Abstract
AIM: To analyze the expression of 8 putative cancer stem cell (CSC) markers within colorectal cancer tumor buds and to determine their prognostic impact in patients with this disease.
METHODS: Immunohistochemistry was performed on 101 colorectal cancer resections for CK22 (to identify tumor buds) as well as CD133, CD166, CD24, CD44s, CD90, EpCAM, ALDH1, and ABCG5, and their expression within tumor buds was evaluated.
RESULTS: CD90, CD44s, and CD133 expression in tumor buds was found in less than 5% of all cases. ALDH1, CD24, CD166 were expressed in 16.5%, 16.2%, and 34% cases, respectively, while ABCG5 and EpCAM expression was more frequent and found in 35% and 69% of cases, respectively. Of the 8 markers studied, EpCAM and ABCG5 positivity in tumor buds were significantly associated with poor prognosis (P = 0.023, P = 0.038, respectively) in multivariable analysis with pT and pN classification [P = 0.048; hazard ratio (HR): 2.64; 95% CI: 1.0-6.9, for EpCAM and P = 0.029; HR: 2.22; 95% CI: 1.0-4.5, for ABCG5]. Poor survival time was particularly striking for lymph node-negative patients with ABCG5-positive buds (P < 0.001).
CONCLUSION: Expression of putative stem cell markers EpCAM and ABCG5 within the tumor buds of colorectal cancer are frequently noted and are associated with poor prognosis.
Keywords: Colorectal cancer, Cancer stem cells, Tumor budding, ABCG5, Prognosis
INTRODUCTION
In 1985, Gabbert and colleagues described a peculiar feature at the invasive border of differentiated colonic tumors: neoplastic glands irregularly arranged into small strands or single cells without junctional complexes and often missing even rudimentary basement membranes[1,2]. Their observation of the tumor front of differentiated adenocarcinomas focally acquiring the phenotype of undifferentiated tumors is credited for pioneering the concept commonly referred to today as epithelial mesenchymal transition (EMT) and represented in colorectal cancer by its histological hallmark “tumor budding”.
Defined as single cells or clusters of up to 4 or 5 cells at the invasive tumor front, tumor budding can easily be spotted using pan-cytokeratin stains and is highly associated with an infiltrating tumor border configuration[3]. The adverse prognostic impact of tumor budding in colorectal cancer has consistently been reported and recognized by the American Joint Committee on Cancer/Union International Contre le Cancer (AJCC/UICC) as an additional prognostic factor to complement Tumor Node Metastases (TNM) staging[4-13]. Moreover, tumor budding is frequently linked to high-grade tumors, lymph node positivity, vascular and lymphatic invasion, as well as to both local tumor recurrence and distant metastasis[11,14-17].
Several lines of evidence seem to suggest that tumor buds may, to some extent, represent malignant colorectal cancer stem cells (CSC) because of their potential for migration and re-differentiation locally and at sites of metastasis[18]. “Pseudopodia-like” cytoplasmic protrusions have been described in tumor buds, which seem to be in direct contact with adjacent interstitial tissue suggesting their formation during cell migration[2,19,20]. Previous studies on EMT and events occurring at the invasive tumor front implicate, in particular, Wnt pathway signaling in the process of tumor budding[21]. This is evidenced by increased β-catenin immunohistochemical staining in tumor buds, a concomitant loss of E-cadherin, as well as overexpression of laminin5γ2 along with activation of transcriptional repressors SLUG, and ZEB1[19,22,23]. Other groups have described changes in the expression of several matrix metalloproteinases (MMP-2, MMP-7, MMP-9), and extensive staining of β(III)-tubulin, a major constituent of microtubules, all suggestive of invasion and migration potential of tumor buds[24-26]. Together with loss of epithelial-like properties and cell-cell adhesion, in addition to the ability to re-differentiate at distant sites, the hypothesis that tumor buds could represent putative migrating stem cells is not far-fetched.
