Autologous hematopoetic stem cell transplantation (HSCT) has been used as a treatment for severe and therapy-refractory autoimmune diseases for more than 13 years.1 The European Bone Marrow Transplantation (EBMT) database ‘PROMISE’ is the largest existing database of transplanted patients with autoimmune diseases, currently including data on more than 1,000 such patients from 172 institutions in 27 countries. In this issue of Haematologica, the results of an 11-year survey of ‘PROMISE’ are presented.2 Patients whose data are recorded in the database suffered from multiple sclerosis, systemic sclerosis, rheumatoid arthritis, systemic lupus erythematosus, juvenile idiopathic arthritis and autoimmune cytopenia. In most cases, these patients had a chronic and progressive autoimmune disease and several lines of established therapies had failed. This reflects that up to now, autologous HSCT has been used as a rescue strategy for patients with an expected poor prognosis and lack of alternative treatment options.
In this regard, the reported overall 5-year survival of 85% and a progression-free survival of 43% are surprisingly positive. Treatment-related mortality did not exceed 5% and was even lower within experienced transplant centers. These are clearly encouraging results supporting further studies of HSCT in selected patients with autoimmune diseases. The best results were reported for patients with systemic sclerosis, multiple sclerosis and systemic lupus erythematosus, whereas HSCT for rheumatoid arthritis was associated with a higher rate of relapses. It should be mentioned that the data on HSCT for rheumatoid arthritis were collected mainly before the era of biological treatments and, since the advent of tumor necrosis factor-α antagonists, HSCT has been widely abandoned in this disease.
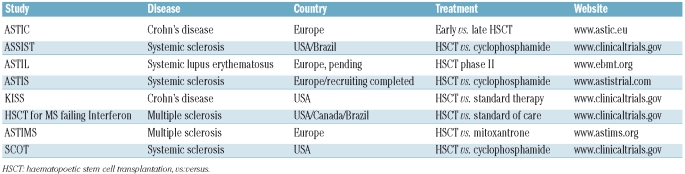
The most significant limitation of the presented survey is its retrospective nature, dealing with retrospective and incomplete data and a heterogeneous study population; the latter is reflected by the different indications for HSCT and the variety of conditioning protocols used. This limitation once more highlights the urgent need for prospective, controlled trials, which are actually underway for several autoimmune diseases (Table 1).
Table 1.
Prospective controlled trials on HSCT in autoimmune diseases.
Mechanistic aspects of autologous hematopoietic stem cell transplantation
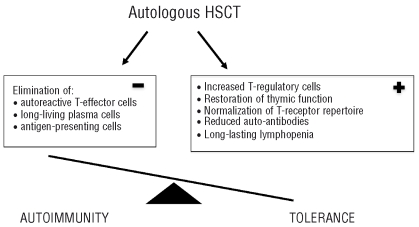
Despite the documented clinical success of HSCT in autoimmune diseases, the exact underlying mechanism of this treatment is still elusive. Obviously, in autologous HSCT a substantial burden of auto-reactive effector and inflammatory cells is eliminated by conditioning. The hope is that the subsequent, de novo generation of naïve T-lymphocytes in the patient will ‘reset’ the immunological clock, leading to restoration of tolerance. The re-infusion of CD34+ hematopoietic stem cells is mainly considered to shorten aplasia rather than being therapeutic. This is supported by studies showing that high dose cyclophosphamide therapy without re-infusion of stem cells can still be an effective treatment for systemic sclerosis.3
There are several factors that may contribute to sustained clinical remissions or even cure in a substantial number of patients. The combination of lymphotoxic chemotherapy, such as cyclophosphamide and antithymocyte globulin, leads to a profound and long-lasting lymphopenia4 and persistently reduced levels of putative pathogenic autoantibodies. Apart from this rather non-specific immunosuppression, there is growing evidence that autologous HSCT can also re-establish immunological tolerance: (i) autologous HSCT leads to an increased number of regulatory, FoxP3-positive T cells5 which are important in the preservation of tolerance; (ii) the reactivation of thymic function after autologous HSCT potentially leads to a tolerant, ‘juvenile’ immune system. This is illustrated by recent thymic emigrating cells, characterized by T-cell receptor excision circles (TREC) and CD31 expression, reestablishing T-cell receptor diversity in the years after HSCT, without relapse of the autoimmune disease;6,7 (iii) antithymocyte globulin directly targets long-living, autoantibody-producing plasma cells by complement-mediated lysis and apoptosis8 (Figure 1).
