Abstract
Background
Pediatric follicular lymphoma has recently been recognized as a novel variant of follicular lymphoma in the World Health Organization classification of lymphomas. Given the rarity of the disease, histopathological and genetic data on this type of lymphoma are still scarce.
Design and Methods
We analyzed 25 cases of pediatric follicular lymphoma (patients aged ≤18 years) by morphology, immunohistochemistry and interphase fluorescence in situ hybridization. All patients analyzed were treated within Non-Hodgkin’s Lymphoma - Berlin-Frankfurt-Münster (NHL-BFM) multicenter trials, and the cohort was representative of the German population.
Results
The genetic hallmark of adult follicular lymphoma, t(14;18)(q32;q21), was not detectable in any of the pediatric cases, although BCL2 protein was expressed in 55% of the latter cases. No correlation was found between BCL2 protein expression and outcome. Chromosomal breaks in the immunoglobulin heavy chain gene (IGH) and the BCL6 locus were detected in 5 of 17 and 1 of 18 cases, respectively. Patients with pediatric follicular lymphoma had long event-free survival and, in contrast to adult follicular lymphoma, the clinical course was not dominated by relapses. A simultaneous diffuse large B-cell lymphoma was frequently detected at initial diagnosis in children but did not indicate an aggressive clinical course.
Conclusions
Our data suggest that pediatric follicular lymphoma is a disease that differs from its adult counterpart both genetically and clinically.
Keywords: pediatric follicular lymphoma, childhood lymphoma, pediatric diffuse large B-cell lymphoma, t(14;18)
Introduction
The vast majority of B-cell non–Hodgkin’s lymphomas in children and adolescents are aggressive lymphomas, predominantly Burkitt’s lymphomas and diffuse large B-cell lymphomas (DLBCL). Follicular lymphomas (FL), although frequent in adults, are rare in children and adolescents and account for not more than 2% of non-Hodgkin’s lymphomas in this age group.1 Moreover, pediatric FL differ genetically from their adult counterpart.2 The translocation t(14;18)(q32;q21), juxtaposing BCL2 next to the immunoglobulin heavy chain gene (IGH) is detectable in more than 80% of adult FL, but was reported to be rare in pediatric FL.2 The rarity of pediatric FL and the absence of the typical diagnostic features of adult FL, such as BCL2 aberrations, render the diagnosis of pediatric FL challenging.3,4 In adults the majority of FL are indolent, low-grade lymphomas of grade 1 or 2 according to the World Health Organization (WHO) classification.1,5 In adults transformation of a low-grade lymphoma to a high-grade lymphoma, usually a DLBCL, is accompanied by rapid clinical progression and an unfavorable prognosis.6,7 However, clinicopathological studies addressing the question of a simultaneous DLBCL component and outcome of FL in children have not been published so far. We have recently shown that pediatric DLBCL differ from adult DLBCL with regard to prognosis, immunophenotype and genetics.8
In this study, we characterized a population-based series of 25 FL in patients aged 18 years or under, using morphological examination, immunohistochemistry studies and fluorescence in situ hybridization (FISH). We examined genetic aberrations such as breaks in the IGH, MYC or BCL6 loci which, remarkably, had not been studied in a larger series of pediatric FL. We evaluated patients who were treated uniformly within clinical trials of the Non-Hodgkin’s Lymphoma – Berlin-Frankfurt-Münster (NHL-BFM) group.
Design and Methods
Patients
All pediatric patients (≤18 years) from Germany with a diagnosis of FL treated in the three consecutive NHL-BFM group multicenter trials, NHL-BFM 90, NHL-BFM 95 and B-NHL BFM-04, were identified. The treatment protocols of these three trials included a common backbone of chemotherapy and outcomes over the last 20 years within these trials were comparable.9–11 The patients’ disease was staged according to the St. Jude’s Hospital system for childhood non-Hodgkin’s lymphomas.12 All biopsies were performed at first diagnosis before any treatment. The size and quality of the biopsy specimens, the tissue processing protocols and the paraffin blocks were heterogeneous. All cases were reviewed by at least two expert pathologists and classified according to the WHO classification.5 Since virtually all pediatric patients with lymphoma in Germany are registered and followed by the BFM group, the samples we analyzed can be considered to have been a representative population-based cohort for Germany.13
The study was carried out in compliance with local ethical guidelines and the ethical guidelines of the studies in which the patients were treated. The scientific studies were done with informed consent of all parents.
