Abstract
AIM: To clarify the therapeutic strategies and prognosis factors of primary clear cell carcinoma of the liver (PCCCL).
METHODS: The clinical pathological data of 64 patients with PCCCL treated with hepatectomy in our hospital from January 2000 to January 2006 were analyzed retrospectively. The patients were divided into two groups to make treatment analysis: curative resection only (n = 40); and curative resection and postoperative chemotherapy with calcium folinate and tegafur (n = 24). Meanwhile, the PCCCL patients were subdivided into two subgroups on the basis of the proportion of clear cells in the tumor for pathological analysis. There were 36 cases in subgroup A for which the proportion of clear cells was more than 70%, and 28 cases in subgroup B for which the proportion was less or equal to 70%, comparing analysis of median survival time of the counterpart groups. Univariate and multivariate analyses were performed to examine factors that affected clinical prognosis, recurrence and metastasis.
RESULTS: Median survival period of the curative surgery group was 38 mo, while the counterpart was 41 mo. Median survival period for group A was 41 mo, while group B was 19 mo. The Kaplan-Meier method showed that capsule formation, preoperative liver function, hepatitis C virus infection, large vascular invasion and multiple tumor occurrences were related to disease-free survival. Cox regression analysis showed that the clear cell ratio, capsule formation, preoperative liver function and large vascular invasion were independent risk factors for overall survival.
CONCLUSION: Postoperative chemotherapy has no obvious effect on survival of patients with PCCCL. Clear cell ratio, capsule formation, preoperative liver function, and vascular invasion were independent risk factors for prognosis.
Keywords: Clear cell carcinoma, Hepatectomy, Prognosis, Treatment, Risk factor
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major cause of cancer mortality worldwide and is increasing in incidence; driven largely by the growing hepatitis B and hepatitis C epidemics[1,2], which comprise approximately 75% of all HCC and liver cirrhosis (70%-80% of all cases)[3,4]. Surgical resection has long been the only curative treatment; conventional chemotherapy and radiotherapy are ineffective for HCC[5]. Despite recent advances in diagnostic and therapeutic modalities, prognosis is usually poor, particularly in patients with coexisting liver cirrhosis. Survival rates are 3%-5% in cancer registries for the United States and developing countries. In total, 55% of cases (and deaths) are in China alone[6]. Many investigations have suggested that tumor size, number of nodules, vascular invasion, tumor encapsulation, blood transfusion, high α-fetoprotein (AFP) level, and resection margin status are the main prognostic factors associated with the postoperative recurrence of HCC[7].
Primary clear cell carcinoma of liver (PCCCL) is a particular histological type of HCC, PCCCL is not frequent and has been reported to account for 7.5%-12.5% of all liver cancer cases[8]. Microscopically, all cases of PCCCL show moderate to marked cytoplasmic accumulation of glycogen and/or macro- and microvascular intracytoplasmic fat droplets that dissolve during hematoxylin-eosin (HE) staining, which leaves behind a clear cytoplasm. Generally, the tumor cells are mainly in the mid-range degree of differentiation, and low-grade malignancy. PCCCL usually has capsule formation and is localized. Surgical resection is the most promising therapeutic method for PCCCL. The outcome for patients with PCCCL is better than for those with common type counterparts, and survival improves with an increasing proportion of clear cells[9,10]. Treatment and prognosis of PCCCL are reported rarely in the literature. Also, treatment and clinical prognostic features are not fully clarified.
MATERIALS AND METHODS
Subjects
The participants of this study were 64 patients [40 male, 24 female (ratio 1.67:1), aged 23-73 years, mean 55.16 ± 10.56 years], who received curative hepatic resection for PCCCL at the Tianjin Medical University Cancer Hospital between January 2000 and January 2006. We used the diagnostic criteria generally accepted by pathologists in China to diagnose PCCCL as follows: (1) only when it contained > 50% clear cells; (2) exception for metastatic clear cell carcinoma from other organs; and (3) diagnosis by more than two pathologists. A total of 64 patients were eligible for this study.