Phenotypic characterization of colorectal CSC is still debated although putative CSC populations have been identified in several solid tumors based on functional stem cell-like properties and expression of specific markers. Recently, 4 such markers have been proposed for colorectal cancer; CD133, a glycoprotein expressed on CD34+ stem and progenitor cells in fetal liver, endothelial precursors and fetal neural stem cells; CD44s, an adhesion molecule with roles in signaling, migration, and homing, EpCAM, a homophilic Ca2+-independent cell adhesion molecule expressed on the basolateral surfaces of most epithelial cells; and CD166 or activated leukocyte cell adhesion molecule (ALCAM) known as a mesenchymal stem cell marker[27]. Other putative stem cell markers have also generated interest in other tumor types including ABCG5, a member of the ATP binding cassette family involved in transport of sterol and other lipids, ALDH1, a member of the aldehyde dehydrogenase family of enzymes with roles in proliferation, differentiation, and survival, CD24, an adhesion molecule and ligand for P-selectin, and CD90, a mediator of thymocyte adhesion to thymic stroma[28].
Considering the apparent stem cell-like properties of tumor buds and adverse effect of budding on clinical outcome, we hypothesized that expression of a subset of these 8 putative stem cell markers could have significant implications for prognosis in patients with positive tumor budding. Thus, the aim of this study was to determine the impact of CD166, CD44s, EpCAM, ALDH1, CD133, CD24, CD90, and ABCG5 expressed within tumor buds on prognosis in patients with colorectal cancer.
MATERIALS AND METHODS
Patients
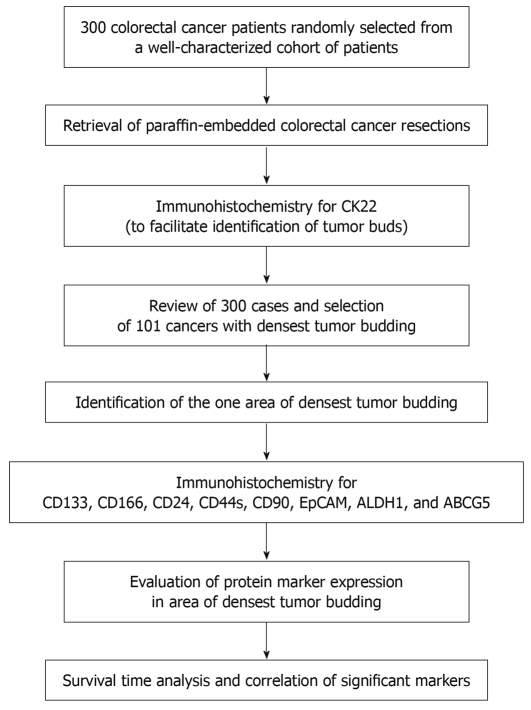
Three hundred patients with pre-operatively untreated tumors who underwent tumor resection between 1987 and 1996 at the University Hospital of Basel, Switzerland were initially included in this study. These patients were randomly selected from a larger previously described cohort of 938 colorectal cancer patients with full clinico-pathological information[29]. Histopathological features were re-reviewed from the corresponding hematoxylin and eosin slides by an experienced gastrointestinal pathologist (LT) and included histological subtype, pT classification, pN classification, tumor grade, and vascular invasion. Tumor border configuration and peritumoral lymphocytic inflammation were diagnosed according to Jass et al[30]. Clinical data were retrieved from patient reports including age at diagnosis, tumor diameter, and tumor location. The clinical endpoint of interest was cancer-specific survival time. Censored observations included patients who died for reasons other than colorectal cancer, who were alive or who were lost to follow-up. The study design is outlined in Figure 1.
Figure 1.
Study design.
Specimen characteristics
The paraffin-embedded colorectal cancer resection specimens for all 300 patients were retrieved from the archives of the Institute of Pathology, University Hospital of Basel as well as at the Institute of Clinical Pathology, Basel, Switzerland. The use of material for this study was approved by the local ethics committee of the University of Basel.
Assay methods
Immunohistochemistry for CK22 staining: All 300 specimens were cut at 4 μm and underwent immunostaining for CK22, a marker of epithelial cells that served to highlight areas of tumor budding, and which is routinely performed in our laboratories for diagnostic purposes. Briefly, tissues were de-waxed and re-hydrated in dH2O. Following pressure cooker-mediated antigen retrieval in 0.001 mol/L ethylenediaminetetraacetic acid pH 8.0, endogenous peroxidase activity was blocked using 0.5% H2O2. Sections were incubated with 10% normal goat serum for 20 min. After incubation with primary antibody (CK22 polyclonal, Genetex, Inc., 1:100), sections were incubated with horseradish peroxidase-conjugated secondary antibody (DakoCytomation) for 30 min at room temperature, immersed in amino-ethylcarbazole (DakoCytomation) for 30 min, and counterstained with hematoxylin.
