Abstract
Background
Hematocrit above the normal range for the population, such as in primary or secondary erythrocytosis, predisposes to both arterial and venous thrombosis. However, little is known about the association between hematocrit and risk of venous thromboembolism in a general population.
Design and Methods
Hematocrit and related hematologic variables such as hemoglobin, red blood cell count, mean corpuscular volume, and baseline characteristics were measured in 26,108 subjects, who participated in the Tromsø Study in 1994–1995. Incident venous thromboembolic events during follow-up were registered up to September 1st, 2007.
Results
There were 447 venous thromboembolic events during a median of 12.5 years of follow-up. Multivariable hazard ratios per 5% increment of hematocrit for the total population, adjusted for age, body mass index and smoking, were 1.25 (95% CI: 1.08–1.44) for total venous thromboembolism and 1.37 (95% CI: 1.10–1.71) for unprovoked venous thromboembolism. In category-based analyses, men with a hematocrit in the upper 20th percentile (≥46% in men) had a 1.5-fold increased risk of total venous thromboembolism (95% CI: 1.08–2.21) and a 2.4-fold increased risk of unprovoked venous thromboembolism (95% CI: 1.36–4.15) compared to men whose hematocrit was in the lower 40th percentile. The risk estimates were higher for men than for women both in continuous and category-based analyses. The findings for hemoglobin and red blood cell count were similar to those for hematocrit, whereas mean corpuscular volume was not associated with venous thromboembolism.
Conclusions
Our findings suggest that hematocrit and related hematologic variables such as hemoglobin and red blood cell count are risk factors for venous thromboembolism in a general population.
Keywords: hematocrit, population range, arterial and venous thrombosis
Introduction
Venous thromboembolism (VTE), manifested as deep vein thrombosis or pulmonary embolism, is a common, multifactorial disease with serious short- and long-term complications and potentially fatal outcome.1 Although increasing age, obesity, surgery, trauma, malignancy, immobility, pregnancy, and acute medical conditions are well-known risk factors for VTE,1,2 up to 30–50% of cases of VTE have no obvious predisposing factors.3
As Virchow postulated, the risk of VTE is primarily related to hypercoagulability, altered blood flow, or endothelial vascular lesions.4 Hematocrit, the proportion of blood volume occupied by red blood cells (RBC), is one of the major determinants of blood viscosity. An increase in the hematocrit is associated with increased blood viscosity,5 reduced venous return,6 and increased adhesiveness of platelets.7 It is well known that subjects with hematocrit levels above the normal range for the population, such as in primary or secondary erythrocytosis, are predisposed to both arterial cardiovascular disease and venous thrombosis.8–12 Hematocrit has also been associated with an increased risk of cardiovascular disease and all-cause mortality in a general population.13
The impact of hemoconcentration on the risk of VTE in a general population has, to our knowledge, not been extensively investigated in prospective studies. We, therefore, wanted to examine whether hematocrit and related hematologic variables, such as hemoglobin, RBC count and mean corpuscular volume (MCV), were associated with the incidence of VTE. To address these questions, we performed a prospective, population-based study in 26,108 adults, followed for a median of 12.5 years, and assessed the impact of hematocrit and related hematologic variables on the risk of VTE.
Design and Methods
Study population
Participants were recruited from the fourth survey of the Tromsø study (conducted in 1994–1995), a single-center prospective, population-based study, with repeated health surveys of inhabitants in Tromsø, Norway. The main focus of the Tromsø study is cardiovascular risk factors and disease. All inhabitants aged over 24 years old were invited to participate, of whom 27,158 agreed to do so (77% of the eligible population). The study was approved by the regional committee for research ethics, and all participants gave their informed, written consent to participation. Subjects who did not consent to medical research (n=300) and subjects not officially registered inhabitants of the municipality of Tromsø (n=43) were excluded from the study. Furthermore, subjects with a known history of VTE (n=47), and subjects for whom values of hematocrit, hemoglobin, RBC count, smoking status, or body mass index (BMI) were lacking (n=660) were excluded. A total of 26,108 subjects were enrolled in the study in 1994–1995, and all first lifetime events of VTE during the follow-up were registered through to the end of the study period, September 1st, 2007.