Immunohistochemistry and interphase cytogenetics
Immunohistochemical studies were performed as described previously.8 Lymphoma samples were scored positive for BCL2, BCL6, CD5 and CD10 if more than 25% of the tumor cells stained positive. Immunohistochemical staining for Ki-67 was assessed as percent of positive tumor cells. Interphase FISH for the detection of breakpoints affecting the IGH, BCL2, BCL6 and MYC loci or fusions of BCL2 and IGH was carried out on paraffin sections of tumor tissues using commercially available probes (Abbott/Vysis, Downers Grove, IL, USA). In one case with a simultaneous IGH and BCL6 break, a home-made IGH-BCL6 double-color double-fusion probe was also applied. FISH was performed according to recently published protocols.14
Statistical analysis
The duration of event-free survival is defined as the time from diagnosis until the date of the first adverse event (tumor failure, death from any cause or the development of a second malignancy), or, if no such event occurred, until the date of latest contact. Probabilities of event-free survival were estimated by the method of Kaplan and Meier, with standard errors according to Greenwood, and were compared using the log-rank test.15 Differences in the distribution of individual parameters among subsets of patients were analyzed using the χ2 test or Fisher’s exact test. The statistical analyses were carried out using SAS (SAS-PC, Version 9.1, Cary, NC: SAS Institute Inc.).
Results
Clinical characteristics
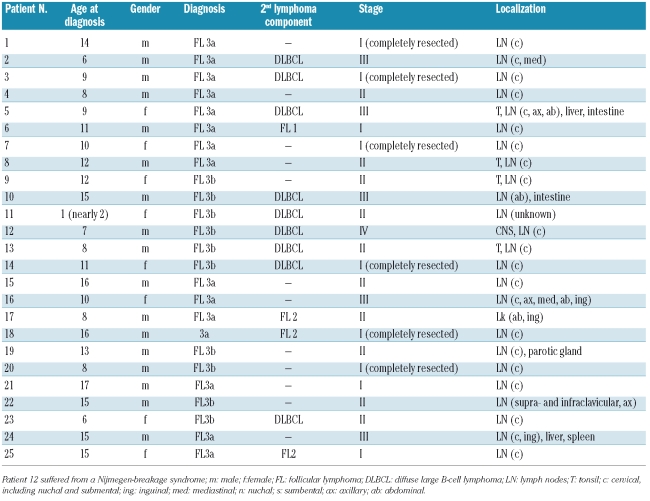
The patients with pediatric FL in our cohort were predominantly male [17 of 25 (68%)] with a median age of 11 years (range, 1–17 years) (Table 1). Pediatric FL presented frequently as localized disease (36% stage I, 40% stage II, 20% stage III and 4% stage IV), and in six patients the diagnostic biopsy represented a complete resection of the lymphoma. Cervical lymph nodes (21 of 25) were the most frequent nodal presentation and the tonsil the most frequent extranodal manifestation. Interestingly, we did not detect any case with testicular involvement. In no cases was bone marrow or bone involved. Central nervous system involvement occurred in one patient with simultaneous DLBCL. Relapse of the disease was described in only one patient, a girl who suffered from the Nijmegen breakage syndrome. Two patients had lactate dehydrogenase concentration greater than 500 IU/L. The major clinical findings are summarized in Table 1.
Table 1.
Characteristics of the patients.
