomised tree. Maximum Likelihood bootstrap values (MLBP) equal or greater than 50 % are given above each node (Fig. 1).
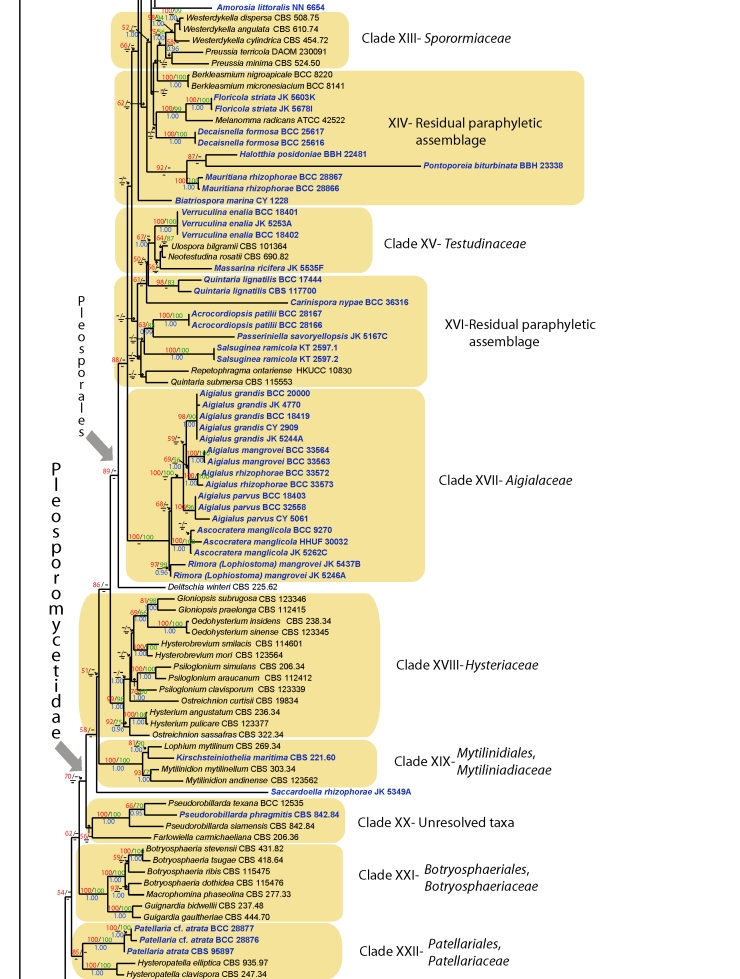
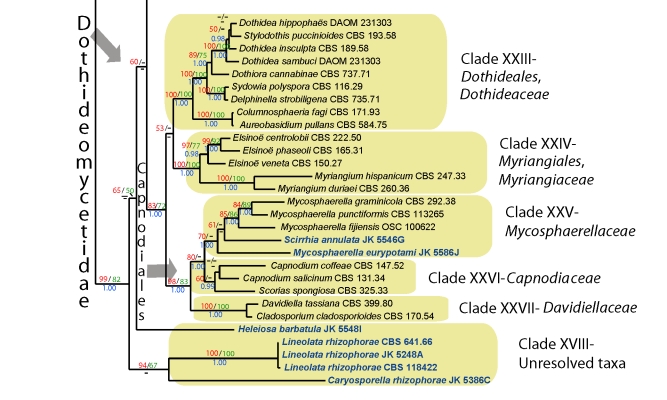
Fig. 1.
RAxML tree of marine Dothideomycetes with bootstrap support values for maximum likelihood and maximum parsimony above the nodes. The values below the nodes are Bayesian posterior probalities. Relevant clades are highlighted in colour.
Phylogenetic trees were drawn using Treeview v. 1.6.6 (Page 2001) and TreeDyn 198.3 (Chevenet et al. 2006). Sequences derived in this study are deposited in GenBank, and the alignments in TreeBASE (www.treebase.org).
RESULTS
Molecular phylogenies
The BLAST search based on SSU and LSU sequences revealed the closest matches with taxa in Dothideomycetes and SSU, LSU, TEF1, and RPB2 sequences generated as part of this study are listed in Table 1. These sequences were combined with previously published data from various orders of the Dothideomycetes (Botryosphaeriales, Capnodiales, Dothideales, Hysteriales, Pleosporales and Myriangiales) obtained from GenBank (Table 1). The data set consisted of 199 taxa, with Opegrapha dolomitica and Roccella fuciformis included as the outgroup taxa. The maximum parsimony dataset consists of 4 141 total characters, 1 890 (45.6 %) characters are constant, 532 (12.8 %) characters are parsimony informative and 1 791 (41.6 %) characters are parsimony uninformative. The heuristic search resulted in a single most parsimonious tree (MPT) with a length of 18 715 steps (CI = 0.208, RI = 0.623, RC = 0.130; data not shown). One hundred successive searches using a rapid hill-climbing algorithm from distinct randomised starting trees in RAxML yielded a best scoring likely tree (Fig. 1) with a log likelihood –84765.605900. The matrix had 2 985 alignment patterns with 32 % of the characters consisting of gaps or undetermined characters. The alignment patterns were distributed across seven partitions as follows: LSU – 859, SSU – 217, TEF1 codon1 – 195, TEF1 codon2 – 309, TEF1 codon3 – 309, RPB2 codon1 – 230, RPB2 codon2 – 203, RPB2 codon1 – 254.
Phylogenetic trees obtained from maximum likelihood, Bayesian and maximum parsimony analyses yielded trees with similar overall topology at subclass, order and family relationship in agreement with previous work based on maximum likelihood (Schoch et al. 2006). However, the internal node relationships of some taxa were resolved differently between the maximum likelihood, Bayesian and maximum parsimony trees. For example: the taxonomic position of Biatriospora marina differed between the maximum likelihood, Bayesian and Maximum parsimony trees In the maximum likelihood and Bayesian tree, B. marina grouped in a basal part of Clade XIV- Residual paraphyletic assemblage. But in the maximum parsimony tree, B. marina grouped in a basal clade to the Testudinaceae. This is not unexpected as divergence in evolutionary rates and the presence of missing data affects all these methods differently. Nevertheless, we describe new taxa based on agreement in support for all three computational methods.
Taxonomy
This study resulted in the sampling of 51 marine dothideomycetous species (Table 1) with most of the marine genera beloning in the Pleosporomycetidae, and only two taxa (Mycosphaerella, Scirrhia) referred to the Dothideomycetidae. Only clades with marine taxa (in blue bold in the tree) are discussed in the text.
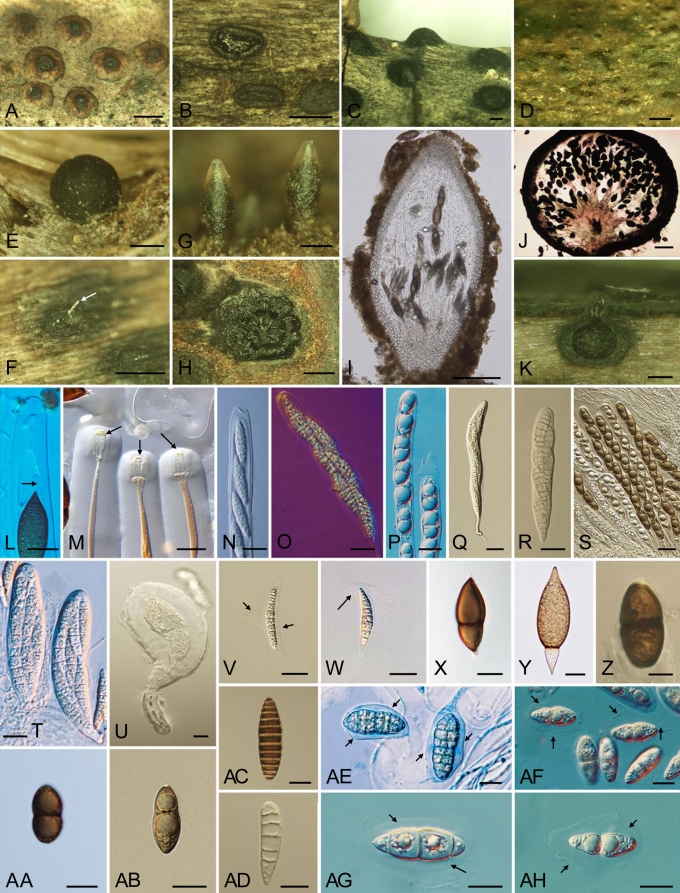
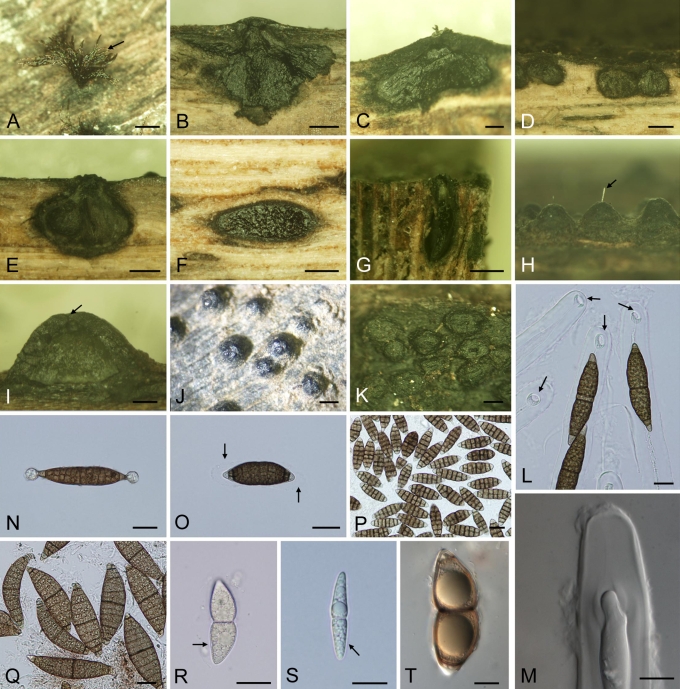
Marine Dothideomycetes show great variation in the morphology of the ascomata, asci and ascospores as illustrated in Figs 2, 3. Many genera possess ascospores with a mucilaginous sheath that swells in water, once released from the asci. In others the sheaths are drawn out to form appendages (e.g. Carinispora nypae, Decorospora gaudefroyi, Falciformispora lignatilis).
Fig. 2.
(p. 162) Morphological features of marine Dothideomycetes. A. Immersed lenticular ascomata beneath clypeus of Carinispora nypae. B. Apothecium of Patellaria cf. atrata (Patellariales). C. Broadly conical ascomata of Halotthia posidoniae. D. Immersed ascomata of Helicascus nypae. E. Globose ascoma of Pontoporeia biturbinata. F. Immersed ascomata of Quintaria lignatilis. Released asci (arrow) from ostiole. G. Mature ascomata of Manglicola guatemalensis (Jahnulales). H. Tangential section of Helicascus nypae through stroma with several loculi. I. Longitudinal section (l.s.) of Manglicola guatemalensis ascoma with asci and pseudoparaphyses. J. Pontoporeia biturbinata, non-ostiolate ascoma, asci originating at the periphery of a hemispherical basal pulvinus. K. Longitudinal section through ascoma of Verruculina enalia. Asci. L–U. Ascus tip of Manglicola guatemalensis. Ascospores show the apical appendage (arrow) in ascus. M. Ascus tip of Salsuginea ramicola, consisting of a large distinctive ocular chamber and prominent ring (arrows). N. Clavate ascus of Quintaria lignatilis with apical plate. O. Clavate ascus of Quintaria lignatilis, with biseriate ascospores, in Nomarski and Quartz. P. Ovoidal or ellipsoidal ascospores in cylindrical asci of Acrocordiopsis patilii. Q. Clavate to long-cylindrical ascus of Carinispora nypae. R. Clavate ascus of Patellaria cf. atrata. S. Subcylindrical asci with pseudoparaphyses of Helicascus nypae. T. Clavate asci of Falciformispora lignatilis (Trematosphaeriaceae). U. Broadly clavate ascus of Pontoporeia biturbinata. V–AH. Ascospores of marine Dothideomycetes: V. Carinispora nypae. Cylindrical and multiseptate ascospore with keel-like mucilaginous sheath (arrows). W. Falciformispora lignatilis. Fusiform ascospores surrounded by thin mucilaginous sheath and single scythe-like appendage (arrow) at the base. X. Salsuginea ramicola. Ovoid, dark brown ascospore with hyaline apical germ pores. Y. Manglicola guatemalensis. Fusiform ascospore with lager, pale brown apical cell and hyaline turbinate basal cell. Z. Halotthia posidoniae. Ellipsoidal, dark brown ascospores, darker around septum. AA. Verruculina enalia. Ellipsoidal, dark brown ascospore, 1-septate. AB. Helicascus nypae. Obovoidal ascospore with persistent mucilaginous sheath. AC. Mauritiania rhizophorae. Fusiform ascospore, 9–13-distoseptate. AD. Patellaria cf. atrata. Clavate ascospore, 5–7-septate. AE. Julella avicenniae. Muriform ascospores with dilated sheath (arrows), straining in ink. AF. Halomassarina (Massarina) thalassiae. Ellipsoidal ascospores with gelatinous sheath (arrows). AG. Morosphaeria (Massarina) velataspora. Fusiform to ellipsoidal ascospores, 3-septate with mucilaginous sheath (arrows). AH. Morosphaeria (Massarina) ramunculicola. Fusiform ascospores with fully dilated mucilaginous sheath (arrows). Habitat: A, D, G, H, I, L, Q, S, V, Y, AB. On the surface of Nypa fruticans. B, F, K, M–P, R, X, AA, AC–AD, AF–AH. On mangrove wood. C, E, J, U, Z. On rhizomes of Posidonia oceanica. T, W. On oil palm (Elaeis guineensis). AE. On Avicennia spp. Scale bars: A–C, E–H = 500 mm; D = 1000 mm; I = 250 mm; K = 200 mm; J = 150 mm; L–Z, AB, AF–AH = 20 mm; AA, AC–AE = 10 mm.
