Abstract
This study investigated temporal integration processes underlying cochlear implant (CI) users’ amplitude modulation processing. Thresholds for modulation detection (AMDTs) and modulation frequency discrimination (AMFDTs) were measured for 50-, 100-, and 200-Hz modulation frequencies with stimulus durations from 50 to 400 ms in eight adult CI users. The results showed significant interactions between modulation frequency and stimulus duration for AMDTs and AMFDTs. The data suggest that temporal integration limits CI users’ sensitivity to low temporal pitch over short durations, and that temporal integration over longer durations may not enhance CI users’ sensitivity to high temporal pitch.
Introduction
Due to the limited spectral cues available with cochlear implants (CIs), pitch information is primarily encoded by temporal amplitude modulation (AM) of electric pulse trains. CI users can typically perceive temporal envelope pitch for modulation frequencies up to ∼300 Hz (Zeng, 2002). CI speech performance has been significantly correlated with thresholds for AM detection and AM frequency discrimination, suggesting the importance of temporal AM processing for CI users (Fu, 2002; Chatterjee and Peng, 2008; Luo et al., 2008). Various stimulation parameters have been shown to affect CI users’ AM detection thresholds (AMDTs), such as AM frequency (Shannon, 1992), stimulation level (Fu, 2002), electrode location (Pfingst et al., 2008), and stimulation rate (Galvin and Fu, 2005, 2009; Pfingst et al., 2007). However, AM frequency discrimination thresholds (AMFDTs) may be a more relevant measure of CI users’ temporal pitch sensitivity, as it may better reflect sensitivity to pitch changes in dynamic stimuli such as speech (e.g., intonation, lexical tones, etc.). CI users’ AMFDTs may also be affected by stimulation parameters, such as AM frequency, electrode location (Chatterjee and Peng, 2008), and stimulation level (Luo et al., 2008).
For AM detection or AM frequency discrimination, listeners must optimally integrate information (stored in short-term memory) from multiple “looks” at the input modulations. A multiple-look model (Viemeister and Wakefield, 1991) has been used to explain increasing sensitivity to AM with increasing stimulus duration in normal-hearing (NH) listeners. As the stimulus duration is increased, more modulation periods are available, allowing NH listeners to better perceive AM. Viemeister (1979) measured NH listeners’ AMDTs for AM frequencies ranging from 2 to 4000 Hz, using stimulus durations of 250, 500, and 1500 ms. Results showed that AM detection improved with stimulus duration, and that the improvement was larger for lower AM frequencies (which contained fewer than 16 modulation periods with short durations). The effect of stimulus duration on NH listeners’ AMFDTs was investigated by Lee (1994) using sinusoidal carriers. In one condition, the carrier frequency was randomized across stimuli so that spectral sideband cues were unavailable, forcing listeners to attend to temporal cues. Results showed that AMFDTs decreased for durations up to about five modulation periods, but were largely unaffected by stimulus durations beyond this “critical” duration. Thus, there seems to be an integration period for NH listeners’ temporal modulation processing.
In this study, we measured AMDTs and AMFDTs in CI users for a range of AM frequencies (50–200 Hz) and stimulus durations (50–400 ms). Given these experimental parameters, the number of modulation periods within the standard stimuli ranged from 2.5 to 80. We hypothesized that there is a critical temporal integration window that underlies CI users’ AM processing, and that CI users’ AM sensitivity will improve with increasing stimulus duration.
Methods
Subjects
Table 1 shows demographic details for the eight post-lingually deafened adult CI subjects. All subjects had extensive experience in psychophysical tests from previous studies. Informed consent was obtained from all subjects.
Table 1.
| CI subject demographics. | ||||||
|---|---|---|---|---|---|---|
| Subject | Age | Gender | Etiology | Device | Strategy | Years with prosthesis |
| S1 | 50 | M | Trauma | Nucleus-22 | SPEAK | 15 |
| S2 | 64 | F | Genetic | Nucleus-24 | ACE | 4 |
| S3 | 76 | M | Noise induced | Nucleus-22 | SPEAK | 10 |
| S4 | 61 | F | Congenital | Nucleus-24 | SPEAK | 9 |
| S5 | 66 | M | Trauma | Nucleus-22 | SPEAK | 17 |
| S6 | 57 | M | Genetic | Freedom | ACE | 2 |
| S7 | 74 | F | Unknown | Nucleus-24 | ACE | 7 |
| S8 | 44 | M | Congenital | Nucleus-24 | ACE | 6 |
Stimuli
All stimuli were bi-phasic pulse trains delivered via custom research interface to electrode 10. The stimulation rate was 2000 pps (pps denotes pulses per second); this relatively high stimulation rate was selected to avoid aliasing effects with amplitude modulation. The AM frequency was 50, 100, or 200 Hz. The stimulus duration was 50, 100, 200, or 400 ms. Table 2 shows other relevant stimulation parameters; note that different parameters were used for different subjects to achieve sufficient loudness for all experimental conditions.