Histopathology
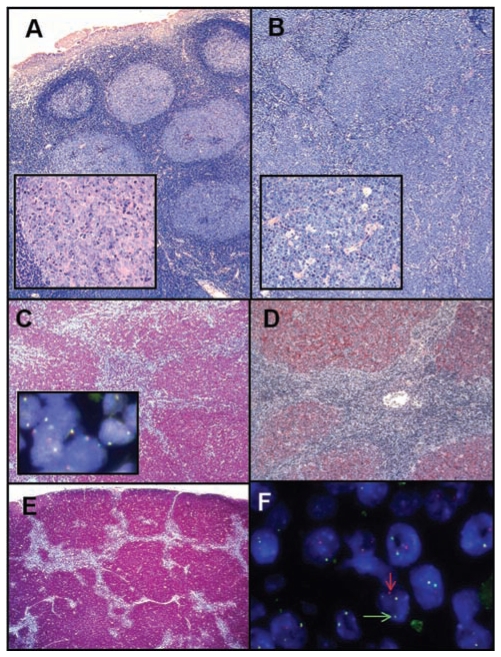
All biopsy specimens were re-evaluated by two hematopathologists according to the 2008 WHO lymphoma classification.5 The pediatric FL were usually composed of large expanded follicles, which displayed architectural features of atypia such as reduced follicle mantles, missing compartmentalization of the follicles into a dark and a light zone or discordant number of large cells and tingible body macrophages (Figure 1). In contrast to reactive follicular hyperplasia, in which a spectrum of variably sized follicles in different functional stages is usually seen, follicles were often homogeneous in size, crowded, frequently back to back and sometimes confluent. In same cases a so-called floral pattern was present (Figure 1). The “starry sky picture”, normally a feature of reactive follicles and caused by tingible body macrophages, was often preserved, especially in areas of high-grade disease. The follicular growth pattern was seen throughout the infiltrated tissues in most cases. Thus, in infiltrated lymph nodes or the tonsils the neoplastic follicles exceeded the physiological B-cell compartments, i.e. the cortical area in lymph nodes and the subepithelial area in the tonsils.
Figure 1.
Follicular lymphoma grade 3a involving the tonsil (A) with a simultanous DLBCL component in deeper parts of the tumor (B) with higher magnification in the inserts, (A+B Giemsa stain). Strong expression of BCL2 (C) in the absence of BCL2 breaks (insert in C) and dense networks of follicular dendritic cells positive for CD23 (D), A-D correspond to case 5 in Tables 1 and 2). A floral follicular growth pattern in case 12 highlighted by staining for CD10 (E). Breaks in the IGH gene of case 13 (F, arrows indicate the split signal).
In several cases small, pre-existing reactive follicles were detectable at the borders of the infiltration. Within the neoplastic follicles cytologically typical centroblasts and centrocytes without atypical features were seen. The criteria of the current WHO classification were used to grade FL according to the number of large cells and to assess a DLBCL component. In all our cases a predominant grade 3 pattern (> 15 centroblasts per high power field of the microscope) was present. Of the 25 FL evaluated, 15 (60%) were classified as grade 3a (FL 3a) and 10 (40%) as grade 3b (FL 3b). Areas of grade 1 or 2 FL as a second lymphoma component were detectable only in cases of FL 3a (4 cases, representing 16% of all FL and 27% of all FL 3a, Table 1).
Although all biopsy specimens were obtained at initial diagnosis before any treatment, a simultaneous DLBCL component, indicated by a diffuse growth pattern5 within the same biopsy, was noted in nine (36%) of the cases. In the majority of our cases of FL with a DLBCL the follicular component dominated the biopsy and usually there was a gradual transition from follicular growth into the diffuse pattern with ill-defined borders between the two components (Figure 1). Nevertheless, in order for a simultaneous DLBCL to be diagnosed, a considerable area with diffuse lymphoma growth had to be present.5 The DLBCL component was more frequently associated with a FL 3b (6 of 10) than a FL 3a (3 of 15) although this association did not reach statistical significance (P=0.09, Fisher’s exact test). There were no statistically significant differences in clinical, immunhistochemical or genetic data between FL 3a and FL3b (Table 1). The DLBCL component was classified as centroblastic subtype in all cases (Table 1).
Immunophenotype
In order to distinguish a pseudo-follicular growth pattern occasionally observed in DLBCL from the follicular growth pattern of a FL, the presence of follicular dendritic cell meshworks was demonstrated in all cases by staining for CD23 (data not shown). Interestingly, in cases with simultaneous FL and DLBCL, both lymphoma components generally had the same immunophenotypic profile (data not shown).