Treatment method
All 64 patients underwent surgical tumor resection. Anatomical resection included hemi-hepatectomy, segmentectomy and sub-segmentectomy, based on Child-Pugh classification. Resection margin was 1 cm beyond the tumor, and surgical margins were negative when examined by the pathologists. Clear cell ratio, large vascular invasion (large blood vessels including the portal vein, hepatic vein and/or first level branch), and lymph node metastasis were confirmed by pathology.
Patients with PCCCL who had undergone their first curative hepatic resection at the Cancer Hospital of Tianjin Medical University were eligible for postoperative adjuvant chemotherapy if they met the following entry criteria: (1) absence of detectable residual or recurrent tumors at 1 mo after curative resection; (2) age < 70 years; (3) liver function belonging to Child A or B class; (4) absence of severe cardiac complications; and (5) general health satisfactory for toleration of the contemplated chemotherapy. The exclusion criteria were the presence of clinically confirmed extrahepatic metastasis, macroscopic evidence of tumor thrombus in the inferior vena cava or the main portal vein, other previous or synchronous malignant disorders, and postoperative dysfunction of any organ. Finally 24 patients were considered as suitable candidates for our studies.
Postoperative chemotherapy of 24 cases consisted of calcium folinate and tegafur. Calcium folinate was administered at a starting dose of 200 mg/m2, as a continuous intravenous infusion over 2 h on days 1-5. Tegafur was administered at a starting dose of 850 mg/m2 given intravenously over 3 h on days 1-5. Adequate intravenous hydration and antiemetic therapy were routinely administered. Chemotherapy courses were repeated every 21 d, provided patients recovered from all toxic effects. Based on the predetermined criteria of toxicity grades, the doses of chemotherapy drugs were increased or decreased by 25%. Criteria for dose reduction included development of grade 3 non-hematological toxicity or grade 4 hematological toxicity. Complete blood, differential, and platelet counts were evaluated at least once weekly and more frequently when patients were myelosuppressed during the rest period. Serum creatinine, blood urea nitrogen, electrolyte, and magnesium levels were monitored regularly during each course.
Survival analysis methods
The prognostic factors were examined in cumulative and disease-free survival, using the following variables: age (older or younger than 50 years); sex (male vs female); serum hepatitis B virus (HBV) surface antigen (HBsAg) (negative vs positive); serum hepatitis C virus (HCV) antibody (HCVAb) (negative vs positive); proportion of clear cell more than vs less than or equal to 70%; tumor size (greater than vs less than or equal to 5.0 cm); liver cirrhosis (negative vs positive); serum levels of AFP (greater than vs less than or equal to 200 ng/mL); operative procedures (anatomical vs non-anatomical resection); lymph node metastases (negative vs positive); vessel invasion (negative vs positive); Child-Pugh classification (Grade A vs Grade B or C); capsule formation (negative vs positive); number of nodules (solitary vs multiple); therapeutic strategies (curative resection vs curative resection and postoperative chemotherapy ); TNM staging (I, II vs III, IV).
Follow-up
All patients were followed up to January 2009, or up to the time of death; all patients were followed up for > 3 years. Patients were examined regularly with measurement of the serum AFP level, hepatic ultrasonography and chest radiography every month after surgical resection to check metastasis and recurrence. Six months later, we examined serum AFP level, hepatic ultrasonography and chest radiography every 3 mo. When recurrence was suspected, further evaluations were made by abdominal, chest and brain enhanced computed tomography (CT), if necessary, by ultrasound-guided biopsy or positive electron tomography/CT examination to confirm the diagnosis. Patients who died of another disease were lost to follow-up.
Statistical analysis
Differences in the means were assessed with the χ2 test. The cumulative survival and the life table and Kaplan-Meier method, calculated recurrence-free survival rates and the difference between the two groups was analyzed by the log-rank test. The survival curve was described using the Kaplan-Meier method. Cox regression (proportional hazard model) was adopted for the multivariate analysis of prognostic factors. Statistical software package SPSS 13.0 (SPSS Inc., Chicago, IL, USA) was employed for all of the analyses. P values less than 0.05 were considered statistically significant. SPSS 13.0 was employed for all of the analyses.