Table 2.
| Experimental stimulation parameters. | |||||||
|---|---|---|---|---|---|---|---|
| Subject | Stimulation mode | Phase duration (μs) | Inter-phase gap (μs) | Loudness-balanced levels at 50% of dynamic range (μA) | |||
| 50 ms | 100 ms | 200 ms | 400 ms | ||||
| S1 | BP+1 | 100 | 20 | 718 | 703 | 638 | 615 |
| S2 | MP1+2 | 50 | 8 | 213 | 215 | 182 | 187 |
| S3 | BP+1 | 100 | 20 | 600 | 579 | 463 | 448 |
| S4 | MP1+2 | 50 | 8 | 676 | 613 | 518 | 500 |
| S5 | BP+1 | 100 | 20 | 600 | 579 | 463 | 448 |
| S6 | BP+1 | 100 | 20 | 657 | 396 | 289 | 274 |
| S7 | BP+1 | 100 | 20 | 137 | 134 | 129 | 137 |
| S8 | BP+1 | 100 | 20 | 208 | 169 | 152 | 150 |
Prior to the AMDT and AMFDT experiments, all stimuli were loudness-balanced across duration conditions. First, the electrode dynamic range (DR) was estimated for the 200-ms stimuli (no modulation), using methods similar to clinical fitting (i.e., “counting” thresholds, then slowly increasing the amplitude until achieving comfortable loudness). The 50% DR level (in linear microamperes) for the 200-ms stimuli was used as the reference for loudness-balancing. The 50-, 100-, and 400-ms stimuli (no modulation) were loudness-balanced to this reference using a two-interval, forced-choice adaptive double-staircase procedure (Jesteadt, 1980). The target stimulation levels (in linear microamperes) were averaged across two sequences and were used as the stimulation levels for the different duration conditions (see Table 2).
Procedures
All subjects (except S1) participated in the AMDT experiment. A 3–interval, forced–choice (3IFC), three-down∕one-up adaptive procedure was used to track the modulation depth corresponding to 79.4% correct AM detection (Levitt, 1971). In each trial, two intervals contained un-modulated stimuli, and the other interval (randomly selected) contained sinusoidal (current) AM. The starting phase of the sinusoidal AM was randomly selected between 0 and 2π. Subjects were asked to identify which interval sounded different. The AM depth was adjusted according to subject response. The adaptive procedure terminated after 12 reversals or after 60 trials, and the AM depths (in percent) for the final 8 reversals were averaged to obtain the AMDT for each run; two to three runs were conducted for each condition.
AMFDTs were measured for a fixed 30% AM depth. This modulation depth was greater than most of the measured AMDTs. A 3IFC, three-down∕one-up procedure was used to track the AM frequency corresponding to 79.4% correct AM frequency discrimination (Levitt, 1971). In each trial, two intervals contained the standard AM frequency, and the other interval (randomly selected) contained the target AM frequency that was always higher than the standard. Subjects were asked to identify which interval was different. The target AM frequency was adjusted according to subject response. The adaptive procedure terminated after 12 reversals or 60 trials, and the target AM frequencies for the final 8 reversals were averaged to obtain the AMFDT for each run; two to three runs were conducted for each condition. AMFDTs were expressed as Weber fractions (Δfmod∕fmod). The test order of conditions was randomized within and across subjects. No preview, training, or feedback was provided during testing.