Measurements
Baseline information was collected from physical examination, blood samples, and self-administered questionnaires. Non-fasting blood samples were collected from an antecubital vein, and analyzed at the Department of Clinical Chemistry, University Hospital of North Norway. For measurements of hematocrit, hemoglobin, RBC count, and MCV, 5 mL of blood were drawn into Vacutainer tubes, containing EDTA as an anticoagulant (K3 –EDTA 40 μL, 0.37 mol/L per tube), and analyzed within 12 h in an automated blood cell counter (Coulter Counter®, Coulter Electronics, Luton, UK). Body height and weight were measured, with subjects wearing light clothing and no shoes. BMI was calculated as weight in kilograms, divided by the square of height in meters (kg/m2). Information on current smoking was collected from a self-administered questionnaire. The smoking questions were “Do you smoke: cigarettes or cigars/cigarillos or a pipe daily?” (Yes= yes to any of these questions, No = No to all of these questions).
Records of venous thromboembolism
All first lifetime events of VTE were identified by searching the hospital discharge diagnosis registry, the autopsy registry, and the radiology procedure registry at the University Hospital of North Norway, from date of enrollment in the Tromsø study, to September 1st, 2007. The University Hospital of North Norway is the only hospital in the region, and all hospital care and relevant diagnostic radiology procedures in the Tromsø municipality are provided exclusively by this hospital. The hospital discharge diagnosis registry includes records on both outpatient clinic visits and inpatient hospital admissions. The relevant discharge codes were ICD-9 codes 325, 415.1, 451, 452, 453, 671.3, 671.4, 671.9 for the period 1994–1998 and ICD-10 codes I80.0-I80.3, I80.8, I80.9, I81, I82.0-I82.3, I82.8, I82.9, I67.6, O22.3, O22.5, O87.1, O87.3, I26.0 and I26.9 for the period 1999–2007. An additional search through the computerized index of autopsy diagnoses was conducted, and cases diagnosed with VTE, either as a cause of death, or as a significant condition, were identified. We also searched the radiology database in order to identify cases of objectively confirmed VTE that had been missed because of coding errors in the hospital discharge diagnosis registry. All relevant diagnostic procedures, performed at the Department of Radiology to diagnose VTE during the 13-year period, were systematically reviewed by trained personnel and cases with objectively confirmed VTE were identified.
The medical records for each potential case of VTE, derived from the hospital discharge diagnosis registry, the autopsy registry, or the radiology procedure registry, were reviewed by trained personnel. For cases identified from the hospital discharge diagnosis registry and the radiology procedure registry, an episode of VTE was verified and recorded as a validated outcome when all four of the following criteria were fulfilled; (i) objectively confirmed by diagnostic procedures (compression ultrasonography, venography, spiral computed tomography, perfusion-ventilation scanning, pulmonary angiography or autopsy); (ii) the medical record indicated that a physician had made a diagnosis of deep vein thrombosis or pulmonary embolism; (iii) signs and symptoms consistent with deep vein thrombosis or pulmonary embolism were present; and (iv) the patient received therapy with anticoagulants (heparin, warfarin, or a similar agent) or thrombolytics, or underwent vascular surgery. For cases identified from the autopsy registry, a venous thromboembolic event was recorded as an outcome when the autopsy record indicated VTE as cause of death or as a significant condition.
A venous thromboembolic event was further classified as unprovoked or provoked, based on the presence of provoking factors at the time of the diagnosis of the VTE. A VTE occurring without any provoking factor was defined as unprovoked, whereas a VTE occurring in the presence of one or more provoking factors was defined as provoked. The following were regarded as provoking factors: recent surgery or trauma (within 8 weeks preceding the event), an acute medical condition (acute myocardial infarction, acute ischemic stroke, major infectious disease), cancer, marked immobilization (bed rest >3 days, wheelchair, long distance travel ≥4 h within the last 14 days), or other provoking factor specifically described by a physician in the medical records (e.g. intravascular catheter).