Selection of densest budding cases: All 300 cases were evaluated using a 10 × magnification for the presence of tumor budding (AL). Since this study was designed to focus on expression of putative stem cell markers within the tumor buds themselves, cases with the densest number of budding cells were selected for the analysis (n = 101). These 101 cases were then carefully re-scored for tumor budding according to the method proposed by Ueno et al[11]. Briefly, the tumor border was scanned at 10 × power and the area of most dense budding identified. In the center of this area, tumor buds (single cells or clusters of up to 5 cells) were counted at 20 × magnification. In order to locate this same region of dense budding on serial sections, the area was circled with a felt-tip pen. The clinico-pathological features for these 101 patients are outlined in Table 1.
Table 1.
Patient characteristics (n = 101)
| Clinico-pathological features | Frequency n (%) | |
| Gender (n = 101) | Female | 63 (62.4) |
| Male | 38 (37.6) | |
| Tumor location (n = 101) | Left-sided | 64 (63.4) |
| Right-sided | 37 (36.6) | |
| Histological subtype (n = 101) | Mucinous | 7 (6.9) |
| Non-mucinous | 94 (93.1) | |
| pT classification (n = 99) | pT1-2 | 16 (16.2) |
| pT3-4 | 83 (83.8) | |
| pN classification (n = 100) | pN0 | 52 (52.0) |
| pN1-2 | 48 (48.0) | |
| Tumor grade (n = 99) | G1-2 | 92 (92.9) |
| G3 | 7 (7.1) | |
| Vascular invasion (n = 99) | Absence | 80 (80.8) |
| Presence | 19 (19.2) | |
| Tumor border configuration (n = 99) | Pushing | 23 (23.2) |
| Infiltrating | 76 (76.8) | |
| Peritumoral lymphocytic inflammation (n = 99) | Absent | 79 (79.8) |
| Present | 20 (20.2) | |
| Age (n = 101) | Mean (range) | 67.4 (41-89) |
| Tumor diameter (n = 101) | Mean (range) | 54.9 (20-170) |
| 5-year survival rate (n = 101) | % (95% CI) | 69.3 (59-78) |
Immunohistochemistry for putative stem cell markers: Following a similar protocol as described above, the 101 cases with densest tumor budding were immunostained for CD166 (clone 110G/07; 1:200; Novocastra), CD44s (clone DF1485; 1:50; Dako), EpCAM (clone VU-1D9; 1:200; Cell Signaling), ALDH1 (isoform α1, clone Polyclonal; 1:500; AbCam), CD133 (clone 24139; 1:100; Cell Signaling), ABCG5 (1:200, Sigma-Aldrich), CD90 (clone 5E10, 1:100, BD Pharmingen), CD24 (clone SN3B, Neomarkers, 1:100). CD133, CD166, CD44, CD24, CD90, EpCAM, and ABCG5 were evaluated for both membrane and cytoplasmic staining; ALDH1 was exclusively evaluated in the cytoplasm of tumor buds. The number of CD166, CD44s, EpCAM, ALDH1, CD133, CD24, CD90, and ABCG5 positive tumor buds was then evaluated in the area of densest tumor budding as determined by CK22 staining.
Statistical analysis
Univariate survival analysis was carried out using the Kaplan-Meier method and log rank test. Two multivariable Cox regression analyses were performed. First, to test the independent prognostic value of tumor budding, the effects of pT stage, pN stage, tumor grade, and vascular invasion were adjusted for. Subsequently, because of the small number of positive cases, only 2 variables could be entered into the multivariable Cox regression analysis along with positive expression of the protein in tumor buds, hence pT classification and pN classification were selected. The assumption of proportional hazards was verified prior to this analysis. Hazard ratios (HR) and 95% CI were obtained to determine the prognostic effect of positive cases adjusting for pT and pN. Kendall’s correlation coefficient (r) was obtained for correlation analysis of markers. P < 0.05 was considered statistically significant.