Figure 1.
Mechanistic aspects of autologous HSCT to restore tolerance in autoimmune disease.
However, relapses are possible and may, in the first instance, be due to the persistence of autoreactive cells such as long-lived plasma cells9 or to de novo emergence of autoimmune disease in a highly predisposed host. Relapses may also be facilitated by genetic polymorphisms of the innate immune system, which is increasingly recognized as playing an important role in autoimmunity and is not the main target of HSCT.10
Clinical data
In systemic sclerosis, initial phase I/II studies and previous EBMT database surveys already showed encouraging results from autologous HSCT, with a 5-year progression-free survival rate of more than 50%.11 Believed irreversible for a long time, structural changes such as fibrosis and micro-vessel rarefaction, both typical features of systemic sclerosis, have been shown to reverse after HSCT. This has been impressively illustrated for skin fibrosis, skin vasculature and digital capillaries12,13 and supports the belief that autologous HSCT has additional effects other than simply immunosuppression. Transplant-related mortality following autologous HSCT in patients with systemic sclerosis has decreased over the years.14 The survey by Farge et al. confirms this trend.2 Apart from growing experience and treatment refinements (e.g. lung shielding during total body irradiation), this is mainly due to the careful selection of patients. Chronic and advanced disease status and co-morbidity increase the risk of transplant-related mortality and patients with these problems are, therefore, now excluded from HSCT trials. Current trials, such as the ASTIS and SCOT trials, are focusing on patients with limited disease duration and preserved organ function but active disease with a poor prognosis.
Among the various autoimmune diseases, multiple sclerosis has been the main indication for autologous HSCT. A recent review15 summarized the data on 400 patients in 12 trials. Patients had either remitting-relapsing or primary progressive disease and conventional immunosuppression had failed. Disease stabilization and improvement occurred in around 70% at least up to 3 years after transplantation. The timing of HSCT in multiple sclerosis may be critical as there is growing evidence that at some stage of the disease neurodegeneration may progress independently of autoreactive processes.16
The majority of the patients with systemic lupus erythematosus who were treated with autologous HSCT had renal or other visceral involvement.17,18 Approximately 50% of patients remained disease-free for at least 5 years. Transplant-related mortality varied from 4% (US single center experience)16 to 12% in a European survey, further confirming the importance of the experience of the centers.17
Severe, therapy-refractory Crohn’s disease has been proposed as a potential indication for autologous HSCT. Results of the largest so far published series of 12 patients showed sustained remission in 11 patients.19
Autologous HSCT has also been used for patients with polyarthritis, both in adults suffering from rheumatoid arthritis and in children with juvenile idiopathic arthritis. Relapse rates in rheumatoid arthritis were, however, considerable.11 For both diseases new biological therapies are now available and the use of autologous HSCT has become less attractive.
Based on the results of small series or single cases, autologous HSCT has been reported to be a potentially effective treatment for a number of other autoimmune diseases such as vasculitis,20 autoimmune cytopenia and diabetes mellitus type I. More than 50% of the patients with this last condition remained insulin-independent at a median of 30 months after HSCT.21
Prospective clinical trials of autologous hematopoietic stem cell transplantation
In the light of the mainly positive clinical results and growing mechanistic understanding, it may be wondered why there is a delay in performing controlled phase three studies and why recruitment into such studies is slow. There are several reasons. First, there is no financial interest in this treatment, as a complex procedure rather than a single pharmaceutical product is investigated. Second, the extensive work to adhere with all regulatory issues within the European Union and sometimes with additional national regulations must be done by enthusiastic physicians who have limited resources as compared to the pharmaceutical industry.22 Third, autologous HSCT is considered to be an expensive therapy and, if not performed for a malignancy, is not covered by health insurances in many countries.
Despite all these hindrances, prospective clinical trials of HSCT in several autoimmune diseases are on the way (Table 1). The ASTIS trial comparing cyclophosphamide pulses with autologous HSCT in patients with systemic sclerosis has completed recruitment at the time of writing this manuscript.