Statistical analyses
For each participant, person-years of follow-up were accrued from the date of enrollment in 1994–95 through to the date a venous thromboembolic event was first diagnosed, the date the participant died or moved from the municipality of Tromsø, or through to the end of the study period, September 1st, 2007.
Statistical analysis was carried out using SPSS version 15.0 (SPSS Inc. Chicago, IL, USA). Cox-proportional hazards regression models were used to estimate hazard ratios, with 95% confidence intervals (95% CI), for total (unprovoked + provoked) and unprovoked VTE. Hazard ratios for hematocrit, hemoglobin, RBC count, and MCV were estimated in analyses adjusted for age, and in a multivariable model including age, smoking, and BMI as covariates. There were no statistical interactions between the hematologic variables and gender. However, gender-specific analyses were carried out for the sake of interest, because the normal ranges of hematocrit, hemoglobin, and RBC count are generally higher in men than in women.13 In order to explore the impact of various levels of hematocrit and hemoglobin on the risk of total VTE, hematocrit and hemoglobin were modeled into three intervals representing the lowest 40th, the middle 40th and the highest 20th percentiles (hematocrit: <39, 39–41, and ≥42 % in women and <43, 43–45, ≥46% in men; hemoglobin: <13.2, 13.2–14.0, ≥14.1 g/dL in women and <14.7, 14.7–15.5, ≥15.6 g/dL in men). In these analyses, the lowest interval was used as the reference group. The proportional hazard assumption was verified by evaluating the parallelism between the curves of the log-log survivor function for quartiles of the hematologic variables.
Results
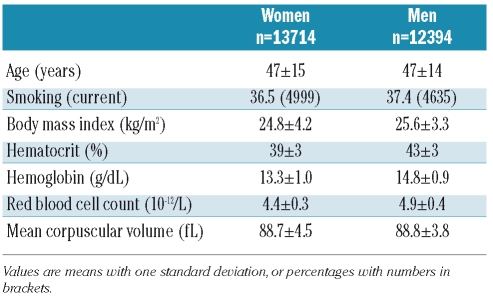
There were 447 validated first venous thromboembolic events during 282,494 person-years of follow-up (median 12.5 years). The overall crude incidence rate of VTE was 1.58 per 1,000 person-years. The baseline characteristics of the men and women enrolled in the study are shown in Table 1.
Table 1.
Baseline characteristics of subjects enrolled in the Tromsø study 1994–95.
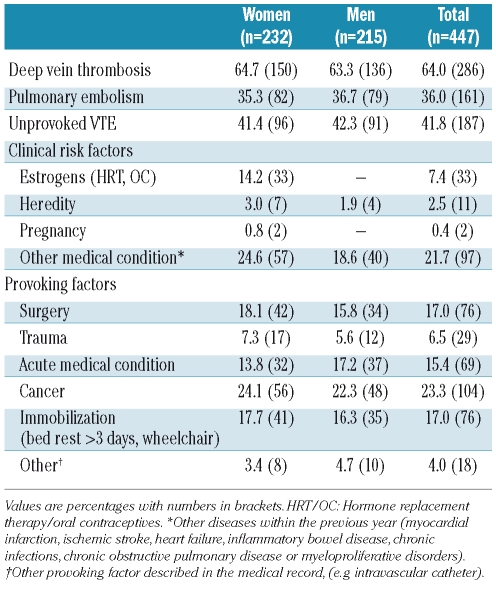
The characteristics of the VTE patients at the time of their venous thromboembolic event are shown in Table 2. Sixty-four percent had deep vein thrombosis and 36% had pulmonary embolism with or without concurrent deep vein thrombosis. A total of 187 (42%) venous thromboembolic events were classified as unprovoked (Table 2). Cancer was the most common provoking factor, and 23.3% of the VTE patients had active cancer at the time of the diagnosis of VTE (Table 2).
Table 2.
Characteristics of VTE patients (n= 447). The Tromsø study, 1994–2007.