RESULTS
Prognostic value of tumor budding
In order to confirm the prognostic value of tumor budding in our series, cases were divided into 3 groups based on the distribution of number of tumor buds: those with < 40 buds, between 41-60 buds, and finally those with > 60 buds per 20 × field. The greater the number of tumor buds the more unfavorable was the prognosis both in univariate (P < 0.001) and multivariable analysis with pT, pN, tumor grade, and vascular invasion (HR: 1.6, 95% CI: 1.2-2.1).
Expression of putative stem cell markers within tumor buds
CK22 staining was used to identify regions of densest tumor budding with epithelial cells exclusively immunoreactive for the protein. Staining for ABCG5, ALDH1, CD133, CD166, CD24, and CD44s could be observed in both tumor cells and inflammatory or stromal cells. EpCAM staining was predominantly limited to expression in tumor cells whereas CD90 was almost always expressed by stromal cells and only in 3 cases in the tumor itself.
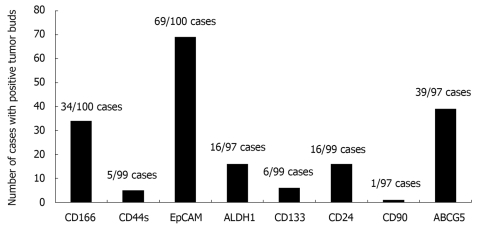
Marker expression was then evaluated in the area of densest budding. Representative immunostains for all markers are shown in Figure 2. Only one case (1.03%) was positive for CD90, while 5 (5.1%) and 6 (6.1%) cases were positive for CD44s and CD133, respectively. On the other hand, a considerably larger number of positive cases was found to express ALDH1 (16/97, 16.5%), CD24 (16/99, 16.2%) and CD166 (34/100, 34%). Finally, ABCG5 and EpCAM staining were frequent events with 39/97 (40.2%) and 69/100 (69%) positive cases, respectively (Figure 3).
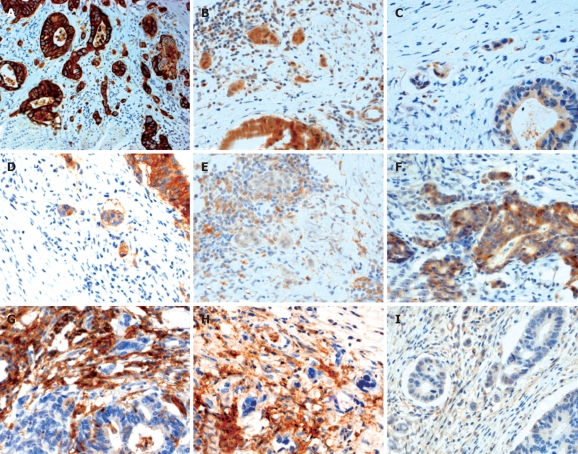
Figure 2.
Immunohistochemical expression of putative cancer stem cell markers by tumor buds in colorectal cancer. A: Cytokeratin 22 staining highlighting the presence of tumor buds in low power magnification (5 ×); B-I: 40 × magnification. Positive expression of ABCG5 (B), ALDH1 (C), and EpCAM (D) in tumor buds with scattered positive staining of stromal cells, absence of staining of CD133 in tumor buds with positive staining of stromal cells (E), positive expression of CD166 in tumor buds with occasional positivity of stromal cells (F), absence of CD24 (G), CD44s (H), and CD90 staining (I) in tumor buds with positive stromal cell expression.
Figure 3.
Histogram showing the number of cases with any degree of positive staining for the 8 putative stem cells markers.