Allogeneic hematopoietic stem cell transplantation
The first indications that allogeneic HSCT could induce durable remissions in patients with autoimmune diseases were obtained in patients with hematologic diseases and concomitant autoimmune diseases such as rheumatoid arthritis or systemic lupus erythematosus.23 However, the relatively high rates of transplant-related mortality and graft-versus-host disease hindered the study of allogeneic HSCT in autoimmune diseases for years. Notwithstanding, allogeneic HSCT has been performed over the years in a small number of patients suffering from rheumatoid arthritis, systemic sclerosis, vasculitis or other autoimmune diseases. Both long-term remissions and relapses have been reported.24,25 A recent review of the EBMT database revealed 35 patients with different autoimmune diseases who had undergone allogeneic HSCT. Seventy-five percent of the patients responded at least partially and the transplant-related mortality was approximately 20%.26 Remarkably, in two patients who suffered from severe systemic sclerosis, non-myeloablative conditioning resulted in a chimerism of 10–15% and was sufficient to achieve complete sustained remission lasting for more than 3 years after transplantation.27,28
Allogeneic HSCT replaces the auto-reactive host immune system by a new, presumably “tolerant”, donor immune system. In contrast to autologous HSCT, the conditioning serves not only to eradicate autoreactive effector cells, but also to allow engraftment of donor hematopoiesis. Both myeloablative and non-myeloablative conditioning have been used. The latter has been shown to be sufficient to yield stable mixed chimerism and is potentially less toxic than myeloablative conditioning.28 A postulated ‘graft-versus-autoimmunity’ effect,29 possibly due to replacement of autoantigen-presenting host cells by donor-derived cells,30,31 may be able to induce tolerance despite persistence of recipient-derived hematopoiesis.
Mesenchymal stem cells
Mesenchymal stem cells are able to differentiate into chondrocytes, osteocytes or fat cells. Apart from their regenerative potential, in vitro results have indicated that mesenchymal stem cells also have immunosuppressive properties. These cells suppress T- and B-cell lymphocyte proliferation when co-cultured with activated lymphocytes, through so far not completely understood mechanisms. In animal models mesenchymal stem cells are enriched in inflamed tissue and are able to attenuate inflammation.32 In a first clinical experience, mesenchymal stem cells were used in the treatment of acute graft-versus-host disease: 30 of 55 steroid-resistant patients were reported to have achieved a complete remission with no immediate toxicity after application of allogeneic mesenchymal stem cells.33 Questions about the immunogenicity and fate of these cells in the host remain and there is still an ongoing discussion about the nature of mesenchymal stem cells. These issues are currently being addressed by internationally cooperating groups.
Outlook
Where do we stand now, more than 13 years after the first autologous HSCT was performed for an autoimmune disease¿ Although not yet an established treatment, autologous HSCT can induce remissions and even cure some selected patients with therapy-refractory autoimmune diseases. Transplant-related mortality following autologous HSCT has decreased significantly over the years due to growing experience and careful selection of patients. To further lower this complication, research must continue on prognostic factors able to identify patients for whom HSCT is suitable early in their disease. Although, in contrast to malignant hematologic diseases, the short-term mortality of patients with autoimmune diseases is low, patients can be severely compromised by their disease and can even die from it. These risks must be weighed against therapy-associated side effects, including transplant-related death and potential long-term toxicity. Data on the latter are only emerging now.
The future of HSCT for autoimmune diseases will depend on information gained in prospective controlled trials and the end-points of such trials should concentrate not only on survival but also on relapse-free survival and quality of life. There is a unique opportunity to learn more about autoimmune diseases and the effect of HSCT on the immune system in such trials and this should stimulate mechanistic side studies.
The still unresolved problem of graft-versus-host disease hampers the use of allogeneic HSCT in autoimmune diseases. Nevertheless, the mechanistic concept of substituting an autoreactive immune system is appealing.
We may hear more about mesenchymal stem cells for the therapy of autoimmune diseases in the future. Preclinical data are accumulating and experience in acute graft-versus-host disease has been positive. Mesenchymal stem cells may complement conventional immunosuppressive therapy with their immediate anti-inflammatory properties.
Thirteen years after the first autologous HSCT for autoimmune diseases we are standing at the crossroads: research activity in this field will only be able to continue if prospective controlled trials can be performed and, even more importantly, be completed in a reasonable time span. To achieve this, interdisciplinary collaboration and support from regulatory authorities and healthcare groups are essential.
Footnotes
Thomas Daikeler is a Junior Consultant at the Department of Rheumatology at the University Hospital of Basel. He is a trained rheumatologist and hematologist and is involved in research and clinical trials concerning stem cell transplantation for autoimmune diseases.