Fig. 3.
Morphological features of marine Dothideomycetes in the Aigialaceae and Coronopapilla mangrovei. A. Aigialus grandis. Immersed ascomata with ascospores (arrow) released from ostiole. B–E. Longitudinal section (l.s.) through ascomata of Aigialus grandis (A), A. parvus (B), A. mangrovis (C) and A. rhizophorae (D). F. A. parvus. Surface wood showing ascoma with thick peridium. G. A. parvus. Sagittal section through ascoma. H. Ascocratera manglicola. Crater-like ascomata with released ascus (arrow) from the ostiole. I. Ascocratera manglicola. l.s. of ascoma filled with gelatinous matrix. J. Coronopapilla mangrovei. Surface view of ascomata. K. Rimora (Lophiostoma) mangrovei. Broadly oblong ascomata. L. Aigialus grandis. Asci with apical refractive ring (arrows) and ascospores. M. Coronopapilla mangrovei. Ascus tip, thick-walled with ocular chamber. N–T. Ascospores of marine Dothideomycetes in Aigialaceae: N. Aigialus grandis. Broadly fusiform (front view), muriform ascospores with drop of mucilage from end cells. O. Aigialus parvus. Ellipsoidal to broadly fusiform (front view), muriform ascospores with a gelatinous cap around apical and subapical cells (arrows). P. Aigialus mangrovis. Ellipsoidal to fusiform (front view), muriform ascospores. Q. Aigialus rhizophorae. Broadly fusiform (front view), muriform ascospores. R. Ascocratera manglicola. Ellipsoidal ascospores, initially 1-septate, later becoming 3-septate with gelatinous sheath (arrow). S. Rimora (Lophiostoma) mangrovei. Fusiform ascospore with gelatinous sheath (arrow). T. Coronopapilla mangrovei. Ellipsoidal ascospore. Habitat A–T. On mangrove wood. Scale bars: A, D–G, J–K = 500 mm; B–C, H = 250 mm; L, N–S = 25 mm; M, T = 10 mm.
Pleosporomycetidae
1. Pleosporales, Fig. 1.
Delineation of families in the Pleosporales previously relied extensively on morphological characters which resulted in 17 to 19 families (Kirk et al. 2001, Lumbsch & Huhndorf 2007). These were poorly resolved at the molecular level and Schoch et al. (2006) could only find reasonable support for seven families in a phylogeny generated from four genes: Leptosphaeriaceae, Lophiostomataceae, Phaeosphaeriaceae, Pleosporaceae, Sporormiaceae, Testudinaceae and Trematosphaeriaceae. A major reassessment of these taxa is needed and attempts are underway to complete this (see Mugambi et al. 2009a, and Zhang et al. 2009; this volume). As part of this process we attempted to place a diverse selection of marine Dothideomycetes using phylogenetic reconstruction. This resulted in 11 supported clades corresponding to families, with marine representatives (Fig. 1) (Didymellaceae-Clade IX, Lentitheciaceae-Clade I, Leptosphaeriaceae-Clade VIII, Lophiostomataceae-Clade XII, Massarinaceae-Clade II, Montagnulaceae-Clade III, Phaeosphaeriaceae-Clade VII, Pleosporaceae-Clade VI, Sporormiaceae-Clade XIII, Testudinaceae-Clade XV, Trematosphaeriaceae-Clade IV) and two new families: 1) Aigialaceae (Clade XVII) for Aigialus and related taxa (Ascocratera manglicola and Lophiostoma mangrovei), and 2) Morosphaeriaceae (Clade V) for the species Morosphaeria (Massarina ramunculicola, Massarina velataspora), Helicascus nypae, H. kanaloanus and Kirschsteiniothelia elaterascus. Further clades are also identified, but their position remains unresolved, e.g. the familial position of the taxa Halotthia posidoniae, Mauritiana rhizophorae and Pontoporeia biturbinata in clade XIV.
Clade I. Lentitheciaceae
The marine Massarina species are not monophyletic which is in agreement with observations on terrestrial and freshwater members of the genus (Zhang et al. 2009b). Consequently a number of taxonomic changes are proposed in this chapter. Zhang et al. (2009a; this volume) erected the family Lentitheciaceae, and the genus Lentithecium for Massarina that do not group in the Massarinaceae. However the monophyly of Lentithecium is not supported in the current study. Massarina phragmiticola was described from the saltmarsh grass Phragmites australis (Poon et al. 1998), and groups within this family. It grouped with M. arundinacea with 84 % MLBP and 98 % MPBP support (Fig. 1). However Zhang et al. (2009a; this volume) refers M. arundinacea to the new genus Lentithecium and we place M. phragmiticola in synonymy with Lentithecium arundinaceum.
Keissleriella (type species K. aesculi) comprises some 25 species (Kirk et al. 2008) and two species group with Lentithecium in clade I, with high support. Keissleriella rara was described from the salt marsh species Juncus roemerianus, a rare halotolerant species (Kohlmeyer et al. 1995c). Zhang et al. (2009a) also included Keissleriella linearis in their phylogenetic analysis and transferred it to Lentithecium.
Clade II. Massarinaceae
Aptroot (1998) reviewed the genus Massarina and reduced the 160 names in the literature to 43 taxa, while others (especially those from aquatic habitats) have been transferred to Lophiostoma (Hyde & Aptroot 1998, Hyde et al. 2002b, Liew et al. 2002). However, subsequent studies indicate that Massarina and Lophiostoma species are polyphyletic (Zhang et al. 2009a; this volume). These genera and the families Lophiostomataceae / Massarinaceae are difficult to separate and often have overlapping characters (Zhang et al. 2009b). In our analysis the type species Massarina eburnea forms a well supported clade (Clade II) with two Helminthosporium species (H. velutinum, H. solani) as a sister group.
Jones et al. (2009) referred the genus Massarina to the Lophiostomataceae based on the molecular evaluation of Hyde et al. (2002b) and Liew et al. (2002). Lophiostoma has been reported as a monophyletic genus (Tanaka & Harada 2003, Tanaka & Hosoya 2008) while Zhang et al. (2009b) have shown that Lophiostoma is phylogenetically divided into two groups: Lophiostoma I which includes the type species L. macrostomum (voucher Lundqvist 20504), and Lophiostoma II which also contains sequences of L. macrostomum (voucher HHUF 27293 and HHUF 27290). Zhang et al. (2009b) were unable to verify the identity of the different strains of L. macrostomum and consequently could not determine the taxonomic position of Lophiostoma s. str. The paraphyletic nature of the Lophiostomataceae has previously been noted (Schoch et al. 2006) and clade XII is likely to represent the narrow concept of the Lophiostomataceae, although it is still too early to draw this conclusion until type material of Lophiostoma (L. macrostomum) is obtained (Zhang et al. 2009b). In our analysis we have selected the accession numbers AB433273 and AB433274 from the voucher specimens HHUF 27290 and HHUF 27293, respectively, and regard this clade as representing the family Lophiostomataceae (Clade XII).
Clade III. Montagnulaceae
Based on morphological data, Jones et al. (2009) referred the genus Tremateia to the Pleosporaceae, but molecular data places it with high support in the Montagnulaceae (100 % MLBP, 94 % MPBP, 1.00 BYPP) with Bimuria novae-zelandiae as a sister taxon. Kohlmeyer et al. (1995a) described Tremateia halophila from senescent leaves of Juncus roemerianus and regarded it as a facultative marine ascomycete. Characteristic features include an apical cap on the ascus, I- ocular chamber, and muriform ascospores with a wide mucilaginous sheath, and a Phoma-like anamorph.
Clade IV. Trematosphaeriaceae
This clade comprises four strains of Massarina thalassiae, a common species on mangrove wood, from Aldabra, Australia, Belize, Brunei, Florida, Galapagos, India, Malaysia, Mexico, Thailand (Kohlmeyer & Volkmann-Kohlmeyer 1987, Hyde 1992d, 1993, Alias & Jones 2000, Jones et al. 2006), with Trematosphaeria pertusa as a sister taxon. Falciformispora lignatilis (Fig. 2T, W) also groups in this clade with high support (94 % MLBP, 90 % MPBP, 1.00 BYPP); a species found on mangrove wood as well as on the fronds of the terrestrial oil palm (U. Pinruan, pers. comm.). As Massarina thalassiae cannot be accommodated in the genus Massarina based on molecular evidence, a new genus Halomassarina, is described.
Halomassarina Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch, gen. nov. MycoBank MB515951. Fig. 2AF.
Etymology: From the Greek hals = salt, in reference to the marine origin of the fungus.
Ascomata subglobosa ad pyriformia, immersa vel erumpentia, ostiolata, periphysata, papillata vel epapillata, clypeata, coriacea, brunnea, singularia. Peridium cellulis applanatis pachydermisque, texturam angularem formans. Hamathecium pseudoparaphysibus simplicibus, rariter anastomosantibus. Asci octospori, cylindrici ad clavati, pedunculati, pachydermi, fissitunicati, camera oculare, sine apparatu apicali, I non reagentes. Ascosporae distichae, ellipsoideae, triseptatae, hyalinae, tunica gelatinosa tectae.
Ascomata subglobose to pyriform, immersed or erumpent, ostiolate, periphysate, papillate or apapillate, clypeate, coriaceous, brown, single. Peridium of flattened, thick-walled cells, forming a textura angularis. Hamathecium of simple, rarely anastomosing pseudoparaphyses. Asci 8-spored, cylindrical to clavate, pedunculate, thick-walled, fissitunicate, with ocular chamber but without apical apparatus, I-negative. Ascospores distichous, ellipsoidal, 3-septate, hyaline, surrounded by a gelatinous sheath.
Type species: Halomassarina thalassiae Kohlm. & Volkm.-Kohlm.), Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch.
Halomassarina thalassiae (Kohlm. & Volkm.-Kohlm.) Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch, comb. nov. MycoBank MB515952.
Basionym: Massarina thalassiae Kohlm. & Volkm.-Kohlm. Canad. J. Bot. 65: 575. 1987.
This is a widely collected tropical species from intertidal and subtidal mangrove wood or fishing crafts (Kohlmeyer & Volkmann-Kohlmeyer 1987).
Clade V. Morosphaeriaceae
This clade, comprising four marine species Massarina ramunculicola, M. velataspora, Helicascus kanaloanus and H. nypae, is well supported (100 % MLBP, 100 % MPBP, 1.00 BYPP) with the Massarinaceae, Montagnulaceae and Trematosphaeriaceae as sister clades. As M. ramunculicola and M. velataspora do not group with other Massarina species, a new family and genus Morosphaeria are proposed.
Morosphaeriaceae Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch, fam. nov. MycoBank MB515953.
Familia Pleosporalium, Ascomycetium. Ascomata subglobosa, conica, lenticulara, immersa ad superficialia, ostiolata, papillata, periphysata, brunnea vel nigra, coriacea vel carbonacea, solitaria, vel gregaria, cum 3–4 loculis, ostiolo communi ad centrum. Hamathecium pseudoparaphysibus filamentosis, numerosis, ramosis ad basem, ramosis anastomosantibusque supra ascos. Asci octospori, clavati vel cylindrici pedunculati, pachydermi, fissitunicati, persistentes, camera apicale et disco apicale, IKI non-reagentes. Ascosporae biseriatae, hyalinae ad brunneae, septatae constrictae ad leviter constrictae, tunica vel calyptra gelatinosa tectae, vel sine tunica.
Family in the Pleosporales, Ascomycota. Ascomata subglobose, conical, lenticular, immersed to superficial, ostiolate, papillate, periphysate, brown to black, coriaceaous or carbonaceous, single to gregarious, stromatic with 3–4 loculi with a common central ostiole. Hamathecium with filamentous pseudoparaphyses, unbranched to branched at the base, anastomosing above the asci, embedded in a gelatinous matrix. Asci 8-spored, clavate to cylindrical, pedunculate, thick-walled, fissitunicate, with an ocular chamber and apical ring, non-amyloid, persistent. Ascospores biseriate, hyaline to brown, septate, with or without a gelatinous sheath or cap.
Type genus: Morosphaeria Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch.
Morosphaeria Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch, gen. nov. MycoBank MB515954.
