Abstract
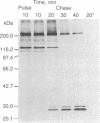
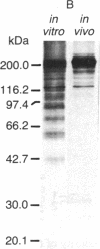
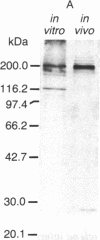
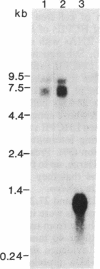
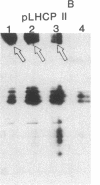
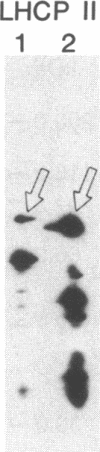
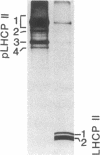
Antibody to the Euglena light-harvesting chlorophyll a/b binding protein of photosystem II (LHCPII) immunoprecipitated 207-, 161-, 122-, and 110-kDa proteins from total Euglena proteins pulse-labeled for 10 min with [35S]sulfate. The 25.6- and 27.2-kDa LHCPII were barely detectable in the immunoprecipitate. During a 40-min chase with unlabeled sulfate, the amount of radioactivity in the high molecular mass proteins decreased, and the amount of radioactivity in the 25.6- and 27.2-kDa LHCPII increased with kinetics consistent with a precursor-product relationship. The half-life of the high molecular mass proteins was ≈20 min. The major proteins immunoprecipitated from a nuclease-treated rabbit reticulocyte cell-free translation system programmed with Euglena whole cell or poly(A)+ RNA had molecular masses corresponding to the molecular masses of the proteins immunoprecipitated from the pulse-labeled in vivo translation products. RNAs of 6.6 and 8.3 kilobases were the only Euglena whole cell and poly(A)+ RNAs that hybridized to a 0.7-kilobase EcoRI-BamHI fragment of plasmid pAB165, which contains a portion of the coding sequence for Arabidopsis LHCPII. RNAs of this size are more than sufficient to code for proteins of 207 kDa. Taken together, these findings demonstrate that the LHCPIIs of Euglena are initially synthesized as slowly processed precursors with molecular masses of 207, 161, 122, and 110 kDa.
Keywords: chloroplast biogenesis, photosynthesis, nuclear-coded chloroplast proteins
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béliveau R., Bellemare G. Light-dependent phosphorylation of thylakoid membrane polypeptides. Biochem Biophys Res Commun. 1979 Jun 13;88(3):797–803. doi: 10.1016/0006-291x(79)91478-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chia C. P., Arntzen C. J. Evidence for two-step processing of nuclear-encoded chloroplast proteins during membrane assembly. J Cell Biol. 1986 Sep;103(3):725–731. doi: 10.1083/jcb.103.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Lubben T. H., Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985 Mar 25;260(6):3691–3696. [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Cunningham F. X., Schiff J. A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b: I. Pigment Composition. Plant Physiol. 1986 Jan;80(1):223–230. doi: 10.1104/pp.80.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham F. X., Schiff J. A. Chlorophyll-Protein Complexes from Euglena gracilis and Mutants Deficient in Chlorophyll b: II. Polypeptide Composition. Plant Physiol. 1986 Jan;80(1):231–238. doi: 10.1104/pp.80.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr S. C., Somerville S. C., Arntzen C. J. Monoclonal antibodies to the light-harvesting chlorophyll a/b protein complex of photosystem II. J Cell Biol. 1986 Sep;103(3):733–740. doi: 10.1083/jcb.103.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmuir P. The petunia chlorophyll a/b binding protein genes: a comparison of Cab genes from different gene families. Nucleic Acids Res. 1985 Apr 11;13(7):2503–2518. doi: 10.1093/nar/13.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlne G., Schantz R. Molecular analysis of the transcripts encoding the light-harvesting chlorophyll a/b protein in Euglena gracilis: unusual size of the mRNA. Curr Genet. 1987;12(8):611–616. doi: 10.1007/BF00368064. [DOI] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Tobin E. M. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986 Jan;5(1):9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Tobin E. M. Chloroplast Import of Light-Harvesting Chlorophyll a/b-Proteins with Different Amino Termini and Transit Peptides. Plant Physiol. 1986 Dec;82(4):1172–1174. doi: 10.1104/pp.82.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K., Morelli G., Chua N. H. Structure and developmental regulation of a wheat gene encoding the major chlorophyll a/b-binding polypeptide. Mol Cell Biol. 1985 Jun;5(6):1370–1378. doi: 10.1128/mcb.5.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leutwiler L. S., Meyerowitz E. M., Tobin E. M. Structure and expression of three light-harvesting chlorophyll a/b-binding protein genes in Arabidopsis thaliana. Nucleic Acids Res. 1986 May 27;14(10):4051–4064. doi: 10.1093/nar/14.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkind M. L., Wessler S. R., Schmidt G. W. Functional determinants in transit sequences: import and partial maturation by vascular plant chloroplasts of the ribulose-1,5-bisphosphate carboxylase small subunit of Chlamydomonas. J Cell Biol. 1985 Jan;100(1):226–234. doi: 10.1083/jcb.100.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- Reisfeld A., Mattoo A. K., Edelman M. Processing of a chloroplast-translated membrane protein in vivo. Analysis of the rapidly synthesized 32 000-dalton shield protein and its precursor in Spirodela oligorrhiza. Eur J Biochem. 1982 May;124(1):125–129. doi: 10.1111/j.1432-1033.1982.tb05914.x. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Schiff J. A. The development, inheritance, and origin of the plastid in Euglena. Adv Morphog. 1973;10:265–312. doi: 10.1016/b978-0-12-028610-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. The transport of proteins into chloroplasts. Annu Rev Biochem. 1986;55:879–912. doi: 10.1146/annurev.bi.55.070186.004311. [DOI] [PubMed] [Google Scholar]
- Sheen J. Y., Bogorad L. Differential expression of six light-harvesting chlorophyll a/b binding protein genes in maize leaf cell types. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7811–7815. doi: 10.1073/pnas.83.20.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A., Arntzen C. J. Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol. 1983 Nov;97(5 Pt 1):1327–1337. doi: 10.1083/jcb.97.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J., Brandt P. Stage-Specific State I-State II Transitions during the Cell Cycle of Euglena gracilis. Plant Physiol. 1986 Jun;81(2):548–552. doi: 10.1104/pp.81.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]