Prognostic differences with putative stem cell marker expression in tumor buds
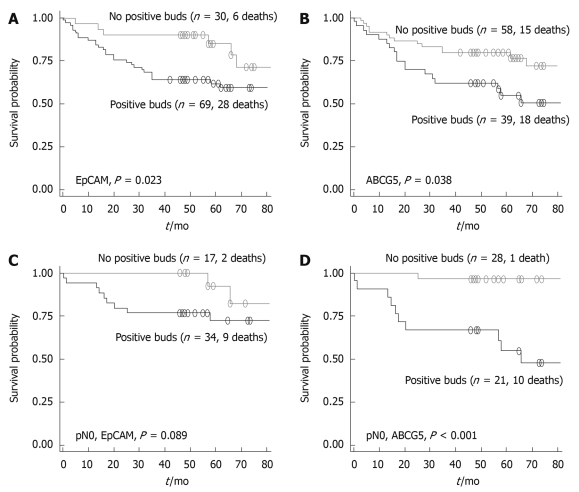
No relationship between survival time and ALDH1, CD24 and CD166 was observed. Patients with positive EpCAM or ABCG5 within tumor buds had a significantly poorer outcome in comparison to patients with no expression of these markers (P = 0.023 and P = 0.038, respectively) (Figure 4). Multivariable analysis was performed for EpCAM and ABCG5 along with pT and pN classification. EpCAM maintained its significant association with a negative effect on outcome (HR: 2.64, 95% CI: 1.0-6.9, P = 0.048), adjusted for pT and pN classification, a result which was also pronounced in patients with lymph node-negative disease. Similarly, positive ABCG5 expression in tumor buds was again associated with a poor patient prognosis (P = 0.029) underlined by a relative risk of death of 2.22 (95% CI: 1.0-4.5) compared to patients lacking expression of ABCG5. ABCG5-positive patients with lymph node-negative cancers had a particularly poor outcome in comparison to their node-negative and ABCG5-negative counterparts (P < 0.001).
Figure 4.
Kaplan-Meier survival curves illustrating the prognostic differences in patients with or without positive staining of EpCAM (A) and positive staining of ABCG5 (B) in tumor buds; differences in prognosis are further analyzed for lymph node-negative patients differing in EpCAM (C) and ABCG5 (D) expression in tumor buds.
Correlation between EpCAM and ABCG5 expression
In order to determine whether the same cases expressed both EpCAM and ABCG5, the correlation between these markers was tested. The correlation coefficient r = 0.17 and P = 0.08, indicated a positive but non-significant trend in the expression of these markers. Of the 96 patients evaluable for both EpCAM and ABCG5, 31 (32.3%) were positive and 21 (21.9%) were negative for both markers. We subsequently tested whether the combination of these markers could additionally stratify patients into prognostic subgroups. Prognosis was worse in patients positive for both EpCAM and ABCG5 (P = 0.013) with a relative risk of death of 2.39 (95% CI: 1.2-4.7) compared to patients negative for both. In comparison to the relative risk of death for either EpCAM or ABCG5 alone, the combination of both markers does not suggest a superior discrimination of patients into better and worse prognostic subgroups. A negative but statistically non-significant correlation between CD44s and EpCAM (r = -0.15, P = 0.145) and ABCG5 (r = -0.1, P = 0.328) was observed.
DISCUSSION
In this study we evaluated 8 of the most promising putative cancer stem cell markers CD166, CD44s, EpCAM, ALDH1, CD133, CD24, CD90, and ABCG5 and their expression in colorectal tumor buds using 101 whole tissue sections from a well-characterized cohort of patients. Our main findings suggest that positive expression of EpCAM and ABCG5 within tumor buds is a frequent event and may confer a significant and adverse prognosis in patients with colorectal cancer, particularly in lymph node-negative patients expressing ABCG5.
Several of these putative CSC markers have previously been evaluated in tumor buds. Horst et al[31] assessed CD133 in colorectal cancers using 3 different antibodies. They reported pronounced expression of CD133 in tumor glands close to the invasive margin but restricted to glandular differentiated cells and a general lack of CD133 in the tumor buds themselves. They further found that nuclear β-catenin expression and CD133 were not correlated and that the 2 protein markers may stain different, yet overlapping populations of tumor cells[32]. Our results of only a few CD133-positive tumor budding cases and no prognostic differences between patients with CD133-positive and -negative tumor budding are in line with these findings. Investigating rectal cancers, Gosens et al[33] found strong membranous EpCAM staining in the tumor center and a progressive loss at the tumor front associated with high tumor grade, tumor budding, and a poor local and distant recurrence-free survival. This was also accompanied by a concomitant increase in cytoplasmic EpCAM staining as well as overexpression of β-catenin. We also observed a pronounced loss of EpCAM toward the invasive tumor front, particularly in tumors with infiltrating margins, as well as a shift in localization of EpCAM expression from membrane to cytoplasm. The findings of this study indicate that despite this loss towards the border, patients with EpCAM-positive tumor buds have a most unfavorable survival time, a result which was maintained in multivariable analysis. Although EpCAM, like CD44, is known for its cell-adhesion function, it seems to have versatile roles in signaling, cell migration, proliferation, and differentiation depending on the microenvironment[34]. In the normal epithelium, EpCAM supports adhesion, whereas in carcinoma it seems to prevent strong cell-cell adhesion, enabling cell migration and metastasis similar to E-cadherin. The intracellular localization of EpCAM and its identification by immunohistochemistry may represent differential roles of this protein in colorectal cancer progression and partially explain why, despite loss of expression from normal at tumor center to tumor border, the positive expression in buds is linked to a poorer patient outcome.