Etymology: Named after Mor = sea in Welsh in reference to its marine habitat and sphaeria in reference to the perithecial ascomata
Ascomata solitaria vel gregaria, subglobosa vel lenticularia, immersa, erumpentia, ostiolata, papillata, coriacea, brunnea ad nigra, pseudoparaphysibus angusti, hyalinis, simplicibus et numerosis. Asci octospori, clavati vel cylindrici, pedunculati, bitunicati, pachydermi, fissitunicati, cum camera apicale et aparatu apicale, IKI non reagentes. Ascosporae uniseriatae vel biseriatae, fusiformes vel ellipsoidales, 1–3 septatae, constrictae ad septae, cum tunica gelatinosae.
Ascomata solitary or gregarious, subglobose to lenticular, immersed becoming superficial, ostiolate, papillate, coriaceous, brown to black, pseudoparaphyses filamenatous, anastomosing, branching, and numerous. Asci 8-spored, clavate to cylindrical, short pedunculate, thick-walled, bitunicate, fissitunicate, with an ocular chamber and apical apparatus, persistent. Ascospores hyaline, 1–3 septate, constricted at the septa, fusiform to ellipsoidal, surrounded by a mucilaginous sheath.
Type species: Morosphaeria velataspora (K.D. Hyde & Borse) Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch.
Morosphaeria velataspora (K.D. Hyde & Borse) Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch, comb. nov. MycoBank MB515955. Fig. 2 AG.
Basionym: Massarina velataspora K.D. Hyde & Borse, Mycotaxon 27: 163. 1986.
Morosphaeria ramunculicola (K.D. Hyde) Suetrong, Sakayaroj, E.B.G. Jones & C.L. Schoch, comb. nov. MycoBank MB515956. Fig. 3A, H.
Basionym: Massarina ramunculicola K.D. Hyde, Mycologia 83: 839. 1992.
Both species are common and frequently collected on dead wood of various mangrove trees in tropical and subtropical localities (Hyde & Borse 1986b, Hyde 1992a, Schmit & Shearer 2003, Jones & Abdel-Wahab 2005, Jones et al. 2006). Ascospores of both species possess a well-developed sheath (Au et al. 2001, Au & Vrijmoed 2002), while in M. ramunculicola polar appendages are formed as outgrowth of the fibrillar material within the inner regions of the sheath through polar discontinuities (Read et al. 1997a, b).
The taxa Helicascus kanaloanus and H. nypae form a sister group to Morosphaeria species with high bootstrap support. Jones et al. (2009) referred this genus to the Pleosporaceae as in previous analyses (Tam et al. 2003) and grouped it with Kirschsteiniothelia elaterascus (Shearer 1993a). However, Kirschsteiniothelia is polyphyletic with the marine species K. maritima grouping in our analysis in the Mytilinidaceae (Clade XIX, Fig. 1). In addition to this the type species of the genus, K. aethiops and its anamorph, Dendryphiopsis atra, are placed outside of the Pleosporales as currently defined, always in close association with an isolate of Phaeotrichum benjaminii, originally isolated from dung (Lumbsch & Lindemuth 2001, Kruys et al. 2006, Schoch et al. 2009b). This continues to demonstrate the polyphyletic nature of this genus in agreement with clear morphological differences alluded to earlier (Shearer 1993a). There is great morphological variation in the three genera assigned to this family, especially the ascospores, hyaline in Morosphaeria, brown to dark-brown in K. elaterascus and Helicascus species, respectively.
Clade VI. Pleosporaceae
Jones et al. (2009) referred five genera with marine representatives in this family: Decorospora, Helicascus, Falciformispora, Pleospora and Tremateia. The current study confirms the placement of D. gaudefroyi in this family (Inderbitzin et al. 2002), along with the two anamorphic species, Dendryphiella arenaria and D. salina, that form a sister group to Pleospora herbarum and Pleospora sedicola (Jones et al. 2008). Alternaria maritima groups as a sister taxon with Alternaria alternata and Lewia species with moderate support (74 % MLBP, 60 % MPBP). The current study refers Tremateia to the Montagnulaceae (Clade II) and Helicascus to the new family Morosphaeriaceae (Clade V), respectively, while Falciformispora groups in a sister group to Halomassarina thalassiae and Trematosphaeria pertusa (Clade IV, Fig. 1). (Zhang et al. 2009a; this volume). The identity of the Alternaria maritima strain is questioned as this taxon was regarded as nomen dubium by Kohlmeyer & Kohlmeyer (1979) since there is no type material to verify the original description by Sutherland (1916).
Clade VII. Phaeosphaeriaceae
The families Leptosphaeriaceae and Phaeosphaeriaceae are closely related as recent sequence data have shown (Khashnobish & Shearer 1996, Cámara et al. 2002, Kodsueb et al. 2006, Schoch et al. 2006). The consensus was that they should both be retained (Câmara et al. 2002, Cannon & Kirk 2007).
Loratospora aestuarii, Phaeosphaeria albopunctata, Ph. olivacea, and Ph. spartinicola are the only marine species represented in the Phaeosphaeriaceae in this data set. Based on ITS2 and partial 28S nrDNA sequences Khashnobish & Shearer (1996) confirmed the inclusion of Ph. albopunctata and Ph. typharum in the Phaeosphaeriaceae, and suggested that Leptosphaeria orae-maris had a closer relationship with Phaeosphaeria than Leptosphaeria. Jones et al. (2009) tentatively referred the genera Carinispora, Lautitia and Phaeosphaeria to this family, with Loratospora aestuarii in the Planistromellaceae (Dothideomycetidae, family incertae sedis), based on morphological observations. Barr (1996) erected the Planistromellaceae for six genera in the Dothideales based on brown-celled pseudoparenchymatous ascostroma with one or more locules which open schizogeneously and contain asci, which are separated and overtopped by interthecial tissues at maturity. However molecular data suggests that species in some currently accepted genera sensu Lumbsch & Huhndorf (2007) e.g. Comminutispora, are unrelated (Schoch et al. 2009a; this volume).
Zhang et al. (2009a; this volume) include the following marine species in the Phaeosphaeriaceae: Leptosphaeria albopunctata, Ph. spartinae, Ph. spartinicola, Ph. typharum as well as Amarenomyces ammophilae. Eriksson (1981) established the new genus Amarenomyces for Ph. ammophilae, but molecular data places it in Phaeosphaeria and thus the earlier name as proposed by Kohlmeyer & Kohlmeyer (1965) and Leuchtmann (1984) should be retained. Phaeosphaeria olivacea is a facultative marine species collected on Juncus roemerianus throughout the year (Kohlmeyer et al. 1997a). Of the marine taxa included in this family all occur on salt marsh plants: L. aestuarii, Ph. olivacea on J. roemerianus, Ph. spartinae, and Ph. spartinicola on Spartina spp., while Ph. ammophilae occurs on a range of grasses and sedges, but primarily on Ammophila arenaria (Kohlmeyer & Kohlmeyer 1979).
Clade VIII. Leptosphaeriaceae
Currently five Leptosphaeria species are referred to this family (Jones et al. 2009), but no sequences of marine Leptosphaeria are available for any of these, and therefore their taxonomic position cannot be verified.
Clade IX. Didymellaceae
The family Didymellaceae was recently described for the teleomorphic genera Didymella, Leptosphaerulina, including several Phoma anamorphs (de Gruyter et al. 2009). Four marine Didymella species have been described, three from brown or red seaweeds and D. avicenniae from wood of Avicennia (Patil & Borse 1985, Jones et al. 2009). In our analyses it forms a well-supported basal clade (99 % MLBP, 97 % MPBP, 1.00 BYPP) to the families Phaeosphaeriaceae, Pleosporaceae, and Leptosphaeriaceae. Kohlmeyer & Volkmann-Kohlmeyer (2003) questioned the taxonomic position of Didymella magnei, a species found on the red seaweed Palmaria palmata, because the ascospores differed morphologically from those of other Didymella species.
Clade X. Julella clade
The genus Julella was previously assigned to the Pleosporales incertae sedis and Phaeosphaeriaceae, respectively (Jones et al. 2009). Julella avicenniae (Fig. 2 AE) was initially described as a Pleospora species but because the ascomata develop on woody substrata, immersed beneath a clypeus with narrow pseudoparaphyses, Hyde (1992b) transferred it to Julella. However, ascomata can be superficial on well-decayed mangrove wood. Although regarded as an obligate marine ascomycete (Hyde 1992b), it may be implicated in the dieback of young shoots of Avicennia marina, at Morib mangrove, Malaysia, not submerged in seawater (Jones 2007). Julella avicenniae strains form a monophyletic clade with an unidentified pleosporaceous sequence (OSC 100706). This forms a moderately supported clade separated from other families in the Pleosporales (67 % MLBP).
Clade XII. Lophiostomataceae
In our analyses the families Lophiostomataceae and Massarinaceae are distinct, and distantly placed within the Pleosporales. This is confirmed elsewhere (Zhang et al. 2009a; this volume). Jones et al. (2009) referred seven genera with marine species to this family (Decaisnella-Clade XIV, Unresolved, Herpotrichia-Clade XI, Melanommataceae, Lophiostoma, Massarina-Clade II, Massarinaceae, Paraliomyces, Platystomum, Quintaria-Clade XVI Residual assemblage). However, molecular data places some of these in other families, as indicated in the above sentence (Fig. 1). Of these genera, only Platystomum and Paraliomyces (Tam et al. 2003) were included in the present analysis. Currently four marine Lophiostoma species are recognised: L. acrostichi, L. armatisporum, L. rhizophorae and Platystomum scabridisporum; however, Suetrong et al. (pers. obs.) propose the transfer of the latter species to Lophiostoma based on morphological and molecular data. Other Lophiostoma species have been transferred to Astrosphaeriella (A. asiana, A. mangrovis) by Hyde et al. (2002b) and Liew et al. (2002). In our analysis, based on molecular data, Lophiostoma mangrovei is referred to the family Aigialaceae (Clade XVII, Fig. 1), while other Massarina species are placed in the Lentitheciaceae (Clade I) [Lentithecium (Massarina) phragmiticola], or the new family Morosphaeriaceae (clade V) [Morosphaeria (Massarina) ramunculicola, M. (Massarina) velataspora]. No molecular data is available for the marine species Herpotrichia nypicola which occurs on the palm Nypa fruticosa, while Quintaria lignatilis forms a sister group to the Testudinaceae with low support (Schoch et al. 2006).
Clade XIV. Residual paraphyletic assemblage
Several unresolved species form part of a poorly resolved group that includes some members of the Lophiostomataceae and it is not clear whether missing data influenced this result. One of these is the marine anamorphic species Amorosia littoralis (isolated from the littoral zone in the Bahamas) and referred to the Sporormiacaeae based on molecular data (Mantle et al. 2006). Another anamorphic species, Floricola striata, is a facultative marine coelomycete from Juncus roemerianus, which grouped with Melanomma radicans with high support (100 % MLBP, 99 % MPBP, 1.00 BYPP). The teleomorph genera forming part of this poorly resolved group include: Decaisnella (Lophiostomataceae), Halotthia (Fig. 2C) (Pleosporales incertae sedis), Mauritiana (Requienellaceae) (Fig. 2AC) and Pontoporeia (Fig. 2E, J, Z) (Zopfiaceae) with weak support and previously assigned to the families listed in brackets (Jones et al. 2009). Morphologically they differ radically with perithecioid or cleistothecial ascomata, clavate to cylindrical asci and ascospores that are 3-septate and thick-walled in Halotthia posidoniae and Pontoporeia biturbinata, muriform in Decaisnella formosa and with 9–13 distosepta in Mauritiana rhizophorae. They also occur on different substrata: Decaisnella formosa on wood associated with sand, Mauritiana rhizophorae on mangrove wood, and Halotthia and Pontoporeia on submerged rhizomes of the seagrasses Posidonia oceanica and Cymodocea nodosa. The latter are temperate hosts, while D. formosa and M. rhizophorae are from the tropics.
Clade XV. Testudinaceae
Verruculina and Massarina ricifera (Fig. 2K, AA) are the only marine genera referred to this family, poorly supported in the current analysis, but confirming the results of a previous study (Schoch et al. 2006). In their analysis the family formed the basal node to the Pleosporales. Members of the Testudinaceae form a monophyletic clade and are characterised by ascospores that are 1-septate, brown without germ slits and with or without ornamentation (Kruys et al. 2006). However, Verruculina enalia shares few characters with members of the Testudinaceae, it differs especially by its marine habitat and persistent asci. Massarina ricifera is an obligate marine ascomycete growing on Juncus roemerianus and referred by Kohlmeyer et al. (1995b) to the Lophiostomataceae “with hesitation” as it did not fully agree with the type species Massarina eburnea. Molecular data presented here clearly indicates that it does not belong in Massarina, but further assignment must await additional collections.