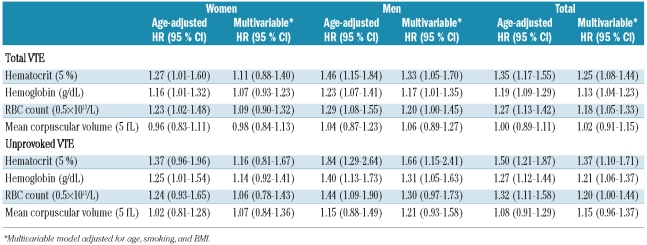
Hematocrit was significantly associated with an increased risk of VTE (Table 3). A 5% increase in hematocrit resulted in an age-adjusted hazard ratio of total VTE of 1.27 (95% CI: 1.01–1.60) in women, 1.46 (95% CI: 1.15–1.84) in men, and 1.35 (95% CI: 1.17–1.55) in both genders pooled together (Table 3). In men, the association remained significant in the multivariable model adjusted for age, smoking, and BMI (hazard ratio: 1.33, 95% CI: 1.05–1.70). When analyzing the related hematologic variables, hemoglobin and RBC count were significantly associated with increased risk of VTE, whereas MCV did not show any association with VTE (Table 3). For hemoglobin, a 1 g/dL increase was associated with an age-adjusted hazard ratio of total VTE of 1.16 (95% CI: 1.01–1.32) in women, 1.23 (95% CI: 1.07–1.41) in men, and 1.19 (95% CI: 1.09–1.29) in the total population. An increase in RBC count of 0.5×1012/L resulted in a hazard ratio of total VTE of 1.23 (95% CI: 1.02–1.48) in women and 1.29 (95% CI: 1.08–1.55) in men. When adjusted for age, smoking, and BMI in the multivariable model, the hazard ratios for hematocrit, hemoglobin, and RBC count were all slightly attenuated, but remained statistically significant in men and in both genders pooled together, whereas they were no longer statistically significant in women only. A similar tendency was apparent in separate analyses of unprovoked VTE. In these analyses, the estimated hazard ratios were slightly higher than for total VTE, and hematocrit and hemoglobin were significantly associated with unprovoked VTE in men and in the total population, but not in women. No association was found between MCV and either total or unprovoked VTE (Table 3).
Table 3.
Gender-specific hazard ratios (HR) with 95% confidence interval (CI) for total and unprovoked VTE. The Tromsø study 1994–2007.
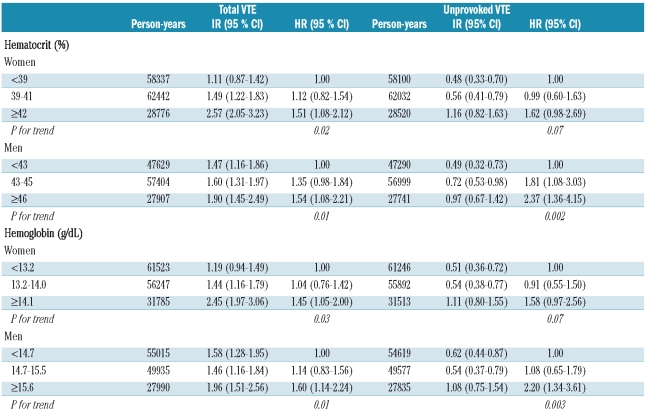
Crude incidence rates and age-adjusted hazard ratios for total and unprovoked VTE by categories of hematocrit and hemoglobin are shown in Table 4. The incidence rate of total VTE increased across categories of hematocrit from 1.11 to 2.57 per 1,000 in women, and from 1.47 to 1.90 in men. Women with a hematocrit of 42% or more had an age-adjusted 1.51-fold higher risk of total VTE (95% CI: 1.08–2.12) and 1.62-fold higher risk of unprovoked VTE (95% CI: 0.98–2.69) compared to women with a hematocrit less than 39% (P for trend: 0.02 and 0.07, respectively) (Table 4). Similarly, men with a hematocrit of 46% or more had a 1.54-fold higher risk of total VTE (95% CI: 1.08–2.21) and 2.37-fold higher risk of unprovoked VTE (95% CI: 1.36–4.15) compared to those with a hematocrit less than 43% (P for trend: 0.01 and 0.002, respectively).