Masaki et al[35] have also described associations between membranous CD44 and CD44v6 expression and a higher degree of tumor budding. However, it is unclear from these studies whether expression was evaluated in the tumor center, then correlated with tumor budding or whether expression was evaluated in buds themselves. Our group has also previously found that loss of membranous expression of both CD44s and CD44v6 within the tumor center is highly correlated with an infiltrating tumor border configuration, a result which is in line with the findings of this study showing only rare cases expressing CD44s in tumor buds, too few in fact for adequate survival analysis.
ABCG5 is a member of the ATP-binding cassette subfamily G and plays a role in the efflux transport of cholesterol[36,37]. Its expression has been correlated with clinical melanoma progression and it is hypothesized to contribute to the refractoriness of metastatic cancer to chemotherapy[38]. Indeed, specific targeting of ABCG5 with monoclonal antibodies appears to significantly inhibit cell growth. To date, ABCG5 does not appear to have been investigated in colorectal cancer, and moreover in tumor buds. However, our findings of ABCG5 expression in a considerable number of colorectal cancer tumor buds as well as an adverse prognosis in particular in patients with lymph node-negative disease suggests that the role of ABCG5 in colorectal pathogenesis warrants further investigation.
Our results of adverse prognosis in EpCAM-positive and ABCG5-positive patients may be to some extent affected by the lack of information regarding cancer treatment. Despite this limitation, the unfavorable outcome associated with EpCAM and, particularly with ABCG5-positivity was maintained in patients with lymph node-negative colorectal cancers who, by today’s treatment guidelines, are not generally considered for adjuvant chemotherapy[39]. The findings of this study regarding the prognostic value and expression of EpCAM and ABCG5 within colorectal tumor buds should be considered preliminary and require validation on independent patient cohorts.
To summarize, in contrast to CD133, CD166, CD24, CD44s, CD90, and ALDH1, the expression of putative stem cell markers EpCAM and ABCG5 within the tumor buds of colorectal tumors are frequent events indicating poor prognosis. In particular, patients with lymph node-negative disease expressing EpCAM or ABCG5 have a particularly unfavorable prognosis suggesting that the immunohistochemically analyzed EpCAM and ABCG5 in tumor buds may be useful biomarkers of poor outcome in this subgroup of patients. Further studies are necessary to address the important issue of whether EpCAM- or ABCG5-positive tumor buds indeed represent migrating colorectal CSC.
COMMENTS
Background
Tumor budding at the invasive tumor front of colorectal cancer is recognized as an important independent prognostic factor. Several lines of evidence seem to suggest that tumor buds may to some extent represent malignant colorectal cancer stem cells because of their potential for migration and re-differentiation locally and at sites of metastasis.
Research frontiers
Phenotypic characterization of cancer stem cells is still debated although at least 8 putative stem cell markers have been suggested including CD166, CD44s, EpCAM, ALDH1, CD133, CD24, CD90, and ABCG5. The research hotspot is how the expression of putative cancer stem cell markers can be potentially used as prognostic biomarkers in patients with colorectal cancer.
Innovations and breakthroughs
Considering the apparent stem cell-like properties of tumor buds and adverse effect of budding on clinical outcome, in this study the authors performed immunohistochemical staining of 8 promising putative cancer stem cell markers, namely CD166, CD44s, EpCAM, ALDH1, CD133, CD24, CD90, and ABCG5 and assessed their expression within tumor buds to determine their frequency and potential prognostic significance in patients with colorectal cancer.
Applications
The study results suggest that, in contrast to CD133, CD166, CD24, CD44s, CD90, and ALDH1, the expression of putative cancer stem cell markers EpCAM and ABCG5 within the tumor buds of colorectal cancer are frequent events associated with poor prognosis.