Clade XVI. Residual paraphyletic assemblage
Several unresolved species form part of a poorly resolved group that includes the Testudinaceae and it is not clear whether missing data played a role in this. The genera in question include: Carinispora (Fig. 2AV), Massarina ricifera, Passeriniella, Salsuginea and Quintaria (Fig. 2F). Jones et al. (2009) referred Salsuginea ramicola (Fig. 2M, X) to the Pleosporales incertae sedis; a genus with similarities to Helicascus (Kohlmeyer 1969, Hyde 1991) while Hyde (1991) suggested the Dothideales incertae sedis. Both genera occur on mangrove wood but differ in that Salsuginea lacks a stroma, the ascomata form under a clypeus, asci have a distinctive ocular chamber and ascospores with prominent apical pores and lacking a mucilaginous sheath. It is a species collected from various mangrove tree species with ascospore measurements differing, but whether this is in response to the host remains to be evaluated (Hyde 1991).
The genera Acrocordiopsis (Fig. 3P) and Passeriniella form an unsupported clade with both taxa known from mangrove wood in the tropics (Hyde & Mouzouras 1988, Borse & Hyde 1989, Alias et al. 1999) and referred previously to the Melanommataceae and Dothideales incertae sedis, respectively (Jones et al. 2009). Morphologically they would appear to share few common characters. Acrocordiopsis species are characterised by large (< 2 mm) ascomata that are conical, superficial on the host and carbonaceous with the asci formed on a thin layer of peridial tissue on the host substratum while the ascospores are hyaline and 1-septate (Alias et al. 1999). Currently two Passeriniella species are accepted (Jones et al. 2009), namely P. mangrovei and P. savoryellopsis, with coriaceous, globose to subglobose, immersed ascomata, and ascospores that are 3-septate, central cells brown, and hyaline end cells (Hyde & Mouzouras 1988, Maria & Sridhar 2002). The taxonomic characterisation of the genus Passeriniella is confusing and has been discussed by Hyde & Mouzouras (1988) and Kohlmeyer & Volkmann-Kohlmeyer (1991).
Byssothecium (Passeriniella) obiones, a common species on senescent culms of Spartina, has a checkered history, assigned to Pleospora, Leptosphaeria, Didymosphaeria, Metasphaeria and Passeriniella (Jones et al. 2009). Khashnobish & Shearer (1996) showed that based on ITS sequence data, Byssothecium (Passeriniella) obiones did not belong in either Leptosphaeria or Phaeosphaeria. Subsequently, Barr (2002) assigned it to Byssothecium, based on the vericolourous ascospores in the Teichosporaceae. In our original data set, it grouped with Mycosphaerella species in the Capnodiales. As the origin of this sequence (JK 4748) cannot be verified, and because of the distinctive morphology of B. obiones which has little in common with those of Mycosphaerella and other members in the Capnodiales, we did not present these data here.
Two sequences of Quintaria lignatilis form a sister group to the Testudinaceae but with moderate support for all analyses. The genus has previously been referred to the Lophiostomataceae (Cai et al. 2006) and shares features in common with Trematosphaeria. Quintaria differs from Trematosphaeria by having completely immersed ascomata with rounded bases, black incrustations lining the sides of the ostiolar canal, a non-amyloid plate in the ascus and hyaline ascospores (Kohlmeyer & Volkmann-Kohlmeyer 1991).
Carinispora nypae is another anomalous taxon whose taxonomic position cannot be resolved at this time. It is placed in the paraphyletic assemblage XVI by maximum likelihood and Bayesian derived phylogenies, but not for those obtained by maximum parsimony. This may be due to artifacts associated with long branch lengths and its placement will require more in depth analysis. Carinispora nypae is found growing on the marine palm Nypa fruticans and has raised crust-like spots covered in a soft crust-like stroma, with lenticular ascomata under a clypeus, cylindrical and narrow asci, and yellow to pale-brown ascospores with a pronounced sheath drawn out on one side into a spine-like polar appendage (Hyde 1992a). Hyde (1992a) commented that it was close to Phaeosphaeria, but our data do not support this view.
Clade XVII. Aigialaceae Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & C.L. Schoch, fam. nov. MycoBank MB515957.
Etymology: Named after the type genus.
Familia Pleosporalium, Ascomycetium. Ascomata subglobosa, conica, immersa ad superficialia, ostiolata, ostiolum rotundum vel fissuriforme, epapillata, periphysata. Hamathecium pseudoparaphysibus trabeculatis, eramosis ad basem, ramosis anastomosantibusque supra ascos. Asci octospori, cylindrici pedunculati, pachydermi, fissitunicati, disco apicale, IKI non-reagentes. Ascosporae biseriatae vel uniseriatae, hyalinae ad atro-brunneae, septatae vel muriformes, constrictae ad leviter constrictae, tunica vel calyptra gelatinosa tectae.
Family in the Pleosporales, Ascomycota. Ascomata subglobose and immersed to superficial or conical, ostiolate, ostiolum round or cleft-like, apapillate, black, carbonaceous to coriaceous, single to gregarious. Periphysate. Hamathecium trabeculate, unbranched at the base, anastomosing above the asci, embedded in a gelatinous matrix. Asci 8-spored, cylindrical, pedunculate, thick-walled, fissitunicate, with a refractive apical ring, non-amyloid. Ascospores biseriate or monostichous, hyaline to brown, septate to muriform, with a gelatinous sheath or cap.
Type genus: Aigialus Kohlm. & Schatz.
Aigialus Kohlm. & S. Schatz, Trans. Brit. Mycol. Soc. 85: 699. 1985.
A. grandis Kohlm. & S. Schatz, Trans. Brit. Mycol. Soc. 85: 699. 1985 (Type species). Fig. 3A–B, L, N
A. mangrovis Borse, Trans. Brit. Mycol. Soc. 88: 424. 1987. Fig. 3D, P
A. parvus S. Schatz & Kohlm., Trans. Brit. Mycol. Soc. 85: 704. 1985. Fig. 3C, F–G, O
A. rhizophorae Borse, Trans. Brit. Mycol. Soc. 88: 424. 1987. Fig. 3E, Q
A. striatispora K.D. Hyde, Mycol. Res. 96: 1044. 1992.
Jones et al. (2009) accepted four species in this genus, but rejected A. rhizophorae as it shared a number of features with A. grandis, but only differed in the vertical septation in the subapical cell. Recent collections made in Thailand have enabled us to sequence this species and it is clearly distinct from A. grandis. This is a commonly encountered genus on mangrove wood and widely reported in the literature (Borse 1987, Schmit & Shearer 2003, Abdel-Wahab 2005, Jones et al. 2006). Aigialus striatispora was described from Ranong mangrove, Thailand, but no further collections have been made (Hyde et al. 1990, 1993).
Ascocratera Kohlm., Canad. J. Bot. 64: 3036. 1986.
A. manglicola Kohlm., Canad. J. Bot. 64: 3036. 1986 (Type species).
Ascocratera manglicola is characterised by carbonaceous, black, gregarious ascomata that are conical, crater-like, superficial on wood, on a black stroma, by trabeculate pseudoparaphyses, by asci with a refractive apical ring, and hyaline ascospores, surrounded by a gelatinous evanescent sheath (Kohlmeyer 1986). It is a common species on mangrove wood in the intertidal zone, and known from various tropical geographic locations (Schmit & Shearer 2003).
Rimora Kohlm., Volkm-Kohlm., Suetrong, Sakayaroj & E.B.G. Jones, gen. nov. MycoBank MB515958.
Etymology: From the Latin rima = cleft, fissure and os = mouth, in reference to the cleft-like ostiole, a unique feature among marine ascomycetes.
Ascomata erumpentia, apice plano, elongata, epapillata, ostiolo fissuriforme, periphysata, nigra, gregaria. Peridium cellulis pachydermis, texturam angularem formans. Hamathecium pseudoparaphysibus ramosibus. Asci octospori, cylindrici, pedunculati, pachydermi, fissitunicati, sine apparatu apicali. Ascosporae distichae, fusiformes, triseptatae, hyalinae, tunica gelatinosa tectae.
Ascomata erumpent, with flat tops, elongated, apapillate, opening with a periphysate cleft-like ostiole, black, gregarious. Peridium of thick-walled cells, forming a textura angularis. Hamathecium of branched pseudoparaphyses. Asci 8-spored, cylindrical, pedunculate, thick-walled, fissitunicate, without apical apparati. Ascospores biseriate, fusiform, 3-septate, hyaline, surrounded by an evanescent sheath.
Type species: Rimora mangrovei (Kohlm. & Vittal) Kohlm.,Volkm-Kohlm., Suetrong, Sakayaroj, E.B.G. Jones.
Rimora mangrovei (Kohlm. & Vittal) Kohlm.,Volkm-Kohlm., Suetrong, Sakayaroj & E.B.G. Jones, comb. nov. MycoBank MB515959. Fig. 3K, S. Basionym: Lophiostoma mangrovei Kohlm. & Vittal, Mycologia 78: 487. 1986.
≡ Astrosphaeriella mangrovei (Kohlm. & Vittal) Aptroot & K.D. Hyde, in K.D. Hyde, Fungi in Marine Environments. Fungal Diversity Press 7: 106. 2002.
Rimora mangrovei was described from collections of bark and wood of mangrove trees from Belize and India (Kohlmeyer & Vittal 1986) as Lophiostoma. It was subsequently transferred to Astrosphaeriella (Hyde et al. 2002b) based on the trabeculate morphology of the pseudoparaphyses. However, the aforementioned authors conceded that A. mangrovis (and A. asiana) differed from other Astrosphaeriella species by their round flattened ascomata, slit-like ostioles and non monocotyledonous hosts.
All three genera Aigialus, Ascocratera and Rimora share features such as carbonaceous, apapillate ascomata, trabeculate pseudoparaphyses, cylindrical asci with an apical apparatus and ascospores with a sheath. However, they differ in the morphology of their ascospores: brown and muriform in Aigialus, hyaline and 1–3-septate in Ascocratera and Rimora.
2. Mytilinidiales, Fig. 1
Clade XIX. Mytilinidiaceae
The common bitunicate ascomycete Kirschsteiniothelia maritima groups with Lophium mytilinum, with Mytilinidion mytilinellum and Hysterium andinense as a sister group. The genus Kirschsteiniothelia has been referred to the Pleosporaceae (Eriksson & Hawksworth 1998, Kirk et al. 2001), Pleomassariaceae (Barr 1993), and questionably the Massarinaceae (Kodsueb et al. 2006). The genus appears to be polyphyletic, and Shearer (1993a) and Schoch et al. (2006) are of the opinion that K. aethiops does not belong in the Pleosporaceae. Kodsueb et al. (2006) show that K. elaterascus (a freshwater species) clusters with Morosphaeria (Massarina) ramunculicola in a sister clade to the Melanommataceae (see also clade XI, Fig. 1). However, K. elaterascus differs from K. maritima, and other Kirschsteiniothelia species in ascus structure, its unusual endoascus with a long, coiled base that uncoils during ascus dehiscence, ascospore measurements, the presence of an ascospore sheath and its freshwater occurrence (Shearer 1993a).
Clade XX. Unresolved taxa
Included in this clade are three coelomycete species of which Pseudorobillarda phragmitis has been reported from pine and yellow poplar test panels from estuarine waters (Salinity 3–16 ppt) (Jones et al. 2009). This monophyletic group formed a well-supported clade and a sister group to the Mytilinidiales. However in the current study they form a weakly supported clade with Farlowiella carmichaeliana and are basal to the Mytilinidiales in all analyses.
3. Patellariales, Fig. 1
Clade XXII. Patellariaceae
Patellaria cf. atrata (Fig 2B, R, AD), a species found growing on various mangrove wood species collected in Hong Kong and Thailand, forms a sister group to Hysteropatella species, taxa normally assigned to the Hysteriales, but recently removed (Boehm et al. 2009a, b; this volume). Morphologically, little distinguishes Gloniella clavatispora and Patellaria atrata; paraphyses in the latter species are distinctly branched and club-shaped (Suetrong & Jones 2006). The paraphyses illustrated by Steinke & Hyde (1997) are simple and not branched (Suetrong & Jones 2006). Boehm et al. (2009a; this volume) refer Gloniella to the Hysteriaceae, and Patellaria in the Patellariaceae; further collections of the marine taxa are required to resolve their identification.
A number of marine species do not group within existing orders of Dothideomycetes and this may indicate new supergeneric taxa not yet circumscribed. The lack of sufficient protein coding gene sequences for these in our analysis and the tendency for these species to be associated with fast evolving branches on our trees further complicates the development of phylogenetic hypotheses for these taxa.
Biatriospora marina (Clade XIV), in all analyses, forms a distinct long branch and is a basal taxon to the Pleosporomycetidae without any closely related taxa (Fig. 1). It is an unusual species described from Sonneratia alba mangrove wood collected in the Seychelles and India (Hyde & Borse 1986a). It has immersed subglobose to pyriform ascomata that are black and carbonaceous, cylindrical asci and brown, septate ascospores with hyaline, globose refractive chamber or an appendage at each end. Septation is unusual in that ascospores are non-septate in the center but septate at both ends and not constricted at the septa. Additional collections have been made from mangroves in Hong Kong, Malaysia and Thailand (Jones et al. 2006, E.B.G. Jones unpubl. data).