Table 4.
Gender-specific crude incidence rates (IR) and age-adjusted hazard ratios (HR) with 95% confidence interval (CI), by levels of hematocrit and hemoglobin. The Tromsø study, 1994–2007.
Similar patterns for risk of VTE were found for hemoglobin. Women with a hemoglobin concentration of 14.1 g/dL or more had a 1.45-fold (95% CI: 1.05–2.00) higher risk of total VTE compared to women with a hemoglobin level less than 13.2 g/dL (P for trend: 0.03), and men with a hemoglobin 15.6 g/dL or more had a 1.6-fold (95% CI: 1.14–2.24) higher risk of total VTE and 2.2-fold higher risk of unprovoked VTE compared to men with whose hemoglobin concentration was less than 14.7 g/dL (P for trend: 0.01 and 0.003, respectively) (Table 4).
Discussion
The purpose of our prospective cohort study was to investigate the impact of hematocrit and related hematologic variables on the risk of VTE in a general population. The risks of total and unprovoked VTE were significantly associated with hematocrit in both continuous and category-based analyses. Risk estimates were higher for men than for women. In the multivariable model treating hematocrit as a continuous variable, hematocrit remained significant in men but not in women, suggesting that hemorheological factors might contribute more to the risk of VTE in men than in women. Similar findings were found for hemoglobin and RBC count, whereas MCV was not associated with VTE. In category-based analysis, men and women whose hematocrit values were in the upper 20th percentile had a 1.5-fold higher risk of total VTE and a 2.4-fold and 1.6-fold, respectively, higher risk of unprovoked VTE compared to subjects whose hematocrit was in the lowest 40th percentile. Our findings suggest that hematocrit is preferable to other related hematologic variables for assessing risk of VTE because of stronger risk estimates in both genders.
The risk of VTE by hematocrit has not been extensively examined in prospective studies of general populations. The LITE study,14 a prospective cohort of 19,923 subjects aged 45 years and above, which primarily investigated the association between traditional cardiovascular risk factors and VTE, found no increased risk of VTE by increasing tertiles of either hematocrit or hemoglobin. However, the cut-off levels for the highest tertile were 43.5% for hematocrit and 14.5 g/dL for hemoglobin, and further levels of these variables were not explored.14 In a small case-control study by Vaya et al.,15 the proportion of subjects with a hematocrit above 45% was significantly greater among VTE cases than among healthy controls.
Recent studies have suggested a link between VTE and arterial cardiovascular disease.16–18 Whether this apparent association is caused by sharing of common traditional cardiovascular risk factors or through other yet unknown risk factors or mechanism(s) is unknown. Hematocrit levels above the normal range are associated with increased risk of both arterial and venous thrombosis.8–12 High levels of hematocrit have also shown significant associations with coronary heart disease in some studies of general populations,19 implying that hematocrit, a factor that affects hemorheology, might contribute to the observed link between arterial and venous thrombosis.
Several studies support the concept that hematocrit has an influence on hemostasis and thrombosis. Low hematocrit is associated with an increased risk of bleeding, and this effect can be corrected by transfusion.7 Furthermore, studies of thrombocytopenic patients have shown improvement in bleeding times after transfusion, despite a lack of increase in platelet count.20 Increased hematocrit has been the only predictive factor of venous and arterial thrombosis in subjects with polycythemia vera, whereas high platelet counts and abnormal platelet function were not significantly linked to polycythemia vera-associated thrombosis.9,11,12 Rheological therapies, including compression devices21 and hemodilution with dextran,22 have been shown to reduce the risk of deep vein thrombosis in patients with polycythemia vera.