Terminology
Tumor budding: single cells or clusters of up to 4 or 5 cells at the invasive tumor front of colorectal cancer which are diagnosed at high magnification and highly associated with an infiltrating tumor growth pattern. Cancer stem cells: tumorigenic cell populations with the potential to self-renew and differentiate.
Peer review
The study is characterized technically by an excellent application of immunohistochemistry and provides interesting evidence to aid in understanding the correlation between cancer stem cell markers in the invasive front of colorectal cancer and prognosis.
Footnotes
Supported by The Krebsliga Beider Basel (Zlobec I, Terracciano L and Lugli A)
Peer reviewers: Dr. Abdel-Majid Khatib, PhD, INSERM, UMRS 940, Equipe Avenir, Cibles Thérapeutiques, I.G.M. 27 Rue Juliette Dodu, 75010 Paris, France; Evangelos Tsiambas, MD, PhD, Lecturer in Molecular Cytopathology, Department of Pathology, Medical School, University of Athens, Athens 15341, Greece; Toshiyuki Ishiwata, Associate Professor, Department of Pathology, Integrative Oncological Pathology, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8602, Japan
S- Editor Wang JL L- Editor Cant MR E- Editor Ma WH
References
- 1.Gabbert H. Mechanisms of tumor invasion: evidence from in vivo observations. Cancer Metastasis Rev. 1985;4:293–309. doi: 10.1007/BF00048094. [DOI] [PubMed] [Google Scholar]
- 2.Gabbert H, Wagner R, Moll R, Gerharz CD. Tumor dedifferentiation: an important step in tumor invasion. Clin Exp Metastasis. 1985;3:257–279. doi: 10.1007/BF01585081. [DOI] [PubMed] [Google Scholar]
- 3.Prall F. Tumour budding in colorectal carcinoma. Histopathology. 2007;50:151–162. doi: 10.1111/j.1365-2559.2006.02551.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS. Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis. 2007;22:863–868. doi: 10.1007/s00384-006-0249-8. [DOI] [PubMed] [Google Scholar]
- 5.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 6.Hase K, Shatney C, Johnson D, Trollope M, Vierra M. Prognostic value of tumor "budding" in patients with colorectal cancer. Dis Colon Rectum. 1993;36:627–635. doi: 10.1007/BF02238588. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama T, Oya M, Ishikawa H. Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J Surg Oncol. 2003;83:42–47. doi: 10.1002/jso.10230. [DOI] [PubMed] [Google Scholar]
- 8.Prall F, Nizze H, Barten M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology. 2005;47:17–24. doi: 10.1111/j.1365-2559.2005.02161.x. [DOI] [PubMed] [Google Scholar]
- 9.Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, Jung A, Kirchner T, Brabletz T. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology. 2006;131:830–840. doi: 10.1053/j.gastro.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Ueno H, Mochizuki H, Hashiguchi Y, Hatsuse K, Fujimoto H, Hase K. Predictors of extrahepatic recurrence after resection of colorectal liver metastases. Br J Surg. 2004;91:327–333. doi: 10.1002/bjs.4429. [DOI] [PubMed] [Google Scholar]
- 11.Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Wang LM, Kevans D, Mulcahy H, O'Sullivan J, Fennelly D, Hyland J, O'Donoghue D, Sheahan K. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009;33:134–141. doi: 10.1097/PAS.0b013e318184cd55. [DOI] [PubMed] [Google Scholar]
- 13.Baker K, Zlobec I, Tornillo L, Terracciano L, Jass JR, Lugli A. Differential significance of tumour infiltrating lymphocytes in sporadic mismatch repair deficient versus proficient colorectal cancers: a potential role for dysregulation of the transforming growth factor-beta pathway. Eur J Cancer. 2007;43:624–631. doi: 10.1016/j.ejca.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Yokoo T, Ishii T. Histopathologic determinants of regional lymph node metastasis in early colorectal cancer. Cancer. 2008;112:924–933. doi: 10.1002/cncr.23248. [DOI] [PubMed] [Google Scholar]