Saccardoella rhizophorae Clade XIX. Saccardoella species have been regarded as having unitunicate asci and thus classified in the Clypeosphaeriaceae (Barr 1994). However, Mathiassen (1989) was of the opinion that the asci are bitunicate and this would appear to be supported by the current study. Saccardoella species are known from terrestrial, marine and freshwater habitats (Hyde 1992c, Tsui et al. 1998). However in all phylogenetic analyses to date this species does not group within any known family or order, and further studies are required to determine its phylogenetic relationship.
4. Jahnulales
Aliquandostipitaceae (data not shown)
The family Aliquandostipitaceae was established for species in the genus Aliquandostipite based on the phylogenetic analyses of SSU nrDNA sequences (Inderbitzin et al. 2001). Subsequently Pang et al. (2002) introduced the new order Jahnulales into the Dothideomycetes, Ascomycota, based on phylogenetic analysis of SSU nrDNA sequences of Aliquandostipite, Jahnula and Patescospora. More recently, Campbell et al. (2007) studied the phylogenetic relationships of taxa in the Jahnulales inferred from SSU and LSU nrDNA sequences and recognised four groups: 1) a basal group with Megalohypha aqua-dulces; 2) a Jahnula group comprising the type species J. aquatica; 3) five Aliquandostipite species; and 4) four Jahnula species and the anamorphic genera Brachiosphaera and Xylomyces. They emended the ordinal description to include brown, wide hyphae (>10 μm) and greater variation of ascospore morphology.
Three marine fungi belong in the Jahnulales, the teleomorph Manglicola guatemalensis and the anamorphic species Xylomyces chlamydosporus and X. rhizophorae (Suetrong et al. 2010). Manglicola guatemalensis is a poorly known species with only three previous collections (Kohlmeyer & Kohlmeyer 1971, Hyde 1988, Jones et al. 2009, Suetrong et al. 2010). The type strain was collected from dead roots of Rhizophora mangle in Guatemala (Kohlmeyer & Kohlmeyer 1971). Subsequent collections have been made on intertidal prop roots of Rhizophora apiculata at Kpg. Danau, Brunei (Hyde 1988) and frond bases of Nypa fruticans (Jones et al. 2009). Common features M. guatemalensis shares with the Jahnulales include stipitate ascomata, bitunicate asci, reticulate pseudoparaphyses and 1-septate brown ascospores. Manglicola guatemalensis differs from other bitunicate ascomycetes by its large ascomata, wide ostiole, large unequally 1-septate ascospores and mangrove habitat on R. mangle and the frond bases of N. fruticans.
Huhndorf (1994) referred Manglicola to the Hypsostromataceae, a family with no known relationship to any group in the Dothideomycetes (Loculoascomycetes) but “probably with affinities to the Melanommatales” (Mugambi & Huhndorf 2009; this volume). Characteristics that unite Manglicola and the Hypsostromataceae include superficial, large, elongate ascomata (stalked) with a soft-texture, trabeculate pseudoparaphyses, stipitate asci attached in a basal arrangement in the centrum and fusiform, septate ascospores (Huhndorf 1994).
Dothideomycetidae
5. Capnodiales, Fig. 1
Fourteen genera, such as Belizeana, Caryosporella, Coronopapilla, Lautospora, Loratospora, Pontoporeia and Thalassoascus, assigned to the subclass Dothideomycetidae, have only marine species, and represent new lineages of fungi that may be associated with the Capnodiales (Jones et al. 2009). Importantly, few have been studied at the molecular level. Placement of the genera Passeriniella and Pontoporeia has already been discussed above.
Clade XXV. Mycosphaerellaceae
Mycosphaerella eurypotami, a halotolerant terrestrial species found on Juncus roemerianus, was tentatively referred to the genus by Kohlmeyer et al. (1997b). In the current study it is a sister taxon to all Mycosphaerella species with moderate support. Jones et al. (2009) list three marine Mycosphaerella species (M. salicorniae, M. staticiola, M. suaedae-australis) found on salt marsh plants (Armeria, Limonium, Salicornia and Suaeda), while M. pneumatophorae is a common species on the pneumatophores of Avicennia species in Asia and the Carribean (Kohlmeyer & Kohlmeyer 1979, Schmit & Shearer 2003, E.B.G. Jones, pers. comm.). However recent molecular phylogenies containing a single culture did not support the placement of M. pneumatophorae in Mycosphaerella (Schoch et al. 2006); instead it was found on a poorly resolved branch within Dothideomycetes.
In our analysis, Scirrhia annulata, described from senescent leaves of Juncus roemerianus (Kohlmeyer et al. 1996), groups with various Mycosphaerella species with moderate support. Diagnostic features are the linear stromata, 1–3 mm long, generally superficial, multiloculate with ascomata in longitudinal rows, asci clavate with apical apparatus (several rings), ascospores 3-septate, brown, with a thin evanescent sheath, and measuring 46–60 x 9–11.5 μm.
Clade XVIII. Unresolved taxa (Fig. 1)
The taxonomic position of Heleiosa barbatula (Fig. 1) is unresolved as observed by its swapping position in different analyses (data not shown) and previously referred to the Dothideales and Pleosporales incertae sedis, respectively (Kohlmeyer et al. 1996, Jones et al. 2009). This species, collected on Juncus roemerianus, is rare and is not obligately marine. Characteristics include immersed ostiolate epapillate ascomata formed beneath a clypeus, with pseudoparaphyses, asci cylindrical with short pedicel, refractive apical apparatus and ascospores that are pale brown, ellipsoid, 1-septate with 10 or more cilia-like polar appendages at each end.
The genera Caryosporella, and Lineolata form a basal clade in all analyses with weak support, genera previously assigned to Melanommataceae and Pleosporales incertae sedis, respectively (Jones et al. 2009). Both occur on mangrove substrata and have been widely reported from different geographical locations (Schmit & Shearer 2003).
Caryosporella was thought to be related to Caryospora, with which it shares a number of common features (Kohlmeyer 1985). It is found on dead wood of intertidal roots and branches of mangrove trees and has large ascomata and 1-septate, dark-brown ascospores that are thickened at their apices.
Lineolata was initially described as a Didymosphaeria but transferred to this genus (Kohlmeyer & Volkmann-Kohlmeyer 1990) as it differs in the following respects: no clypeus, almost superficial ascomata, hamathecium with a gelatinous matrix, asci with an apical ring-like structure around the ocular chamber and ornamented brown ascospores. It remains enigmatically placed here, although three monophyletically placed isolates obtained from different geographic locations heighten our confidence in the provenance of these sequences.
DISCUSSION
Marine lineages of the Dothideomycetes
The study confirms the occurrence of several marine Dothideomycetes with well supported sequence data. The Pleosporales includes ten families and three unresolved clades with marine species, while the orders Capnodiales, Jahnulales, Mytilinidiales, and Patellariales are represented by few taxa. This is in common with their known diversity (?) in nature (Kohlmeyer & Kohlmeyer 1979, Jones et al. 2009). While many terrestrial genera have marine members, e.g. Mycosphaerella, Passeriniella, Lophiostoma, Massarina, Trematosphaeria and Phaeosphaeria, others have no known terrestrial counterparts. The uniqeness of these has necessitated the introduction of two new families in the Pleosporales, Aigialaceae (all marine genera: Aigialus, Ascocratera, Rimora) and Morosphaeriaceae (marine genera Helicascus, Morosphaeria and the freshwater species Kirschsteiniothelia elaterascus). The taxonomic position of other exclusively marine genera/species remains to be resolved e.g. the seagrass ascomycetes Halotthia posidoniae, Pontoporeia biturbinata (CladeXIV), and Lineolata rhizophorae (Clade XVIII) and Biatriospora marina (Clade XIV).
A number of new marine lineages have been highlighted as result of molecular studies including Manglicola guatemalensis, the first member of the Jahnulales reported from marine habitats (Suetrong et al. 2010). This is of particular interest as all other Jahnulales members are fresh water or peat swamp species and raises the question as to whether these marine fungi are derived from terrestrial and freshwater taxa that have migrated to the sea. This would support earlier phylogenetic analyses (Spatafora et al. 1998) that strongly suggest a terrestrial origin of another marine ascomycete family in the Sordariomycetes, the Halosphaeriaceae. A more recent data set (Schoch et al. 2009a; this volume) continues to support this hypothesis. The marine species M. guatemalensis occurs in estuarine mangrove habitats on the palm fronds of Nypa fruticans and Rhizophora wood and may well form a link between lignicolous freshwater taxa and species from estuarine to marine environments. Another Jahnulales species of interest is the anamorph Xylomyces rhizophorae, found on various marine and mangrove substrata (Kohlmeyer & Volkmann-Kohlmeyer 1998, S. Sivichai, pers. comm.). Campbell et al. (2007) and Prihatini et al. (2008) have shown that Xylomyces chlamydosporus has a teleomorph in the Jahnulales.
A second marine lineage is the Aigialaceae comprising three genera: Aigialus, Ascocratera, and the new genus Rimora, a family within the Pleosporales. Morphologically they show few common characteristics but all are to be found in mangrove habitats.
Schoch et al. (2006) showed that Verruculina enalia is a member of the Testudinaceae, and another marine lineage in the Dothideomycetes. Previously referred to the Didymosphaeriaceae (Kohlmeyer & Volkmann-Kohlmeyer 1990), it forms a well supported basal clade to the Pleosporales. Continued molecular studies of unresolved taxa may yield further lineages of marine ascomycetes.
Taxa for future phylogenetic study
Marine Dothideomycetes include a broad spectrum of genera and a wide variety has been sequenced for the current study. However, several remain to be investigated with DNA sequence data, especially the genera Belizeana, Capillatospora and Thalassoascus (Dothideales incertae sedis); Lautospora (Dothideomycetidae incertae sedis); Bicrouania (Melanommataceae?); Lautitia (Phaeosphaeriaceae?) and Tirisporella (Pleosporales incertae sedis). Most are only rarely collected, have yet to be isolated, are intertidal, or rarely totally submerged. Other more frequently collected taxa also require further analysis: Quintaria lignatilis (mangrove species), Decaisnella formosa (wood in association with sand) and Byssothecium obiones (on Spartina grass).
Adaptation to the marine environment
Of the 64 genera (108 species) of marine Dothideomycetes nearly all are intertidal species found in mangrove habitats, with the exception of those that occur on marine algae, saltmarsh plants or seagrasses, e.g. Thalassoascus, Lautitia, Pharcidia (algae), Bicrouania (marsh plants), Halotthia, Pontoporeia (seagrasses); Caryospora australiensis, Decaisnella formosa and Platystomum scabridisporum (wood associated with sand) (Abdel-Wahab & Jones 2000, 2003). Most of them would appear to be well adapted to intertidal estuarine habitats with active discharge of their ascospores. Although they lack the elaborate ascospore appendages found in the Halosphaeriaceae (Jones 1994, 1995) many have mucilaginous sheaths, often elaborated to form polar appendages (Yusoff et al. 1994, Read et al. 1997a, b, Alias et al. 2001, Au et al. 1999). Ascospores within the ascus are surrounded by a well-defined delimiting membrane which prevents the mucilaginous sheath from expanding, thus ensuring effective ascospore discharge (Read et al. 1994, Yusoff et al. 1994). Once ejected from the ascus the sheaths (and appendages) take up water, swell and help in the attachment of the spores to suitable substrata (Jones 1995).
Some species form ascospore appendages by fragmentation of a sheath e.g. Capronia ciliomaris (Au et al. 1999) and Tirisporella beccariana (Jones et al. 1996). A similar mechanism of appendage unfolding appears to occur in Heleiosa barbatula (Kohlmeyer et al. 1996). As with the ensheathed ascospores, the appendages do not dilate until they are dispersed into water.
Few marine anamorphic fungi have been reported in comparison to those found in freshwater habitats (Marvanová 1997, Belliveau & Bärlocher 2005, Cai et al. 2006). Currently some 94 marine anamorphs are known, but only a few have been linked to teleomorphs in the Dothideomycetes: Amorosia littoralis (Mantle et al. 2006), Dendryphiella arenaria, D. salina (Jones et al. 2008), Xylomyces spp. (Campbell et al. 2007, Prihatini et al. 2008), Pseudorobillarda phragmitis (Rungjindamai, pers. comm.), and Robillarda rhizophorae (Rungjindamai, pers. comm.). A strain of Alternaria maritima groups within the Pleosporaceae in the current study, while other marine anamorphic species e.g. Stemphylium spp. Stagonospora spp., may also be linked to teleomorphs in the Dothideomycetes.
Freshwater anamorphic fungi are uniquely adapted to their habitat with branched, sigmoid and tetraradiate conidia (Jones 2006, Campbell et al. 2007); many have teleomorphs in the Dothideomycetes (Webster & Descals 1979, Tsui & Berbee 2006, Tsui et al. 2006). In contrast few of the marine hyphomycetes appear to be adapted to their milieu, lacking any elaboration of their conidia (except e.g. Varicosporina ramulosa and Dwayaangam junci). This is particularly so for species with recorded teleomorphs in the Dothideomycetes (Jones et al. 2008).
Specific habitats of marine Dothideomycetes
Marine Dothideomycetes are generally intertidal ascomycetes and more common in mangroves, with only a few documented from temperate climates.