Hematocrit is one of the major determinants of blood viscosity,5 and increases in the hematocrit might favor clot formation by increasing the residence time of circulating platelets and coagulation factors adjacent to dysfunctional endothelium. Furthermore, elevated hematocrit has been shown to promote the transport of platelets towards the vessel wall, thereby increasing the interaction with the vasculature.23 Blood clotting is also dependent on the velocity gradient at which the clotting takes place. The influence of the hematocrit on blood viscosity increases when the shear rate decreases.5,24 Under low-flow conditions, such as in the venous system, increases in hematocrit may have a strong influence on blood flow and, thereby, clinical outcome.
RBC mass may also have a direct effect on thrombotic mechanisms. A recent experimental study investigating the effect of RBC on thrombin generation, showed that the total amount of thrombin activity generated, and the maximum concentration of thrombin achieved, was proportional to the hematocrit.25 RBC may also take part in thrombus formation by other mechanisms, such as stimulation of platelet aggregation through ADP release,26–28 or possibly through the exposure of phosphatidylserine.29
The main strengths of our study are its prospective design, the large number of participants recruited from a general population with high attendance rate, the long-term follow-up, and the validation of the venous thromboembolic events. All hospital care and radiological imaging in the region is provided exclusively by a single hospital, which enhances the possibility of a complete VTE registry. However, the study has some limitations. Modifiable risk factors, such as hematocrit and related hematologic variables, are a potential weakness of cohort studies, especially when the time between exposure and disease manifestation is long. However, this type of non-differential misclassification generally leads to underestimation of the true associations. In our study, smoking was assessed as a dichotomous variable (current smoker: yes/no). Thus, we were unable to take into consideration the potential dose-dependent effect of smoking on hematocrit, and thereby unable to remove all possible confounding by smoking. Underlying medical conditions such as lung, heart and kidney diseases can influence the level of hematocrit. In our study, information on other medical conditions was only available for those who developed VTE. In men, the presence of other medical conditions did not differ across various levels of hematocrit (data not shown). In women, the presence of other medical conditions was slightly higher among those whose hematocrit was in the upper 20th percentile. Since there was no available clinical information on underlying disease during follow-up in the reference group, we did not have sufficient clinical information for the total population to consider underlying disease as a possible cause of high hematocrit and risk of thrombosis in detail.
In conclusion, hematocrit and the related variables hemoglobin and RBC count were identified as risk factors for VTE in our prospective, population-based study. The size of red blood cells, expressed as MCV, was not associated with VTE. Our findings may suggest that hematocrit should be taken into consideration for risk assessment in VTE.
Footnotes
Authorship and Disclosures
SKB and JBH were the principal investigators. SKB was involved in data collection, analysis of the data, and was the leading author in drafting the manuscript. JBH was the main contributor to the conception and design of the study, and participated in interpretation of the results and revision of the manuscript. EBM and IN contributed to the study design and acquisition of data. TW provided statistical assistance. All authors participated in critical revision of the entire manuscript, and approved the final version of the manuscript. The authors reported no potential conflicts of interest.
References
- 1.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–7. doi: 10.1111/j.1538-7836.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosendaal FR. Venous thrombosis: a multi-causal disease. Lancet. 1999;353(9159):1167–73. doi: 10.1016/s0140-6736(98)10266-0. [DOI] [PubMed] [Google Scholar]
- 3.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 4.Virchow R. Thrombose und Embolie (1846–1856) Leipzig, Germany: Verlag von Johann Ambrosius Barth; 1910. [Google Scholar]