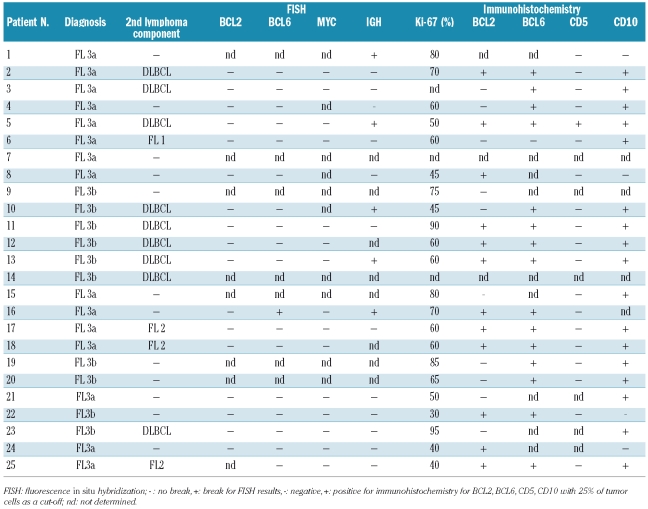
All pediatric FL in our series were positive for CD20. CD5 expression was only detected in one case (Table 2). The expression of Ki-67 was variable, but overall high, ranging from 40 to 95% (Table 2). The germinal center markers CD10 and BCL6 were expressed in the majority of cases [CD10 in 17 of 21 evaluable cases (81%) and BCL6 in 15 of 16 evaluable cases (94%)].
Table 2.
FISH and immunohistochemical results.
We used staining for CD5 to highlight small, reactive T cells, present in the interfollicular areas and within some neoplastic follicles. The amount of physiologically BCL-2 expressed by T cells within the lymphoma follicles was considered when estimating the percentage of BCL2-positive lymphoma cells. This was generally easily applicable as only BCL2-positivity in large cells was evaluated whilst the CD5 staining was confined to small T-cells. Using this approach BCL2 expression of the lymphoma cells was detectable in roughly half of the samples (12 of 22, 55% of evaluable cases, Table 2).
Molecular cytogenetics
Genetic aberrations were studied using FISH to detect breaks in IGH, BCL2, BCL6 and MYC. We did not detect any fusions of BCL2 and IGH (0 of 17 evaluable cases, data not shown) or breaks in BCL2 (0 of 18 evaluable cases), indicating that the translocation t(14;18)(q32;q21) was absent in this series. However, a gain (3–4 copies) of BCL2 was detected in one case (case 25, Table 2). We found no breaks of MYC in our series (0 of 15 evaluable cases), whereas breaks in IGH occurred in 5 of 17 (29%) evaluable cases (Table 2, Figure 1). One of 18 evaluable cases showed a BCL6 break but a fusion with the IGH gene was not detected although this case did show a IGH break (case 16, Table 2). Although there are reports of the presence of trisomy 3 and/or BCL6/3q27 gains in t(14;18)-negative FL, we detected no gains of BCL6/3q27 in our series using a BCL6 break-apart probe. Considering the cases in which any break was found, 5 of 17 FL with evaluable FISH results for at least the IGH and BCL6 assay displayed chromosomal aberrations (Table 2). The tumor cell content in the cases with detectable chromosomal aberrations did not differ from that in cases without aberrations (data not shown).
Correlations with clinical characteristics and outcome
In our series of 25 pediatric FL followed for a median of 6.1 years (range, 0.2 to 10.2 years), only one patient (case 12, Tables 1 and 2) suffered from a relapse. The estimated probability of 5-year event-free survival was 96±4%. Given this excellent outcome of the whole series of patients, with only one event, no significantly different outcomes for FL with a DLBCL component, with chromosomal aberrations or with BCL2 positivity could be demonstrated (data not shown). However, four of 12 (47%) of the BCL2-positive lymphomas were in an advanced stage (stage III or IV), whereas only one of the ten BCL2-negative lymphomas was stage III. In addition, FL with detectable FISH aberrations more often presented in a higher stage (> stage II) than FL without detectable chromosomal aberrations (3 of 5 and 2 of 12 cases, respectively). However, neither association was statistically significant (Fisher’s exact test).
Discussion
Pediatric FL is a rare disease. A recently published review,4 and a review by our group of the literature indicated that fewer than 100 cases of FL in children have been reported so far.2,3,16–21 To our knowledge, our analysis represents the largest series of pediatric FL published so far and our cohort can be considered representative for Germany. In addition, all patients in this study were treated similarly within trials of the NHL-BFM group.
Pediatric FL often present in localized stages with involvement of cervical lymph nodes and the tonsil, a feature that has been recognized previously.2,5 Interestingly, although there are well documented cases of testicular FL in children,2,21 we did not find any FL with a manifestation in the testis in our population-based series. Thus, it is possible that the frequency of testicular FL has been overestimated, since the published series might represent highly selected case series. Alternatively this specific testicular disease might be dependent on epidemiological factors that are less prevalent in western Europe.