Nypa fruticans: Currently some 100 saprophytic fungi have been documented from Nypa fruticans, a brackish water palm that occurs from fully saline conditions to freshwater habitats. Common fungi on this palm include Astrosphaeriella nypae, Astrosphaeriella striatispora, Helicascus nypae, Linocarpon appendiculatum and Tirisporella beccariana. Many of the fungi occurring in Nypa are not found on other mangrove or marine substrata, for example, Linocarpon spp., Astrosphaeriella spp., Oxydothis spp. and Fasciatispora lignicola. Therefore one could ask, are these fungi host-specific or is their occurrence on Nypa determined by the salinity of the habitat? A significant number of fungi on Nypa are unique to the palm, e.g. Helicascus nypae, Tirisporella beccariana and Carinispora nypae while recently Manglicola guatemalensis has been found to be common on this palm in Thailand.
-
Seagrasses: The diversity of fungi in seagrasses has been a neglected field (Raghukumar 2008). Generally, diverse seagrass species support low diversity and density of saprophytic and endophytic fungi, as confirmed by many studies (Wilson 1998, Alva et al. 2002, Devarajan et al. 2002, Rodríguez 2008, Sakayaroj et al. 2010). The most common marine fungi associated with seagrasses include Sordariomycetes, Corollospora maritima, Lindra thalassiae, Lulworthia sp. and anamorphic fungi (Kohlmeyer & Kohlmeyer 1979, Newell & Fell 1980). Cuomo et al. (1982, 1985) reported that the marine Dothideomycetes, Pontoporeia biturbinata, and Halotthia posidoniae were commonly found on Posidonia oceanica and Cymodocea nodosa from Mediterranean coasts (Cuomo et al. 1982, 1985) and Cyprus (Jones et al. 2009). These two obligate marine Dothideomycetes appear to be host specific and are frequently found on rhizomes of seagrass (Kohlmeyer & Kohlmeyer 1979).
Many anamorphic dothideomycetous fungi have been found predominantly as endophytes associated with living seagrass tissues (Sakayaroj et al. 2010). They are mostly sterile mycelia and have only been identified by DNA sequence analysis (Sakayaroj et al. 2010). So far the diversity of marine fungi associated with seagrasses, compared with other substrata, is relatively low (Kohlmeyer & Kohlmeyer 1979). This is probably due to 1) growth inhibiting substances present in seagrass, 2) possibly the frail leaves of seagrass break up before most of the ascomycetes are able to colonise or sporulate and finally 3) they are attacked by other competitors such as bacteria, protozoa, lower fungi, fast growing anamorphic and/or terrestrial fungi (Sakayaroj et al. 2010).
-
Saltmarsh plants: Spartina and Juncus roemerianus: The mycota of the saltmarsh plant Juncus roemerianus, endemic to the U.S. east coast and to the Gulf of Mexico, is unique among herbaceous plants and can only be vaguely compared to that of mangrove trees, which also host obligate marine as well as terrestrial species. The terete leaves of J. roemerianus remain standing for three years or more and the extreme conditions of the habitat are the reason for the unique fungal diversity (117 species, 17 families; Kohlmeyer & Volkmann-Kohlmeyer 2001). Bitunicates appear to be less abundant than other groups of fungi; they range from obligate marine taxa at the base to terrestrial but halotolerant species at the tip of the leaves.
Spartina species are common saltmarsh plants in temperate climates that support a wide range of fungi. Kohlmeyer & Volkmann-Kohlmeyer (2002) list 39 obligate and facultative marine fungi reported from Spartina species, of which 13 are bitunicate species. Phaeosphaeria species appear to be the most common bitunicate genus on this substratum.
Mangroves: Some 54 species of mangrove trees and 60 associates occur in the new and old world (Tomlinson 1986) with senescent wood, leaves and fruits offereing a unique habitat for fungi. It is interesting that maglicolous fungi are predominantly bitunicate species, while unitunicate ascomycetes are more prevalent in other marine habitats. Of the 108 described marine Dothideomycetes, 90 sequences are currently available enabling the taxonomic resolution of a number of genera and species; in particular of Massarina species which are frequently found on mangrove substrata.
Future studies
Many habitats, substrata, geographical locations remain virgin territory for studies on marine fungi. For example, a recent investigation of the fungal diversity associated with the brown alga Fucus serratus found several unknown phylotypes within the Dothideomycetes, including some grouping with an anamorph species isolated from leaf litter (Sporidesmium obclavatulum; Shenoy et al. 2006) without obvious marine assocations (Zuccaro et al. 2008). Previously Zuccaro & Mitchell (2005) isolated fungi from living and cast fronds of the alga, with 33 % belonging in the Dothideomycetes. Many other niches such as endophytes from marine animals and mangroves await intense study (Pang et al. 2008, Schulz et al. 2008, Wang et al. 2008). Practical applications are also possible as marine endophytes from plants and animals have already yielded a wide range of new chemical structures (Jones 2008, Pan et al. 2008). Unknown fungi, including those belonging to the Dothideomycetes, have even been isolated from extreme marine environments, e.g. ocean sediments and deep sea hydrothermal ecosystems (Burgaud et al. 2009). Although it remains to be seen whether these fungi truly qualify as marine fungi the increase in fungal and dothideomycete phylotypes from these environments suggest additional sources of untapped diversity (Le Calvez et al. 2009).
In conclusion, marine bitunicate ascomycetes, (as other marine fungi) is a broadly defined ecological group that occupy a wide range of habitats within the maritime environment. Within this study facultative and halotolerant species from Juncus roemerianus were also included, as well as two genera on submerged seagrasses from European regions. The vast majority of fungi presented are predominantly tropical/subtropical mangrove species. When compared to the other diverse groups of marine fungi in the Sordariomycetes the prevalence of mangrove fungi in Dothideomycetes is even more noticeable. Does this ecological predominance reflect a radiation event of these fungi in the Dothideomycetes? Or is our sampling still biased towards specific geographies and ecologies? Only a renewed focus on the niches described above will provide us with the answer. It is our hope that a broader scope will provide enough resolution to begin to address ecological shifts in this fascinating group of fungi.
Acknowledgments
This work was supported by TRF/BIOTEC Special Program for Biodiversity Research and Training Grant BRT R251006, BRT R351004 BRT R352015, and a TOTAL Corporate Foundation, TOTAL E & P Thailand. We thank Y. Zhang and Dr K.D. Hyde for exchange of data and useful discussions. SS acknowledges the Songklanagarind Scholarship for Graduate Studies from the Prince of Songkla University. We thank Prof. M. Tanticharoen, Dr K. Kirtikara and Dr L. Eurwilaichitr for continued support. We thank A. Klaysuban for technical assistance, and U. Pinruan, N. Rungjindamai, R. Choeyklin, A. Loilong, S. Preedanon and O. Supaphon for assistance with the field work. Work performed by CLS after 2008 was supported in part by the Intramural Research Program of the NIH, National Library of Medicine. Part of this work was also funded by a grant from NSF (DEB-0717476) to JWS and CLS (until 2008).
Taxonomic novelties: Aigialaceae Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & Schoch, fam. nov., Halomassarina Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & Schoch, gen. nov., Halomassarina thalassiae (Kohlm. & Volkm.-Kohlm.), Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm. & Schoch, comb. nov., Suetrong, Sakayaroj, E.B.G. Jones, Kohlm., Volkm.-Kohlm.,comb. nov., Clade V. Morosphaeriaceae Suetrong, Sakayaroj, E.B.G. Jones, & Schoch, fam. nov., Morosphaeria velataspora (K.D. Hyde & Borse) Suetrong,· Sakayaroj, E.B.G. Jones & Schoch, comb. nov., Morosphaeria ramunculicola (K.D. Hyde) Suetrong,· Sakayaroj, E.B.G. Jones & Schoch, comb. nov., Rimora Kohlm., Volkm-Kohlm., Suetrong, Sakayaroj, E.B.G. Jones, gen. nov., Rimora mangrovei (Kohlm. & Vittal) Kohlm.,Volkm-Kohlm., Suetrong, Sakayaroj, E.B.G. Jones, comb. nov.
References
- Abdel-Wahab MA (2005). Diversity of marine fungi from Egyptian Red Sea mangroves. Botanica Marina 48: 348–355. [Google Scholar]
- Abdel-Wahab MA, Jones EBG (2000). Three new marine ascomycetes from driftwood in Australian sand dunes. Mycoscience 41: 379–388. [Google Scholar]
- Abdel-Wahab MA, Jones EBG (2003). Decaisnella formosa sp. nov. (Ascomycota, Massariaceae) from an Australian sandy beach. Canadian Journal of Botany 81: 598–600. [Google Scholar]
- Alias SA, Jones EBG (2000). Colonization of mangrove wood by marine fungi at Kuala Selangor mangrove stand, Malaysia. Fungal Diversity 5: 9–21. [Google Scholar]
- Alias SA, Jones EBG, Torres J (1999). Intertidal fungi from the Philippines, with a description of Acrocordiopsis sphaerica sp. nov. (Ascomycota). Fungal Diversity 2: 35–41. [Google Scholar]
- Alias SA, Moss ST, Jones EBG (2001). Cucullosporella mangrovei, ultrastructure of ascospores and their appendages. Mycoscience 42: 405–411. [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990). Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Alva P, Mckenzire EHC, Pointing SP, Pena-Murala R, Hyde KD (2002). Do seagrasses habour endophytes? In: Fungi in Marine Environments (Hyde KD, ed.). Fungal Diversity Research Series 7: 167–178. [Google Scholar]
- Aptroot A (1998). A world revision of Massarina (Ascomycota). Nova Hedwigia 66: 89–162. [Google Scholar]
- Au DWT, Jones EBG Vrijmoed LLP (1999). The ultrastructure of Capronia ciliomaris, an intertidal marine fungi from San Juan Island. Mycologia 91: 326–333. [Google Scholar]
- Au DWT, Vrijmoed LLP, Jones EBG (2001). Ultrastructure of asci and ascospores of Massarina velataspora from intertidal mangrove wood. Botanica Marina 44: 261–266. [Google Scholar]
- Barr ME (1993). Notes on the Pleomassariaceae. Mycotaxon 49: 129–142. [Google Scholar]
- Barr ME (1994). Notes on the Amphisphaeriaceae and related families. Mycotaxon 51: 191–224. [Google Scholar]
- Barr ME (1996). Planistromellaceae, a new family in the Dothideales. Mycotaxon 60: 433–442. [Google Scholar]
- Barr ME (2002). Teichosporaceae, another family in the Pleosporales. Mycotaxon 72: 373–389. [Google Scholar]
- Belliveau MJ-R, Bärlocher F (2005). Molecular evidence confirms multiple origins of aquatic hyphomycetes. Mycological Research 109: 1407–1417. [DOI] [PubMed] [Google Scholar]
- Boehm EWA, Mugambi GK, Huhndorf SM, Marubcowits SL, Schoch CL (2009a). A phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with key to world species: Studies in Mycology 64: 49–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm EWA, Schoch CL, Spatafora JW (2009b). On the evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycological Research 113 461–479. [DOI] [PubMed] [Google Scholar]
- Borse, BD (1987). New species Aigialus from India. Transaction of the British Mycological Society 88: 424–426. [Google Scholar]
- Borse BD, Hyde KD (1989). Marine fungi from India III. Acrocordiopsis patillii gen. et sp. nov. from mangrove wood. Mycotaxon 34: 535–540. [Google Scholar]
- Burgaud G, Le Calvez T, Arzur D, Vandenkoornhuyse P, Barbier G. (2009). Diversity of culturable marine filamentous fungi from deep-sea hydrothermal vents. Environmental Microbiology 11 1588–1600. [DOI] [PubMed] [Google Scholar]
- Bunyard BA, Nicholson MS, Royse DJ (1994). A systematic assessment of Morchella using RFLP analysis of the 28S ribosomal RNA gene. Mycologia 86: 762–772. [Google Scholar]
- Cai L, Hyde KD, Tsui CKM (2006). Genera of freshwater fungi. Fungal Diversity Research Series 18: 1–261. [Google Scholar]
- Cámara MPS, Palm ME, Berkum P van, O'Neill NR (2002). Molecular phylogeny of Leptosphaeria and Phaeosphaeria. Mycologia 94: 630–640. [DOI] [PubMed] [Google Scholar]
- Campbell J, Ferrer A, Raja HA, Sivichai S, Shearer CA (2007). Phylogenetic relationships among taxa in the Jahnulales inferred from 18S and 28S nuclear ribosomal DNA sequences. Canadian Journal of Botany 85: 873–888. [Google Scholar]
- Campbell J, Shearer CA, Marvanová L (2006). Evolutionary relationships among aquatic anamorphs and teleomorphs: Lemonniera, Margaritispora, Goniopila. Mycological Research 110: 1025–1033. [DOI] [PubMed] [Google Scholar]
- Cannon PF, Kirk PM (2007). Fungal Families of the World. CABI, Egham, U.K.