- 5.Wells RE, Jr, Merrill EW. Influence of flow properties of blood upon viscosity-hematocrit relationships. J Clin Invest. 1962;41:1591–8. doi: 10.1172/JCI104617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyton AC, Richardson TQ. Effect of hematocrit on venous return. Circ Res. 1961;9:157–64. doi: 10.1161/01.res.9.1.157. [DOI] [PubMed] [Google Scholar]
- 7.Hellem AJ, Borchgrevink CF, Ames SB. The role of red cells in haemostasis: the relation between haematocrit, bleeding time and platelet adhesiveness. Br J Haematol. 1961;7:42–50. doi: 10.1111/j.1365-2141.1961.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 8.Gruppo Italiano Studio Policitemia. Polycythemia vera: the natural history of 1213 patients followed for 20 years. Ann Intern Med. 1995;123(9):656–64. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Boneu B. Laboratory investigations and prediction of thrombotic risk in polycythemia vera. Nouv Rev Fr Hematol. 1994;36(2):183–5. [PubMed] [Google Scholar]
- 10.Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;2(8102):1219–22. doi: 10.1016/s0140-6736(78)92098-6. [DOI] [PubMed] [Google Scholar]
- 11.Schafer AI. Bleeding and thrombosis in the myeloproliferative disorders. Blood. 1984;64(1):1–12. [PubMed] [Google Scholar]
- 12.Wehmeier A, Daum I, Jamin H, Schneider W. Incidence and clinical risk factors for bleeding and thrombotic complications in myeloproliferative disorders. A retrospective analysis of 260 patients. Ann Hematol. 1991;63(2):101–6. doi: 10.1007/BF01707281. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon DR, Zhang TJ, Brand FN, Kannel WB. Hematocrit and the risk of cardiovascular disease--the Framingham study: a 34-year follow-up. Am Heart J. 1994;127(3):674–82. doi: 10.1016/0002-8703(94)90679-3. [DOI] [PubMed] [Google Scholar]
- 14.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162(10):1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 15.Vayá A, Mira Y, Martínez M, Villa P, Ferrando F, Estellés A, et al. Biological risk factors for deep vein trombosis. Clin Hemorheol Microcirc. 2002;26(1):41–53. [PubMed] [Google Scholar]
- 16.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 17.Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, Lensing AW, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348(15):1435–41. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet. 2007;370(9601):1773–9. doi: 10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- 19.Danesh J, Collins R, Peto R, Lowe GD. Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J. 2000;21(7):515–20. doi: 10.1053/euhj.1999.1699. [DOI] [PubMed] [Google Scholar]
- 20.Ho CH. The hemostatic effect of adequate red cell transfusion in patients with anemia and thrombocytopenia. Transfusion. 1996;36(3):290. doi: 10.1046/j.1537-2995.1996.36396182154.x. [DOI] [PubMed] [Google Scholar]
- 21.Allan A, Williams JT, Bolton JP, Le Quesne LP. The use of graduated compression stockings in the prevention of postoperative deep vein thrombosis. Br J Surg. 1983;70(3):172–4. doi: 10.1002/bjs.1800700311. [DOI] [PubMed] [Google Scholar]
- 22.Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al. Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis. Health Technol Assess. 2005;9(49):1–78. doi: 10.3310/hta9490. [DOI] [PubMed] [Google Scholar]
- 23.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207(4430):541–3. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 24.Dintenfass L. Viscosity and clotting of blood in venous thrombosis and coronary occlusions. Circ Res. 1964;14:1–16. doi: 10.1161/01.res.14.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Horne MK, 3rd, Cullinane AM, Merryman PK, Hoddeson EK. The effect of red blood cells on thrombin generation. Br J Haematol. 2006;133(4):403–8. doi: 10.1111/j.1365-2141.2006.06047.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaarder A, Jonsen J, Laland S, Hellem A, Owren PA. Adenosine diphosphate in red cells as a factor in the adhesiveness of human blood platelets. Nature. 1961;192:531–2. doi: 10.1038/192531a0. [DOI] [PubMed] [Google Scholar]
- 27.Santos MT, Valles J, Marcus AJ, Safier LB, Broekman MJ, Islam N, et al. Enhancement of platelet reactivity and modulation of eicosanoid production by intact erythrocytes. A new approach to platelet activation and recruitment. J Clin Invest. 1991;87(2):571–80. doi: 10.1172/JCI115032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valles J, Santos MT, Aznar J, Marcus AJ, Martinez-Sales V, Portoles M, et al. Erythrocytes metabolically enhance collagen-induced platelet responsiveness via increased thromboxane production, adenosine diphosphate release, and recruitment. Blood. 1991;78(1):154–62. [PubMed] [Google Scholar]
- 29.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89(4):1121–32. [PubMed] [Google Scholar]