The immunohistochemical profile of pediatric FL has been described before.2 Our data confirm that the majority of cases of pediatric FL express the germinal center marker CD10. However, the percentage of BCL2-positive cases was higher in our series than in the series analyzed so far.2 The differences between the studies might be due to different immunohistochemical techniques or evaluation criteria. We used a threshold of 25% positive cells, which is widely accepted for the evaluation of the BCL2-staining. In our series with an excellent outcome under BFM-therapy, we did not find a correlation between BCL2 expression and outcome as was stated in the WHO classification.5 Nevertheless there was a trend towards higher stage disease in BCL2-positive FL, indicating more aggressive tumor growth in these cases.
Using the FISH technique, we excluded the presence of fusions between the IGH and BCL2 loci, which are indicative of the t(14;18)(q32;q21) translocation, in all evaluable pediatric FL. Moreover, we excluded any variant translocations affecting the BCL2 locus by a specific break-apart probe. A previously published study demonstrated BCL2 translocations in two pediatric FL (patients aged 13 and 17 years) using a polymerase chain reaction technique.2 A screening of healthy individuals indicated that t(14;18)(q32;q21)-positive B cells are detectable in the peripheral blood starting from the age of 10 years.22 Thus, positive polymerase chain reaction results suggestive of a t(14;18)(q32;q21) in pediatric patients should be validated by an in situ technique, such as FISH, to prove that the translocation is a feature of the lymphoma cells.
The lack of BCL2 translocations raises the question of the molecular mechanisms involved in the pathogenesis of pediatric FL. We were able to demonstrate recurrent aberrations of IGH in pediatric FL. Although, the translocation partner of IGH remains to be determined, our finding might point the way towards a understanding of the genetic basis of pediatric FL. Furthermore, our genetic findings might be of help in a diagnostic setting. Considering the fact that some lymphomas are immunohistochemically negative for BCL2, the diagnosis of pediatric FL, especially the distinction from atypical hyperplasia, can be quite challenging.3,4 Analyses of clonal rearrangements of IGH or monoclonal light chain expression are useful tools for the differential diagnosis of FL from atypical hyperplasia. However, clonality does not definitely distinguish between atypical hyperplasia and lymphoma.3,23
The diagnosis of FL is based primarily on the recognition of atypical follicles, as described above. In addition to the morphological criteria several features assessed in our study can be considered as reliable markers of malignancy that are useful for the differential diagnosis: (i) the presence of any chromosomal aberration, (ii) a DLBCL component, and (iii) immunohistochemical expression of BCL2 in centroblasts or centrocytes (using any level of expression, including cases with <25% positive cells). In our cohort, 23 cases showed at least one of these markers of malignancy, and the diagnosis of FL was based on morphological features alone in only two cases (cases 7 and 21). Unfortunately, in these two cases VDJ rearrangement analysis of IGH was not assessable.
In adults FL is considered an incurable disease and thus its clinical course differs from that of DLBCL if treated with comparable therapy.24–27 However, in children, the outcome of both diseases, FL and DLBCL, is equivalent if the patients are treated according to the NHL-BFM protocols for mature B-cell non-Hodgkin’s lymphoma.8 Relapses, which are frequent in adult FL, are rare in children. More differences in the clinical course between children and adults became evident in our study. In our cohort of pediatric FL a large proportion (36%) of patients had a DLBCL as a second lymphoma component at the time of the primary biopsy. In adults the presence of a DLBCL component is considered to be a transformation of the FL. The rate of transformation of adult FL is low and was estimated to be in the range of 3% per year.7 Transformation in adults is usually associated with a very poor outcome.6,7 Here again, pediatric FL differs from the adult counterpart, in that the presence of a DLBCL component does not indicate an adverse outcome.
In summary, our data suggest that pediatric FL is a disease that differs from the adult counterpart genetically and clinically. The genetic hallmark of adult FL, the translocation t(14;18)(q32;q21), is typically not detectable in pediatric FL. The molecular mechanisms involved in the pathogenesis of pediatric FL still remain to be elucidated. The outcome of pediatric FL is excellent and, in contrast to adult FL, the clinical course is not dominated by relapses, if the pediatric FL are treated according to NHL-BFM protocols. Furthermore, in pediatric FL a simultaneous DLBCL can frequently be detected at initial diagnosis but does not indicate a more aggressive clinical course in children.
