Abstract
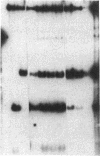
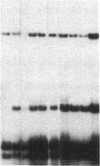
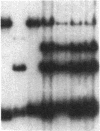
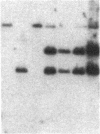
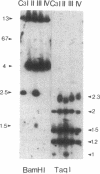
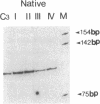
We transformed the ciliate Tetrahymena thermophila by microinjection of circular plasmids containing the ribosomal RNA gene (rDNA). In the somatic macronucleus of Tetrahymena, the rDNA is in the form of linear palindromic molecules. The rDNA molecules from the C3 strain have a replication advantage over rDNA from both B strain and the C3 rDNA mutant rmm1. We constructed two circular plasmids carrying replication origin sequences from C3 rDNA and a point mutation (Pmr) in the 17S rRNA gene that confers resistance to the antibiotic paromomycin. One plasmid contained a single complete copy of the rRNA gene and its flanking sequences, while the other had an additional rDNA origin of replication. In all B or rmm1 Tetrahymena cell lines transformed with the plasmids, rDNA sequences from the plasmid were found in palindromic rDNA molecules. In one transformant line, a small amount of the plasmid was also retained in a form with the original circular restriction map. Our results show that the plasmids underwent homologous recombination with one arm of the endogenous rDNA to give heteropalindromic rDNA, or with both arms of the palindrome to form homopalindromic rDNA. The resulting recombinant molecules were able to replace the recipient's original rDNA completely, providing strong evidence that C3 rDNA sequences in the donor DNAs confer a replication advantage over recipient rDNA. Thus microinjection of circular plasmids provides a method for replacement of an endogenous gene or gene fragment with exogenous sequences.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H., Budarf M. L., Challoner P. B., Cherry J. M., Howard E. A., Katzen A. L., Pan W. C., Ryan T. DNA termini in ciliate macronuclei. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1195–1207. doi: 10.1101/sqb.1983.047.01.135. [DOI] [PubMed] [Google Scholar]
- Brunk C. F., Bohman R. E. Analysis of nuclei from exponentially growing and conjugated Tetrahymena thermophila using the flow microfluorimeter. Exp Cell Res. 1986 Feb;162(2):390–400. doi: 10.1016/0014-4827(86)90344-7. [DOI] [PubMed] [Google Scholar]
- Challoner P. B., Amin A. A., Pearlman R. E., Blackburn E. H. Conserved arrangements of repeated DNA sequences in nontranscribed spacers of ciliate ribosomal RNA genes: evidence for molecular coevolution. Nucleic Acids Res. 1985 Apr 11;13(7):2661–2680. doi: 10.1093/nar/13.7.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg J. Strong sequence conservation of a 38 bp region near the center of the extrachromosomal rDNA palindrome in different Tetrahymena species. Nucleic Acids Res. 1983 Jul 25;11(14):4939–4946. doi: 10.1093/nar/11.14.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Kiss G. B., Pearlman R. E. Extrachromosomal rDNA of Tetrahymena thermophila is not a perfect palindrome. Gene. 1981 Apr;13(3):281–287. doi: 10.1016/0378-1119(81)90032-9. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Løvlie A., Haller B. L., Orias E. Molecular evidence for somatic recombination in the ribosomal DNA of Tetrahymena thermophila. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5156–5160. doi: 10.1073/pnas.85.14.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W. C., Orias E., Flacks M., Blackburn E. H. Allele-specific, selective amplification of a ribosomal RNA gene in Tetrahymena thermophila. Cell. 1982 Mar;28(3):595–604. doi: 10.1016/0092-8674(82)90214-8. [DOI] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Tondravi M. M., Yao M. C. Transformation of Tetrahymena thermophila by microinjection of ribosomal RNA genes. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4369–4373. doi: 10.1073/pnas.83.12.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]