Thomas Huegle is currently a Research Fellow at the Musculoskeletal Research Group of the Institute of Cellular Medicine at Newcastle University. He is working on scleroderma skin pathology and is involved in clinical research concerning the use of stem cells in autoimmune diseases.
No potential conflicts of interests relevant to this article were reported.
References
- 1.Tyndall A, Black C, Finke J, Winkler J, Mertlesmann R, Peter HH, et al. Treatment of systemic sclerosis with autologous haemopoietic stem cell transplantation. Lancet. 1997;349(9047):254. doi: 10.1016/s0140-6736(05)64864-7. [DOI] [PubMed] [Google Scholar]
- 2.Farge D, Labopin M, Tyndall A, Fassas A, Mancardi GL, van Laar JM, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years of experience from the European Group for Blood and Marrow Transplantation (EBMT) Working Party on Autoimmune Diseases. Haematologica. 2010;95(1):284–292. doi: 10.3324/haematol.2009.013458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tehlirian CV, Hummers LK, White B, Brodsky RA, Wigley FM. High-dose cyclophosphamide without stem cell rescue in scleroderma. Ann Rheum Dis. 2008;67(6):775–81. doi: 10.1136/ard.2007.077446. [DOI] [PubMed] [Google Scholar]
- 4.Farge D, Henegar C, Carmagnat M, Daneshpouy M, Marjanovic Z, Rabian C, et al. Analysis of immune reconstitution after autologous bone marrow transplantation in systemic sclerosis. Arthritis Rheum. 2005;52(5):1555–63. doi: 10.1002/art.21036. [DOI] [PubMed] [Google Scholar]
- 5.Roord ST, de Jager W, Boon L, Wulffraat N, Martens A, Prakken B, et al. Autologous bone marrow transplantation in autoimmune arthritis restores immune homeostasis through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2008;111(10):5233–41. doi: 10.1182/blood-2007-12-128488. [DOI] [PubMed] [Google Scholar]
- 6.Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113(1):214–23. doi: 10.1182/blood-2008-07-168286. [DOI] [PubMed] [Google Scholar]
- 7.Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani-Ingoni R, et al. Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med. 2005;201(5):805–16. doi: 10.1084/jem.20041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zand MS, Vo T, Pellegrin T, Felgar R, Liesveld JL, Ifthikharuddin JJ, et al. Apoptosis and complement-mediated lysis of myeloma cells by polyclonal rabbit antithymocyte globulin. Blood. 2006;107(7):2895–903. doi: 10.1182/blood-2005-06-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyer BF, Mumtaz IM, Yoshida T, Hiepe F, Radbruch A. How to cope with pathogenic long-lived plasma cells in autoimmune diseases. Ann Rheum Dis. 2008;67(Suppl 3):iii87–9. doi: 10.1136/ard.2008.098418. [DOI] [PubMed] [Google Scholar]
- 10.Allam R, Anders HJ. The role of innate immunity in autoimmune tissue injury. Cur Opin Rheumat. 2008;20(5):538–44. doi: 10.1097/BOR.0b013e3283025ed4. [DOI] [PubMed] [Google Scholar]
- 11.Gratwohl A, Passweg J, Bocelli-Tyndall C, Fassas A, van Laar JM, Farge D, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases. Bone Marrow Transplant. 2005;35(9):869–79. doi: 10.1038/sj.bmt.1704892. [DOI] [PubMed] [Google Scholar]
- 12.Verrecchia F, Laboureau J, Verola O, Roos N, Porcher R, Bruneval P, et al. Skin involvement in scleroderma--where histological and clinical scores meet. Rheumatology (Oxford) 2007;46(5):833–41. doi: 10.1093/rheumatology/kel451. [DOI] [PubMed] [Google Scholar]
- 13.Aschwanden M, Daikeler T, Jaeger KA, Thalhammer C, Gratwohl A, Matucci-Cerinic M, et al. Rapid improvement of nailfold capillaroscopy after intense immunosuppression for systemic sclerosis and mixed connective tissue disease. Ann Rheum Dis. 2008;67(7):1057–9. doi: 10.1136/ard.2007.082008. [DOI] [PubMed] [Google Scholar]
- 14.Farge D, Passweg J, van Laar JM, Marjanovic Z, Besenthal C, Finke J, et al. Autologous stem cell transplantation in the treatment of systemic sclerosis: report from the EBMT/EULAR Registry. Ann Rheum Dis. 2004;63(8):974–81. doi: 10.1136/ard.2003.011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogojan C, Frederiksen JL. Hematopoietic stem cell transplantation in multiple sclerosis. Acta Neurol Scand. 2009;120(6):371–82. doi: 10.1111/j.1600-0404.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- 16.Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS, et al. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. 2007;130(Pt 5):1254–62. doi: 10.1093/brain/awl370. [DOI] [PubMed] [Google Scholar]