Acknowledgments
the authors thank Hans-Heinrich Wacker, Olivera Batic, Edda Sevecke-Wessel, Michael Weiss, Monika Hauberg, Christiane Stange, Claudia Becher, Dorit Schuster and Reina Zühlke-Jenisch for their excellent support and Ulrike Meyer and Bettina Paul for excellent data management.
Footnotes
Funding: WK and RS are supported by the Kinderkrebsinitative Buchholz, Holm-Seppensen and are members of the Deutsche Krebshilfe (70-3173-Tr3) project “Molecular Mechanisms in Malignant Lymphomas”. IS is supported by a fellowship from the Alexander Von Humboldt Foundation.
Authorship and Disclosures
IO, RS, and WK contributed to the conception and design of the study, acquisition, analysis and interpretation of the data, drafting the article and revising it critically for important intellectual content. IS, FM, MK and SG analyzed and interpreted FISH data and revised the draft of the article. BB, WW, AM MZ and AR provided clinical data, performed the statistical analyses and revised the draft of the article. The order of authorship reflects the contribution of each author to the design of the study, data interpretation and writing of the manuscript. All authors approved the last version of the manuscript.
The company Abbott/Vysis discounts the Deutsche Krebshilfe (70-3173-Tr3) project “Molecular Mechanisms in Malignant Lymphomas” for FISH probes. RS has received speaker’s honoraria from Abbott/Vysis. None of the other authors reported any potential conflicts of interest.
References
- 1.Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br J Haematol. 2008;142(3):329–47. doi: 10.1111/j.1365-2141.2008.06988.x. [DOI] [PubMed] [Google Scholar]
- 2.Lorsbach RB, Shay-Seymore D, Moore J, Banks PM, Hasserjian RP, Sandlund JT, et al. Clinicopathologic analysis of follicular lymphoma occurring in children. Blood. 2002;99(6):1959–64. doi: 10.1182/blood.v99.6.1959. [DOI] [PubMed] [Google Scholar]
- 3.Swerdlow SH. Pediatric follicular lymphomas, marginal zone lymphomas, and marginal zone hyperplasia. Am J Clin Pathol. 2004;122 (Suppl):S98–109. doi: 10.1309/4BKNAKE4D7CT3C1B. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal R, Wang J. Pediatric follicular lymphoma: a rare clinicopathologic entity. Arch Pathol Lab Med. 2009;133(1):142–6. doi: 10.5858/133.1.142. [DOI] [PubMed] [Google Scholar]
- 5.WHO Classification of Tumors of the Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. [Google Scholar]
- 6.Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25(17):2426–33. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 7.Al Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5165–9. doi: 10.1200/JCO.2008.16.0283. [DOI] [PubMed] [Google Scholar]
- 8.Oschlies I, Klapper W, Zimmermann M, Krams M, Wacker HH, Burkhardt B, et al. Diffuse large B-cell lymphoma in pediatric patients belongs predominantly to the germinal-center type B-cell lymphomas: a clinicopathologic analysis of cases included in the German BFM (Berlin-Frankfurt-Munster) Multicenter Trial. Blood. 2006;107(10):4047–52. doi: 10.1182/blood-2005-10-4213. [DOI] [PubMed] [Google Scholar]
- 9.Woessmann W, Seidemann K, Mann G, Zimmermann M, Burkhardt B, Oschlies I, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms - a report of the BFM group study NHL-BFM95. Blood. 2005;105(3):948–58. doi: 10.1182/blood-2004-03-0973. [DOI] [PubMed] [Google Scholar]
- 10.Reiter A, Schrappe M, Tiemann M, Ludwig WD, Yakisan E, Zimmermann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood. 1999;94(10):3294–306. [PubMed] [Google Scholar]
- 11.Reiter A, Schrappe M, Parwaresch R, Henze G, Muller-Weihrich S, Sauter S, et al. Non-Hodgkin’s lymphomas of childhood and adolescence: results of a treatment stratified for biologic subtypes and stage–a report of the Berlin-Frankfurt-Munster Group. J Clin Oncol. 1995;13(2):359–72. doi: 10.1200/JCO.1995.13.2.359. [DOI] [PubMed] [Google Scholar]