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R (2006). TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics 7: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian RR, Bryant WL Jr, Brinson MM (1990). Juncus roemerianus production and decomposition along gradients of salinity and hydroperiod. Marine Ecology Progress Series 68: 137–145. [Google Scholar]
- Cuomo V, Vanzanella F, Fresi E, Cinelli F, Mazzella L (1985). Fungal flora of Posidonia oceanica and its ecological significance. Transactions of the British Mycological Society 84: 35–40. [Google Scholar]
- Cuomo V, Vanzanella F, Fresi E, Mazzella L, Scipione MB (1982). Micoflora delle fenerogame dell `Isola d'Ischia: Posidonia oceanica (L.) Delile Cymodocea nodosa (Ucria) Aschers. Bulletin Musea Institute Biologia, Universiti Genova 50: 162–166. [Google Scholar]
- Devarajan PT, Suryanarayanan TS, Geetha V (2002). Endophytic fungi associated with the tropical seagrass Halophila ovalis (Hydrocharitaceae). Indian Journal of Marine Sciences 31: 73–74. [Google Scholar]
- Edgar RC (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson OE (1981). The families of bitunicate ascomycetes. Opera Botanica 60: 1–220. [Google Scholar]
- Eriksson OE (2006). Outline of Ascomycota 2006. Myconet 12: 1–82. Available at www.fieldmuseum.org/myconet/printed_v12_a.asp
- Eriksson OE, Hawksworth DL (1991). Notes on ascomycete systematics. Systema Ascomycetum 10: 135–150. [Google Scholar]
- Farris JS (1989). The retention index and the rescaled consistency index. Cladistics 5: 417–419. [DOI] [PubMed] [Google Scholar]
- Felsentstein J (1985). Confidence intervals on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Ferrer A, Sivichai S, Shearer CA (2007). Megalohypha, a new genus in the Jahnulales from aquatic habitats in the tropics. Mycologia 99: 456–460. [DOI] [PubMed] [Google Scholar]
- Gruyter J de, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009). Molecular phylogeny of Phoma and allied anamorph genera: Towards a reclassification of the Phoma complex. Mycological Research 113: 508–519. [DOI] [PubMed] [Google Scholar]
- Hall T (2004). BioEdit v. 7.0.1. Department of Microbiology, North Carolina State University. Available at www.mbio.ncsu.edu/BioEdit/bioedit.html.
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, et al. (2007). A higher-level phylogenetic classification of the Fungi. Mycological Research 84: 509–547. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist FR (2001). MrBayes: Bayesian inference of 380 phylogenetic trees. Biometrics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Huhndorf SM (1992). Neotropical Ascomycetes 2. Hypsostroma, a new genus from the Dominican Republic and Venezuela. Mycologia 84: 750–758. [Google Scholar]
- Huhndorf SM (1994). Neotropical Ascomycetes 5. Hypsostromataceae, a new family of Loculoascomycetes and Manglicola samuelsii, a new species from Guyana. Mycologia 86: 266–269. [Google Scholar]
- Hyde KD (1988). Studies on the tropical marine fungi of Brunei. Botanical Journal of the Linnean Society 98: 135–151. [Google Scholar]
- Hyde KD (1991). Helicascus kanaloanus, Heliascus nypae sp. nov. and Salsuginea ramicola gen. et sp. nov. from intertidal mangrove wood. Botanica Marina 4: 311–318. [Google Scholar]
- Hyde KD (1992a). Fungi from decaying intertidal fronds of Nypa fruticans, including three new genera and four new species. Botanical Journal of the Linnean Society 110: 95–110. [Google Scholar]
- Hyde KD (1992b). Julella avivenniae (Borse) comb. nov. (Thelenellaceae) from intertidal mangrove wood and miscellaneous fungi from the North East Coast of Queensland. Mycological Research 95: 939–942. [Google Scholar]
- Hyde KD (1992c). Intertidal mangrove fungi from the West Coast of Mexico, including one new genus and two new species. Mycological Research 96: 25-30. [Google Scholar]
- Hyde KD (1992d). The genus Saccardoella from intertidal mangrove wood. Mycologia 84: 803–810. [Google Scholar]
- Hyde KD (1993). Tropical Australian freshwater fungi V. Bombardia sp., Jahnula australiensis sp. nov., Savoryella aquatica sp. nov. and S. lignicola sp. nov. Australian Systematic Botany 6: 161–167. [Google Scholar]
- Hyde KD, Alias SA (1999). Linocarpon angustatum sp. nov., and Neolinocarpon nypicola sp. nov. from petioles of Nypa fruticans, and a list of fungi from aerial parts of this host. Mycoscience 40: 145–149. [Google Scholar]
- Hyde KD, Alias SA (2000). Biodiversity and distribution of fungi associated with decomposing Nypa fruticans. Biodiversity and Conservation 9: 393–402. [Google Scholar]
- Hyde KD, Aptroot A (1998). Tropical freshwater species of the genera Massarina and Lophiostoma (ascomycetes). Nova Hedwigia 66: 489–502. [Google Scholar]
- Hyde KD, Borse BD (1986a) Marine fungi from Seychelles. V. Biatriospora marina gen. et sp. nov. from mangrove wood. Mycotaxon 26: 263–270. [Google Scholar]
- Hyde KD, Borse BD (1986b). Marine fungi from Seychelles. VI. Massarina velataspora a new marine ascomycotina from mangrove wood. Mycotaxon 27: 161–167. [Google Scholar]
- Hyde KD, Chalermpongse A, Boonthavikoon T (1990). Ecology of intertidal fungi at Ranong mangrove, Thailand. Transactions of the Mycological Society of Japan 31: 17–27. [Google Scholar]
- Hyde KD, Chalermpongse A, Boonthavikoon T (1993). The distribution of intertidal fungi on Rhizophora apiculata. In: The Marine Biology of the South China Sea, Proceedings of the First International Conference on the Marine Biology of Hong Kong and South China Sea (Morton B, ed.). University of Hong Kong Press, Hong Kong: 643–652.
- Hyde KD, Jones EBG (1989). Intertidal mangrove fungi from Brunei. Lautospora gigantea gen. et sp. nov., a new Loculoascomycete from prop roots of Rhizophora sp. Botanica Marina 32: 479–482. [Google Scholar]
- Hyde KD, Jones EBG, Moss S (1986). Mycelial adhesion to surfaces. In: The Biology of Marine Fungi (ST Moss, ed.). Cambridge Univ. Press, Cambridge, U.K.: 331–340.
- Hyde KD, Mouzouras R (1988). Passeriniella savoryellopsis sp. nov., a new ascomycete from intertidal mangrove wood. Transactions of the British Mycological Society 91: 179–185. [Google Scholar]
- Hyde KD, Sarma VV, Jones EBG (2002a). Morphology and taxonomy of higher marine fungi. In: Marine Mycology, a Practical Approach (Hyde KD, Pointing SP, eds) Fungal Diversity Research Series 1: 172–204. [Google Scholar]
- Hyde KD, Wong WSW (1999). Tropical Australian freshwater fungi XV. The ascomycete genus Jahnula, with five new species and one new combination. Nova Hedwigia 68: 489–509. [Google Scholar]
- Hyde KD, Wong WSW, Aptroot A (2002b). Marine and estuarine species of Lophiostoma and Massarina. In: Fungi in Marine Environments (Hyde KD, ed.). Fungal Diversity Research Series 7: 93–109. [Google Scholar]
- Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Berbee ML (2002). Decorospora, a new genus for the marine ascomycetes Pleospora gaudefroyi. Mycologia 91: 651–659. [DOI] [PubMed] [Google Scholar]
- Inderbitzin P, Landvik S, Abdel-Wahab A, Berbee ML (2001). Aliquandostipitaceae, a new family for two new tropical ascomycetes with unusually wide hyphae and dimorphic ascomata. American Journal of Botany 88: 52–61. [PubMed] [Google Scholar]
- Jones EBG (1994). Fungal adhesion. Mycological Research 98: 961–981. [Google Scholar]
- Jones EBG (1995). Ultrastructure and taxonomy of the aquatic ascomycetous order Halosphaeriales. Canadian Journal of Botany 73: S790–S801. [Google Scholar]
- Jones EBG (2006). Form and function of fungal spore appendages. Mycoscience 47: 167–183. [Google Scholar]
- Jones EBG (2007). Marine and mangrove fungi. In: Malaysian Fungal Diversity (Jones EBG, Hyde KD, Vikineswary S, eds). Mushroom Research Center, University Malaya, and Ministry of Natural Resources and Environment, Malaysia: 185–200.
- Jones EBG (2008). Marine compounds in marine organisms. Botanica Marina 51: 161–162. [Google Scholar]
- Jones EBG, Abdel-Wahab MA (2005). Marine fungi from the Bahamas Islands. Botanica Marina 48: 356–373. [Google Scholar]
- Jones EBG, Klaysuban A, Pang K-L (2008). Ribosomal DNA phylogeny of marine anamorphic fungi: Cumulospora varia, Dendryphiella species and Orbimyces spectabilis. The Raffles Bulletin of Zoology suppl. 19: 11–18. [Google Scholar]
- Jones EBG, Hyde KD, Read SJ, Moss ST, Alias SA (1996). Tirisporella gen. nov., an ascomycete from the mangrove palm Nypa fruticans. Canadian Journal of Botany 74: 1487–1495. [Google Scholar]
- Jones EBG, Pilantanapak A, Chatmala I, Sakayaroj J, Phongpaichit S, Choeyklin R (2006). Thai marine fungal diversity. Songklanakarin Journal of Science and Technology 28: 687–708. [Google Scholar]
- Jones EBG, Sakayaroj J, Suetrong S, Somrithipol S, Pang K-L (2009). Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Diversity 35: 1–203. [Google Scholar]
- Khashnobish A, Shearer CA (1996). Phylogenetic relationships in some Leptosphaeria and Phaeosphaeria species. Mycological Research 100: 1355–1363. [Google Scholar]
- Katoh K, Toh H (2008). Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinformatics 9: 286–298. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, David JC, Stalpers JA (2001). Ainsworth and Bisby's Dictionary of the Fungi, 8th edn. CABI Publishing, London, U.K.
- Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008). Ainsworth and Bisby's Dictionary of the Fungi, 10th edn. CABI International, Wallingford, U.K.
- Kluge AG, Farris JS (1969). Quantitative physics and the evolution of anurans. Systematic Zoology 18: 1–32. [Google Scholar]
- Kodsueb R, Dhanasekaran V, Aptroot A, Lumyong S, McKenzie EHC, Hyde KD, Jeewon R (2006). The family Pleosporaceae: intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 98: 571–583. [DOI] [PubMed] [Google Scholar]
- Kohlmeyer J (1969). Marine fungi of Hawaii including the new genus Helicascus. Canadian Journal of Botany 47: 1469–1487. [Google Scholar]
- Kohlmeyer J (1985). Caryosporella rhizophorae gen. et sp. nov. (Massariaceae), a marine Ascomycete from Rhizophora mangle. Proceedings of the Indian Academy of Science (Plant Science) 94: 355–361. [Google Scholar]
- Kohlmeyer J (1986). Ascocratera manglicola gen. et sp. nov. and key to the marine Loculoascomycetes on mangroves. Canadian Journal of Botany 64: 3036–3042. [Google Scholar]
- Kohlmeyer J, Kohlmeyer E (1965). New marine fungi from mangroves and trees along eroding shorelines. Nova Hedwigia 9: 89–104. [Google Scholar]
- Kohlmeyer J, Kohlmeyer E (1971). Marine fungi from tropical America and Africa. Mycologia 63: 831–861. [PubMed] [Google Scholar]
- Kohlmeyer J, Kohlmeyer E (1979). Marine Mycology. The Higher Fungi. Academic Press, New York, U.S.A.