- 17.Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295(5):527–35. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- 18.Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R, et al. Autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13(3):168–76. doi: 10.1191/0961203304lu525oa. [DOI] [PubMed] [Google Scholar]
- 19.Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, et al. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn’s disease. Gastroenterology. 2005;128(3):552–63. doi: 10.1053/j.gastro.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Daikeler T, Kotter I, Bocelli Tyndall C, Apperley J, Attarbaschi A, Guardiola P, et al. Haematopoietic stem cell transplantation for vasculitis including Behcet’s disease and polychondritis: a retrospective analysis of patients recorded in the European Bone Marrow Transplantation and European League Against Rheumatism databases and a review of the literature. Ann Rheum Dis. 2007;66(2):202–7. doi: 10.1136/ard.2006.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301(15):1573–9. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 22.Tyndall A. Why do we need noncommercial, investigator-initiated clinical trials? Nat Clin Pract Rheumatol. 2008;4(7):354–5. doi: 10.1038/ncprheum0814. [DOI] [PubMed] [Google Scholar]
- 23.Hinterberger W, Hinterberger-Fischer M, Marmont A. Clinically demonstrable anti-autoimmunity mediated by allogeneic immune cells favorably affects outcome after stem cell transplantation in human autoimmune diseases. Bone Marrow Transplant. 2002;30(11):753–9. doi: 10.1038/sj.bmt.1703686. [DOI] [PubMed] [Google Scholar]
- 24.Marmont AM, Gualandi F, Piaggio G, Podestà M, Teresa van Lint M, Bacigalupo A, et al. Allogeneic bone marrow transplantation (BMT) for refractory Behcet’s disease with severe CNS involvement. Bone Marrow Transplant. 2006;37(11):1061–3. doi: 10.1038/sj.bmt.1705372. [DOI] [PubMed] [Google Scholar]
- 25.Snowden JA, Kearney P, Kearney A, Cooley HM, Grigg A, Jacobs P, et al. Long-term outcome of autoimmune disease following allogeneic bone marrow transplantation. Arthritis Rheum. 1998;41(3):453–9. doi: 10.1002/1529-0131(199803)41:3<453::AID-ART11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Daikeler T, Hügle T, Farge D, Andolina M, Gualandi F, Baldomero H, et al. Allogeneic hematopoietic SCT for patients with autoimmune diseases. Bone Marrow Transplant. 2009;44(1):27–33. doi: 10.1038/bmt.2008.424. [DOI] [PubMed] [Google Scholar]
- 27.Khorshid O, Hosing C, Bibawi S, Ueno N, Reveille J, Mayes MD, et al. Nonmyeloablative stem cell transplant in a patient with advanced systemic sclerosis and systemic lupus erythematosus. J Rheumatol. 2004;31(12):2513–6. [PubMed] [Google Scholar]
- 28.Loh Y, Oyama Y, Statkute L, Verda L, Quigley K, Yaung K, et al. Non-myeloablative allogeneic hematopoietic stem cell transplantation for severe systemic sclerosis: graft-versus-autoimmunity without graft-versus-host disease? Bone Marrow Transplant. 2007;39(7):435–7. doi: 10.1038/sj.bmt.1705611. [DOI] [PubMed] [Google Scholar]
- 29.Marmont AM, Gualandi F, Van Lint MT, Bacigalupo A. Refractory Evans’ syndrome treated with allogeneic SCT followed by DLI. Demonstration of a graft-versus-autoimmunity effect. Bone Marrow Transplant. 2003;31(5):399–402. doi: 10.1038/sj.bmt.1703833. [DOI] [PubMed] [Google Scholar]
- 30.Kamath AT, Henri S, Battye F, Tough DF, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100(5):1734–41. [PubMed] [Google Scholar]
- 31.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 32.Tyndall A, Uccelli A. Multipotent mesenchymal stromal cells for autoimmune diseases: teaching new dogs old tricks. Bone Marrow Transplant. 2009;43(11):821–8. doi: 10.1038/bmt.2009.63. [DOI] [PubMed] [Google Scholar]
- 33.Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]