Results
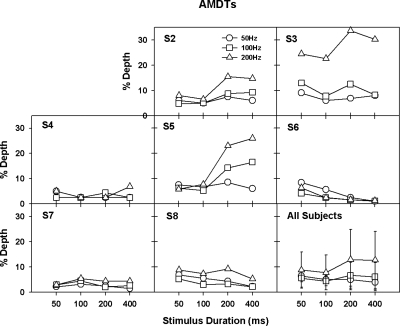
Figure 1 shows AMDTs for individual subjects. There was a large inter-subject variability in AMDTs. A two-way repeated measures analysis of variance (RM ANOVA) showed that AMDTs were significantly affected by AM frequency [F(2,12)=4.16, p=0.04], but not by stimulus duration [F(3,18)=1.14, p=0.36]; there was a significant interaction between AM frequency and stimulus duration [F(6,36)=4.35, p=0.002]. According to post-hoc Bonferroni t-tests, 50- and 100-Hz AMDTs were not significantly affected by stimulus duration, but 200-Hz AMDTs were significantly higher for the 200- and 400-ms durations than for the 100-ms duration (p<0.04), driven mostly by the performance of subjects S2, S3, and S5. Performance for these same three subjects likely caused 200-Hz AMDTs to be significantly higher than 50- and 100-Hz AMDTs with the 200- and 400-ms durations (p<0.05).
Figure 1.
AMDTs (in percent) as a function of stimulus duration. The results from individual subjects are shown in different panels. The right-bottom panel shows group mean data. The open circles, open squares, and open triangles represent the data for 50-, 100-, and 200-Hz AM frequencies, respectively.
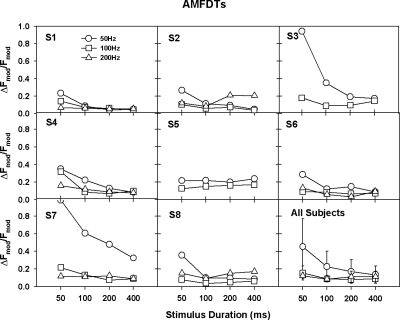
Figure 2 shows AMFDTs for individual subjects. Again, there was large inter-subject variability in AMFDTs. For most subjects, 50-Hz AMFDTs decreased by half or more as the stimulus duration was increased from 50 to 100 ms, but remained constant thereafter, in agreement with the putative critical duration of five modulation periods. For subjects S3, S4, and S7, 50-Hz AMFDTs continued to decrease with durations up to 400 ms, and for subject S5, 50-Hz AMFDTs were similar across all durations. 100-Hz AMFDTs were lower than 50-Hz AMFDTs and were largely unchanged across all durations (except for subjects S1, S4, and S7). 200-Hz AMFDTs were mostly similar to 100-Hz AMFDTs and were constant across all durations. Note that subjects S3 and S5 were unable to discriminate changes in AM frequency above 200 Hz, most likely due to the high AMDTs (see Fig. 1); due to the missing data, subjects S3 and S5 were excluded from the statistical analyses.
Figure 2.
AMFDTs (in Δfmod∕fmod) as a function of stimulus duration. The results from individual subjects are shown in different panels. The right-bottom panel shows group mean data (including those from subjects S3 and S5). The open circles, open squares, and open triangles represent the data for 50-, 100-, and 200-Hz AM frequencies, respectively.
A two-way RM ANOVA showed that AMFDTs were significantly affected by stimulus duration [F(3,15)=16.10, p<0.001], but not by AM frequency [F(2,10)=2.83, p=0.11]; there was a significant interaction between stimulus duration and AM frequency [F(6,30)=9.24, p<0.001]. Post-hoc Bonferroni t-tests showed that 50-Hz AMFDTs were significantly higher for the 50-ms duration than for longer durations, and for the 100-ms duration than for the 400-ms duration (p<0.03). 100-Hz AMFDTs were significantly higher for the 50-ms duration than for the 200- and 400-ms durations (p<0.05). 200-Hz AMFDTs were not significantly different across all durations. Post-hoc analyses also showed that AMFDTs were significantly higher for 50 Hz than for 100 and 200 Hz with the 50-ms duration (p<0.005).