- Kohlmeyer J, Vittal BPR (1986) Lophiostoma mangrovis, a new marine ascomycete from the tropics. Mycologia 78: 485–489. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (1987). Marine fungi from Aldabra, the Galapagos, and other tropical islands. Canadian Journal of Botany 65: 571–582. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (1990). Revision of marine species of Didymosphaeria (Ascomycotina). Mycological Research 94: 685–690. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (1991). Illustrated key to the filamentous marine fungi. Botanica Marina 34: 1–61. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (1998). A new marine Xylomyces on Rhizophora from the Caribbeaen and Hawaii. Fungal Diversity 1: 159–164. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (2001). The biodiversity of fungi on Juncus roemerianus. Mycological Research 105: 1411–1412. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (2002). Fungi on Juncus and Spartina: New marine species of Anthostomella, with a list of marine fungi known from Spartina. Mycological Research 106: 365–374. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B (2003). Marine ascomycetes from algae and animal hosts. Botanica Marina 46: 285–306. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1995a). Fungi on Juncus roemerianus. 2. New dictyosporous ascomycetes. Botanica Marina 38: 165–174. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1995b). Fungi on Juncus roemerianus. 4. New marine ascomycetes. Mycologia 87: 532–542. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1995c). Fungi on Juncus roemerianus. New marine and terrestrial ascomycetes. Mycological Research 100: 393–401. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1996). Fungi on Juncus roemerianus. 8. New bitunicate ascomycetes. Canadian Journal of Botany 74: 1830–1840. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1997a). Fungi on Juncus roemerianus. 9. New obligate and facultative marine ascomycotina. Botanica Marina 40: 291–300. [Google Scholar]
- Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1997b). Fungi on Juncus roemerianus. 12. Two new species of Mycosphaerella and Paraphaeosphaeria (Ascomycotina). Botanica Marina 42: 505–511. [Google Scholar]
- Kruys A, Eriksson OE, Wedin M (2006). Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycological Research 110: 527–536. [DOI] [PubMed] [Google Scholar]
- Larget B, Simon D (1999). Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution 16: 750–759. [Google Scholar]
- Le Calvez T, Burgaud G, Mahe S, Barbier G, Vandenkoornhuyse P (2009). Fungal diversity in deep-sea hydrothermal ecosystems. Applied and Environmental Microbiology 75: 6415–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchtmann A (1984). Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia 37: 75–194. [Google Scholar]
- Liew ECY, Aptroot A, Hyde KD (2002). An evaluation of the monophyly of Massarina based on ribosomal DNA sequences. Mycologia 94: 803–813. [PubMed] [Google Scholar]
- Lindemuth R, Wirtz N, Lumbsch HT (2001). Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that loculoascomycetes (Ascomycota) are not monophyletic. Mycological Research 105: 1176–1181. [Google Scholar]
- Liu YJ, Whelen S, Hall BD (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Huhndorf S (2007). Outline of Ascomycota. Myconet 13: 1–99. [Google Scholar]
- Lumbsch HT, Lindemuth R (2001). Major lineages of Dothideomycetes (Ascomycota) inferred from SSU and LSU rDNA sequences. Mycological Research 105: 901–908. [Google Scholar]
- Mantle PG, Hawksworth DL, Pazoutova S, Collinson LM, Rassing BR (2006). Amorosia littoralis gen. sp. nov., a new genus and species name for the scorpinone and cafferine-producing hyphomycete from the littoral zone in the Bahamas. Mycological Research 110: 1371–1378. [DOI] [PubMed] [Google Scholar]
- Maria GL, Sridhar KR (2002). New ascomycete, Passeriniella mangrovei sp. nov. from the mangrove forest of India. Indian Journal of Forestry 25: 319–322. [Google Scholar]
- Marvanová L (1997). Freshwater hyphomycetes: a survey with remarks on tropical taxa. In: Tropical Mycology (Janardhanan KK, Rahendran C, Natarajan K, Hawksworth DL, eds) Science Publ. U.S.A.: 169–226.
- Mathiassen G (1989). Some corticolous and lignicolous Pyrenomycetes s. lat. (Ascomycetes) on Salix in Troms, N. Norway. Sommerfeltia 9: 1–100. [Google Scholar]
- Mugambi GK, Huhndorf SM (2009). Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae re-circumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Studies in Mycology 64: 103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell SY, Fell JW (1980). Mycoflora of turtlegrass (Thalassia testudinum Konig) as recorded after seawater incubation. Botanica Marina 23: 265–275. [Google Scholar]
- Nylander JAA (2004). MrModeltest 2.2: Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden.
- O'Donnell K, Cigelnik E, Weber NS, Trappe JM (1997). Phylogenetic relationships among ascomycetous truffles and the true and false morels inferred from 18S and 28S ribosomal DNA sequence analysis. Mycologia 89: 48–65. [Google Scholar]
- Page RDM (1996). Treeview: An application to display phylogenetic trees on personal computers. Bioinformatics 12: 357–358. [DOI] [PubMed] [Google Scholar]
- Pan J-Y, Jones EBG, She Z-Y, Ling Y-C (2008). Review of bioactive compounds from fungi in the South China Sea. Botanica Marina 51: 179–190. [Google Scholar]
- Pang KL, Abdel-Wahab MA, Sivichai S, El-Sharouney HM, Jones EBG (2002). Jahnulales (Dothideomycetes, Ascomycota): a new order of lignicolous freshwater ascomycetes. Mycological Research 106: 1031–1042. [Google Scholar]
- Pang KL, Vrijmoed LLP, Goh TK, Plaingam N, Jones EBG (2008). Fungal endophytes associated with Kandelia candel (Rhizophoraceae) in Mai Po Nature Reserve, Hong Kong. Botanica Marina 51: 171–178. [Google Scholar]
- Patil SD, Borse BD (1985). Marine fungi from Maharashtra (India) IV: Some loculoascomycetes. Transactions of the Mycological Society of Japan 26: 271–276. [Google Scholar]
- Pinruan U, Jones EBG, Hyde KD (2002). Aquatic fungi from peat swamp palms: Jahnula appendiculata sp. Sydowia 54: 242–247. [Google Scholar]
- Poon MOK, Hyde KD (1998). Biodiversity of intertidal estuarine fungi on Phragmites at Mai Po marshes, Hong Kong. Botanica Marina 41: 141–155. [Google Scholar]
- Prihatini R, Boonyuen N, Sivichai S (2008). Phylogenetic evidence that two submerged-habitat fungal species, Speiropsis pedatospora and Xylomyces chlamydosporus, belong in the order Jahnulales incerate sedis Dothideomycetes. Microbiology Indonesia 2: 136–140. [Google Scholar]
- Raghukumar C (2008). Marine fungal biotechnology: an ecological perspective. Fungal Diversity 31: 19–35. [Google Scholar]
- Raja HA, Ferrer A, Shearer CA (2005). Aliquandostipite crystallinus, a new ascomycete species from submerged wood in freshwater habitats. Mycotaxon 91: 207–215. [Google Scholar]
- Raja HA, Shearer CA (2006). Jahnula species from North and Central America, including three new species. Mycologia 98: 319–332. [DOI] [PubMed] [Google Scholar]
- Read SJ, Jones EBG, Moss ST (1997a). Ultrastructural observations of asci, ascospores and appendages of Massarina armatispora (Ascomycota). Mycoscience 38: 141–146. [Google Scholar]
- Read SJ, Moss ST, Jones EBG (1994). Ultrastructure of asci and ascospores sheath of Massarina thalassiae (Loculoascomycetes, Ascomycotina). Botanica Marina 37: 547–533. [Google Scholar]
- Read SJ, Moss ST Jones EBG (1997b). Ultrastructure of asci, ascospores and appendages of Massarina ramunculicola (Loculascomycetes, Ascomycota). Botanica Marina 40: 465–471. [Google Scholar]
- Rehner S (2001). Primers for Elongation Factor 1-a (EF1-a). http://ocid.NACSE.ORG/research/deephyphae/EF1primer.pdf.
- Rodríguez GM (2008). Potential of fungal endophytes from Thalassia testudinum Bank ex K.D. Koenig as producers of bioactive compounds. MSc. Thesis, University of Puerto Rico, Puerto Rico.
- Sakayaroj J, Preedanon S, Supaphon O, Jones EBG, Phongpaichit S (2010). Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Diversity 41: In press.
- Schatz S (1984). The life history, developmental morphology, and taxonomy of Lautitia danica gen. nov., comb. nov. Canadian Journal of Botany 62: 28–32. [Google Scholar]
- Schmit JP, Shearer CA (2003). A checklist of mangrove-associated fungi, their geographical distribution and known host plants. Mycotaxon 85: 423–478. [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. (2009a). A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Sung G-H, Lopez-Giraldez F, Townsend JP, Miadlikowska J, et al. (2009b). The Ascomycota tree of life: A phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Systematic Biology 58: 224–239. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Sung G-H, Volkmann-Kohlmeyer B, Kohlmeyer J, Spatafora JW (2007). Marine fungal lineages in the Hypocreomycetidae. Mycological Research 111: 154–162. [DOI] [PubMed] [Google Scholar]
- Schulz B, Draeger S, dela Cruz TE, Rheinheimer J, Siems K, et al. (2008). Screening strategies for obtaining novel, biologically active, fungal secondary metabolites from marine habitats. Botanica Marina 51: 219–234. [Google Scholar]
- Shearer CA (1993a). A new species of Kirschsteiniothelia (Pleosporales) with an unusual fissitunicate ascus. Mycologia 85: 963–969. [Google Scholar]
- Shearer CA (1993b). Pseudohalonectria (Lasiosphaeriaceae), an antagonistic genus from wood in freshwater. Canadian Journal of Botany 67: 1944–1955. [Google Scholar]
- Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, et al. (2009). The molecular phylogeny of freshwater Dothideomycetes. Studies in Mycology 64: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy BD, Jeewon R, Hyde KD (2007). Inpact of DNA sequence-data on the taxonomy of anamorphic fungi. Fungal Diversity 26: 1–54. [Google Scholar]
- Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006). Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycological Research 110: 916–928. [DOI] [PubMed] [Google Scholar]
- Simmons E, Schatz S (1989). Memoir of the New York Botanic Gardens 49: 305. [Google Scholar]
- Spatafora JW, Volkmann-Kohlmeyer B, Kohlmeyer J (1998). Independent terrestrial origins of the Halosphaeriales (marine Ascomycota). American Journal of Botany 85: 1569–1580. [PubMed] [Google Scholar]
- Steinke TD, Hyde KD (1997). Gloniella clavatispora, sp. nov. from Avicennia marina in South Africa. Mycoscience 38: 7–9. [Google Scholar]
- Stamatakis A (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Suetrong S, Jones EBG (2006). Marine discomycetes: A review. Indian Journal of Marine Sciences 35: 1–6. [Google Scholar]
- Suetrong S, Sakayaroj J, Phongpaichit S, Jones EBG (2010). Morphological and molecular characteristics of a poorly known marine ascomycete, Manglicola guatemalensis. Mycologia: doi. 10.3852/07-147. [DOI] [PubMed]
- Sutherland GK (1916). Marine Fungi Imperfecti. New Phytolologist 15: 35–48. [Google Scholar]
- Swofford DL (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts, U.S.A.
- Tam WY, Pang KL, Jones EBG (2003). Ordinal placement of selected marine Dothideomycetes inferred from SSU ribosomal DNA sequence analysis. Botanica Marina 4: 487–494. [Google Scholar]
- Tanaka K, Harada Y (2003). Pleosporales in Japan (1): the genus Lophiostoma. Mycoscience 44: 85–96. [Google Scholar]
- Tanaka K, Hosoya T (2008). Lophiostoma sagittiforme sp. nov., a new ascomycete (Pleosporales, Dothideomycetes) from Island Yakushima in Japan. Sydowia 20: 131–145. [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994). Clustal W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson TB (1986). The Botany of Mangroves. Cambridge University Press, Cambridge, U.K: 1–413.
- Tsui CKM, Berbee ML (2006). Phylogenetic relationships and convergence of helicosporous fungi inferred from ribosomal DNA sequences. Molecular Phylogenetics and Evolution 39: 587–597. [DOI] [PubMed] [Google Scholar]
- Tsui KM, Hyde KD, Hodgkiss IJ, Goh TK (1998). A new freshwater species of Saccardoella from Hong Kong and South Africa. Mycologia 90: 701–704. [Google Scholar]
- Tsui KM, Sivichai S, Berbee M (2006). Molecular systematic of Helicoma, Helicomyces and Helicosporium and their teleomorphs inferred from rDNA sequences. Mycologia 98: 94–104. [DOI] [PubMed] [Google Scholar]
- Wang G, Li Q, Zhu P (2008). Phylogenetic diversity of culturable fungi associated with the Hawaiian sponges Suberites zeteki and Gelliodes fibrosa. Antonie van Leeuwenhoek 93: 163–174. [DOI] [PubMed] [Google Scholar]
- Webster J, Descals E (1979). The teleomorphs of waterborne hyphomycetes from freshwater. In: The Whole Fungus (Kendrick WB, ed.). Ottawa, National Museum of Canada and Kananaski Foundation 2: 419–451. [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocol: A guide to methods and applications (Innis MA, Gelfand DH, Sninsky JS, White TJ, eds), Academic Press, San Diego, U.S.A.: 315–322.
- Wiens JJ (2006). Missing data and the design of phylogenetic analyses. Journal of Biomedical Informatics 39 34–42. [DOI] [PubMed] [Google Scholar]
- Wilson WL (1998). Isolation of Endophytes from Seagrasses from Bermuda. MSc. Thesis. The University of New Brunswick, Canada.
- Yusoff M, Moss ST, Jones EBG (1994). Ascospore ultrastructure of Pleospora gaudefroyi Patouillard (Pleosporaceae, Loculoascomycetes, Ascomycotina). Canadian Journal of Botany 72: 1–6. [Google Scholar]
- Zhang Y, Schoch CL, Fournier J, Crous PW, Gruyter J de, et al. (2009). Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary reevaluation. Studies in Mycology 64: 85–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009b). Towards a phylogenetic clarification of Lophiostoma / Massarina and morphologically similar genera in the Pleosporaceae. Fungal Diversity 38: 225–251. [Google Scholar]
- Zuccaro A, Mitchell JI (2005). Fungal communities of seaweeds. In: The Fungal Community (Dighton J, White JF Jr, Oudemans P, eds). 3rd edn. CRC Press, New York, NY, U.S.A.: 533–579
- Zuccaro A, Schoch CL, Spatafora JW, Kohlmeyer J, Draeger S, Mitchell JI (2008). Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Applied Environmental Microbiology 74: 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]