- 15.Kazama S, Watanabe T, Ajioka Y, Kanazawa T, Nagawa H. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br J Cancer. 2006;94:293–298. doi: 10.1038/sj.bjc.6602927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park SY, Choe G, Lee HS, Jung SY, Park JG, Kim WH. Tumor budding as an indicator of isolated tumor cells in lymph nodes from patients with node-negative colorectal cancer. Dis Colon Rectum. 2005;48:292–302. doi: 10.1007/s10350-004-0773-y. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Hashiguchi Y, Ueno H, Hase K, Mochizuki H. Tumor budding at the invasive margin can predict patients at high risk of recurrence after curative surgery for stage II, T3 colon cancer. Dis Colon Rectum. 2003;46:1054–1059. doi: 10.1007/s10350-004-7280-z. [DOI] [PubMed] [Google Scholar]
- 18.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 19.Shinto E, Baker K, Tsuda H, Mochizuki H, Ueno H, Matsubara O, Foulkes WD, Jass JR. Tumor buds show reduced expression of laminin-5 gamma 2 chain in DNA mismatch repair deficient colorectal cancer. Dis Colon Rectum. 2006;49:1193–1202. doi: 10.1007/s10350-006-0568-4. [DOI] [PubMed] [Google Scholar]
- 20.Shinto E, Mochizuki H, Ueno H, Matsubara O, Jass JR. A novel classification of tumour budding in colorectal cancer based on the presence of cytoplasmic pseudo-fragments around budding foci. Histopathology. 2005;47:25–31. doi: 10.1111/j.1365-2559.2005.02162.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 22.Baldus SE, Mönig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Hölscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790–2796. doi: 10.1158/1078-0432.ccr-03-0163. [DOI] [PubMed] [Google Scholar]
- 23.Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, Kirchner T. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179:56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- 24.Masaki T, Matsuoka H, Sugiyama M, Abe N, Izumisato Y, Sakamoto A, Atomi Y. Laminin-5 gamma2 chain expression as a possible determinant of tumor aggressiveness in T1 colorectal carcinomas. Dig Dis Sci. 2003;48:272–278. doi: 10.1023/a:1021967108134. [DOI] [PubMed] [Google Scholar]
- 25.Masaki T, Sugiyama M, Matsuoka H, Abe N, Izumisato Y, Sakamoto A, Atomi Y. Coexpression of matrilysin and laminin-5 gamma2 chain may contribute to tumor cell migration in colorectal carcinomas. Dig Dis Sci. 2003;48:1262–1267. doi: 10.1023/a:1024142722640. [DOI] [PubMed] [Google Scholar]
- 26.Portyanko A, Kovalev P, Gorgun J, Cherstvoy E. beta(III)-tubulin at the invasive margin of colorectal cancer: possible link to invasion. Virchows Arch. 2009;454:541–548. doi: 10.1007/s00428-009-0764-4. [DOI] [PubMed] [Google Scholar]
- 27.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217:307–317. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 28.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 29.Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260–268. doi: 10.1002/path.2164. [DOI] [PubMed] [Google Scholar]
- 30.Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, Northover JM, Todd IP. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437–459. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 31.Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horst D, Kriegl L, Engel J, Jung A, Kirchner T. CD133 and nuclear beta-catenin: the marker combination to detect high risk cases of low stage colorectal cancer. Eur J Cancer. 2009;45:2034–2040. doi: 10.1016/j.ejca.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Gosens MJ, van Kempen LC, van de Velde CJ, van Krieken JH, Nagtegaal ID. Loss of membranous Ep-CAM in budding colorectal carcinoma cells. Mod Pathol. 2007;20:221–232. doi: 10.1038/modpathol.3800733. [DOI] [PubMed] [Google Scholar]
- 34.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masaki T, Goto A, Sugiyama M, Matsuoka H, Abe N, Sakamoto A, Atomi Y. Possible contribution of CD44 variant 6 and nuclear beta-catenin expression to the formation of budding tumor cells in patients with T1 colorectal carcinoma. Cancer. 2001;92:2539–2546. doi: 10.1002/1097-0142(20011115)92:10<2539::aid-cncr1605>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Hirata T, Okabe M, Kobayashi A, Ueda K, Matsuo M. Molecular mechanisms of subcellular localization of ABCG5 and ABCG8. Biosci Biotechnol Biochem. 2009;73:619–626. doi: 10.1271/bbb.80694. [DOI] [PubMed] [Google Scholar]
- 37.Kusuhara H, Sugiyama Y. ATP-binding cassette, subfamily G (ABCG family) Pflugers Arch. 2007;453:735–744. doi: 10.1007/s00424-006-0134-x. [DOI] [PubMed] [Google Scholar]
- 38.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]