Discussion
The present AMDTs for the 200- and 400-ms durations are comparable to those from previous studies with similar stimulus durations (e.g., Shannon, 1992; Fu, 2002; Chatterjee and Peng, 2008; Luo et al., 2008). Subjects S2, S3, and S5’s AMDTs were higher for 200-Hz AM than for 50- and 100-Hz AMs with the 200- and 400-ms durations, reflecting the low-pass filter characteristics of CI users’ temporal modulation transfer function (TMTF) (Shannon, 1992). However, AMDTs were largely unaffected by AM frequency for the remaining subjects with the 200- and 400-ms durations. AMDTs for all subjects (except for S3) were unaffected by AM frequency with the 50- and 100-ms durations. The results suggest that the cut-off frequency of the TMTF may be greater than 200 Hz for some CI users, and that the TMTF may be influenced by stimulus duration.
While NH listeners’ AMDTs improve with increasing duration (Viemeister, 1979), the present CI subjects’ 50- and 100-Hz AMDTs were largely unchanged across duration conditions. In contrast to NH results, CI users’ mean 200-Hz AMDTs worsened with increasing duration (largely driven by the poorer performance of subjects S2, S3, and S5). According to the multiple-look model (Viemeister and Wakefield, 1991), if the information from each modulation period is mutually independent and optimally combined, the accumulative perceptual sensitivity should have improved with the greater number of modulation periods contained in the longer duration. The present pattern of results may be attributed to potential loudness cues used for AM detection. McKay and Henshall (2009) showed that AM stimuli were perceived louder than un-modulated stimuli with the same average current level. According to their AM loudness model, such loudness cues for AM detection would be similar across the present duration conditions because relative loudness is determined by a short temporal integration window (∼7 ms). In the present study, loudness-balancing with un-modulated stimuli resulted in lower current levels for longer durations (see Table 2). However, according to McKay and Henshall’s (2009) AM loudness model and their experimental data, the lower “absolute” current levels (while providing equal loudness for longer durations) may have reduced loudness difference between modulated and un-modulated stimuli, resulting in higher AMDTs. The reduced loudness cues for AM detection may have offset the benefits of longer duration (i.e., more looks at modulation), especially for subjects S2, S3, and S5 with 200-Hz AM detection.
The present AMFDTs for the 200-ms duration were similar to those measured by Chatterjee and Peng (2008). Performance patterns across different AM frequencies were also similar in both studies. With the 200-ms duration, 200-Hz AMFDTs were similar to 100-Hz AMFDTs; 50-Hz AMFDTs were slightly (but not significantly) higher than 100- and 200-Hz AMFDTs, possibly because the number of modulation periods was smaller for the 50-Hz AM. Also, the difference between 50-, 100-, and 200-Hz AMFDTs increased with shorter durations (i.e., 50 ms).
In line with the multiple-look model and with previous NH data (Lee, 1994), CI subjects’ 50- and 100-Hz AMFDTs decreased with longer durations, showing the benefit of temporal integration when more modulation periods were available. These duration effects are consistent with pulse train rate discrimination performance in CI users (Tong et al., 1982) as well as in NH subjects (Plack and Carlyon, 1995). Similar to NH data, the critical duration for 50-Hz AMFDTs was 100 ms (or five modulation periods) for subjects S1, S6, and S8; 50-Hz AMFDTs continued to decrease with longer durations for the remaining subjects. For 100- and 200-Hz AMFDTs, 50-ms duration (or five to ten modulation periods) was adequate for most CI subjects. The similar critical durations for AMFDTs between CI users and NH listeners suggest that similar temporal integration mechanisms and windows may be used for their AM processing, despite differences in auditory neuron inputs. Unlike AMDTs, AMFDTs are less likely to be affected by loudness cues, as McKay and Henshall’s (2009) AM loudness model predicts little effects of AM frequency on overall loudness of AM stimuli. Chatterjee and Peng (2008) also found that AMFDTs were not significantly affected by 1-dB amplitude roving.
Summary
CI users’ AM detection and AM frequency discrimination were measured as a function of stimulus duration. Significant interactions were found between AM frequency and stimulus duration. Lower-frequency AMFDTs (50 and 100 Hz) improved with stimulus durations up to 100 ms, suggesting that a critical duration (five to ten modulation periods) may be needed by CI users to process changes in AM frequency. Higher-frequency AMFDTs (200 Hz) were fairly constant across duration conditions (50–400 ms). Lower-frequency AMDTs (50 and 100 Hz) were not significantly affected by stimulus duration, while higher-frequency AMDTs (200 Hz) worsened with longer durations (200–400 ms), possibly due to reduced loudness cues used for AM detection. The results suggest that temporal integration limits CI users’ sensitivity to low temporal pitch (50 Hz) over short durations (50–100 ms). In optimal listening conditions (e.g., clear speech with slow speaking rate), temporal integration over longer durations (400 ms) may not enhance CI users’ sensitivity to high temporal pitch (200 Hz).