- 12.Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7(3):332–9. [PubMed] [Google Scholar]
- 13.Klapper W, Szczepanowski M, Burkhardt B, Berger H, Rosolowski M, Bentink S, et al. Molecular profiling of pediatric mature B-cell lymphoma treated in population-based prospective clinical trials. Blood. 2008;112(4):1374–81. doi: 10.1182/blood-2008-01-136465. [DOI] [PubMed] [Google Scholar]
- 14.Ventura RA, Martin-Subero JI, Jones M, McParland J, Gesk S, Mason DY, et al. FISH analysis for the detection of lymphoma-associated chromosomal abnormalities in routine paraffin-embedded tissue. J Mol Diagn. 2006;8(2):141–51. doi: 10.2353/jmoldx.2006.050083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalbfleisch J, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley; 2005. [Google Scholar]
- 16.Frizzera G, Gajl-Peczalska KJ, Bloomfield CD, Kersey JH. Predictability of immunologic phenotype of malignant lymphomas by conventional morphology: a study of 60 cases. Cancer. 1979;43(4):1216–24. doi: 10.1002/1097-0142(197904)43:4<1216::aid-cncr2820430409>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Winberg CD, Nathwani BN, Bearman RM, Rappaport H. Follicular (nodular) lymphoma during the first two decades of life: a clinicopathologic study of 12 patients. Cancer. 1981;48(10):2223–35. doi: 10.1002/1097-0142(19811115)48:10<2223::aid-cncr2820481018>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Pinto A, Hutchison RE, Grant LH, Trevenen CL, Berard CW. Follicular lymphomas in pediatric patients. Mod Pathol. 1990;3(3):308–13. [PubMed] [Google Scholar]
- 19.Ribeiro RC, Pui CH, Murphy SB, Shuster JJ, Hvizdala EV, Falletta J, et al. Childhood malignant non-Hodgkin lymphomas of uncommon histology. Leukemia. 1992;6(8):761–5. [PubMed] [Google Scholar]
- 20.Atra A, Meller ST, Stevens RS, Hobson R, Grundy R, Carter RL, et al. Conservative management of follicular non-Hodgkin’s lymphoma in childhood. Br J Haematol. 1998;103(1):220–3. doi: 10.1046/j.1365-2141.1998.00941.x. [DOI] [PubMed] [Google Scholar]
- 21.Finn LS, Viswanatha DS, Belasco JB, Snyder H, Huebner D, Sorbara L, et al. Primary follicular lymphoma of the testis in childhood. Cancer. 1999;85(7):1626–35. doi: 10.1002/(sici)1097-0142(19990401)85:7<1626::aid-cncr27>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Schuler F, Dolken L, Hirt C, Kiefer T, Berg T, Fusch G, et al. Prevalence and frequency of circulating t(14;18)-MBR translocation carrying cells in healthy individuals. Int J Cancer. 2009;124(4):958–63. doi: 10.1002/ijc.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dongen JJ, Langerak AW, Bruggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17(12):2257–317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 24.Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rube C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004;104(3):634–41. doi: 10.1182/blood-2003-06-2095. [DOI] [PubMed] [Google Scholar]
- 25.Pfreundschuh M, Trumper L, Kloess M, Schmits R, Feller AC, Rudolph C, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of young patients with good-prognosis (normal LDH) aggressive lymphomas: results of the NHL-B1 trial of the DSHNHL. Blood. 2004;104:626–33. doi: 10.1182/blood-2003-06-2094. [DOI] [PubMed] [Google Scholar]
- 26.Lenz G, Dreyling M, Schiegnitz E, Forstpointner R, Wandt H, Freund M, et al. Myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission prolongs progression-free survival in follicular lymphoma: results of a prospective, randomized trial of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:2667–74. doi: 10.1182/blood-2004-03-0982. [DOI] [PubMed] [Google Scholar]
- 27.Gleissner B, Küppers R, Siebert R, Glass B, Trümper L, Hiddemann W, et al. Report of a workshop on malignant lymphoma: a review of molecular and clinical risk profiling. Br J Haematol. 2008;142(2):166–78. doi: 10.1111/j.1365-2141.2008.07138.x. [DOI] [PubMed] [Google Scholar]