Acknowledgments
We are grateful to all subjects for their participation in the experiments. Research was supported in part by NIH (Grant Nos. R03-DC-008192 and R01-DC-004993).
References and links
- Chatterjee, M., and Peng, S. -C. (2008). “Processing F0 with cochlear implants: Modulation frequency discrimination and speech intonation recognition,” Hear. Res. 235, 143–156. 10.1016/j.heares.2007.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Q. -J. (2002). “Temporal processing and speech recognition in cochlear implant users,” NeuroReport 13, 1635–1639. 10.1097/00001756-200209160-00013 [DOI] [PubMed] [Google Scholar]
- Galvin, J. J., and Fu, Q. -J. (2005). “Effects of stimulation rate, mode and level on modulation detection by cochlear implant users,” J. Assoc. Res. Otolaryngol. 6, 269–279. 10.1007/s10162-005-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin, J. J., and Fu, Q. -J. (2009). “Influence of stimulation rate and loudness growth on modulation detection and intensity discrimination in cochlear implant users,” Hear. Res. 250, 46–54. 10.1016/j.heares.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesteadt, W. (1980). “An adaptive procedure for subjective judgements,” Percept. Psychophys. 28, 85–88. [DOI] [PubMed] [Google Scholar]
- Lee, J. (1994). “Amplitude modulation rate discrimination with sinusoidal carriers,” J. Acoust. Soc. Am. 96, 2140–2147. 10.1121/1.410156 [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49, 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Luo, X., Fu, Q. -J., Wei, C. -G., and Cao, K. -L. (2008). “Speech recognition and temporal amplitude modulation processing by Mandarin-speaking cochlear implant users,” Ear Hear. 29, 957–970. 10.1097/AUD.0b013e3181888f61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay, C. M., and Henshall, K. R. (2009). “Amplitude modulation and loudness in cochlear implants,” J. Assoc. Res. Otolaryngol. In press. 10.1007/s10162-009-0188-5 [DOI] [PMC free article] [PubMed]
- Pfingst, B. E., Burkholder-Juhasz, R. A., Xu, L., and Thompson, C. S. (2008). “Across-site patterns of modulation detection in listeners with cochlear implants,” J. Acoust. Soc. Am. 123, 1054–1062. 10.1121/1.2828051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst, B. E., Xu, L., and Thompson, C. S. (2007). “Effects of carrier pulse rate and stimulation site on modulation detection by subjects with cochlear implants,” J. Acoust. Soc. Am. 121, 2236–2246. 10.1121/1.2537501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack, C. J., and Carlyon, R. P. (1995). “Differences in frequency modulation detection and fundamental frequency discrimination between complex tones consisting of resolved and unresolved harmonics,” J. Acoust. Soc. Am. 98, 1355–1364. 10.1121/1.413471 [DOI] [Google Scholar]
- Shannon, R. V. (1992). “Temporal modulation transfer functions in patients with cochlear implants,” J. Acoust. Soc. Am. 91, 2156–2164. 10.1121/1.403807 [DOI] [PubMed] [Google Scholar]
- Tong, Y. C., Clark, G. M., Blamey, P. J., Busby, P. A., and Dowell, R. C. (1982). “Psychophysical studies for two multiple-channel cochlear implant patients,” J. Acoust. Soc. Am. 71, 153–160. 10.1121/1.387342 [DOI] [PubMed] [Google Scholar]
- Viemeister, N. F. (1979). “Temporal modulation transfer functions based upon modulation thresholds,” J. Acoust. Soc. Am. 66, 1364–1380. 10.1121/1.383531 [DOI] [PubMed] [Google Scholar]
- Viemeister, N. F., and Wakefield, G. H. (1991). “Temporal integration and multiple looks,” J. Acoust. Soc. Am. 90, 858–865. 10.1121/1.401953 [DOI] [PubMed] [Google Scholar]
- Zeng, F. -G. (2002). “Temporal pitch in electric hearing,” Hear. Res. 174, 101–106. 10.1016/S0378-5955(02)00644-5 [DOI] [PubMed] [Google Scholar]