RESULTS
In 36 cases (56.25%), the proportion of clear cells was > 70%; 46 cases (71.88%) were positive for HBsAg, 10 cases (14%) were positive for HCVAb, and six cases (9.32%) were negative for both HBsAg and HCVAb. Fifty-six patients had liver cirrhosis (87.50%). In 50 patients, tumor diameter was > 5.0 cm (78.13%). In 26 patients, serum AFP level was > 200 ng/mL (40.63%). Forty-four patients had a solitary tumor (68.75%); 30 had large vessel invasion (46.86%); 12 had lymph node metastasis (18.75%); 24 received postoperative chemotherapy with calcium folinate and tegafur (37.50%); and 40 received curative hepatic resection without chemotherapy (62.50%). The liver function was evaluated using Child-Pugh classification. Forty patients had grade A liver function (62.50%), 21 (32.81%) had grade B, and three (4.69%) had grade C. Forty-six patients had tumor capsule formation (71.88%). Pathological stage of PCCCL was evaluated using TNM staging. Three patients had stage I, nine had stage II, 40 patients had stage III, and two had stage IV.
Postoperative follow-up
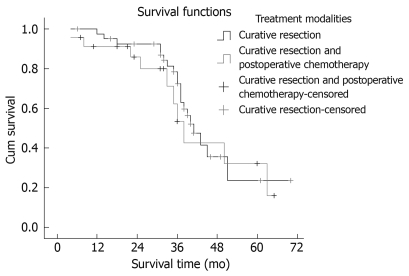
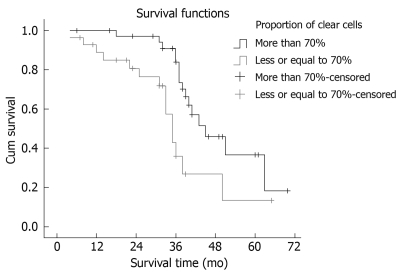
Seventeen patients suffered intrahepatic recurrence during follow-up; extra-hepatic metastasis occurred in 13 cases, and six patients suffered metastasis and recurrence. One patient in the curative surgical group died of perioperative complications, which resulted in a perioperative mortality of 1.56% (1/64). One patient died from traffic accidents, and two from the resection and postoperative chemotherapy group missed postoperative follow-up. After excluding the perioperative deaths and the missing patients, the postoperative cumulative and disease-free survival rates at 1, 3 and 5 years were 78.13%, 49.88% and 37.50% (mean ± SD, 41.56 ± 3.72 mo; median, 39.6 mo) and 71.82%, 40.63% and 25.00% (mean ± SD, 34.56 ± 3.93 mo; median, 33.0 mo), respectively. Median survival period in the curative surgical group was 38 mo, and 41 mo in its counterpart. There was no statistical significance in the survival time between the two groups (χ2 = 0.196, P = 0.658). The survival curves are shown in Figure 1. Median survival period of the group with > 70% clear cells was 41 mo, and 29 mo in its counterpart. The proportion of clear cells had an obvious effect on the median survival period of patients (χ2 = 7.432, P = 0.006). The survival curves are shown in Figure 2. The prognosis of the patients with a higher proportion of clear cells was better than that in patients with a lower proportion of clear cells.
Figure 1.
Comparison of survival rates of different treatment modalities.
Figure 2.
Comparison of survival of patients with different percentage of clear cells.
Prognostic analyses
Univariate and multivariate analyses: For the univariate analysis, age, sex, serum HBsAg, serum HCVAb, tumor diameter, vascular invasion, capsule formation, background of liver cirrhosis, serum AFP level, Child-Pugh classification, vascular invasion, proportion of clear cells, and lymph node metastases were included. We found that the parameters of vascular invasion, capsule formation, background of liver cirrhosis, number of nodules, proportion of clear cells, and Child-Pugh classification were statistically significant in cumulative survival (Table 1). Then, the parameters of significances were all contained in the Cox regression analysis. Using the Cox regression analysis, four clinic pathological variables were shown to have potential of predicting overall or disease-free survival of PCCCL patients, including rate of capsule formation, vascular invasion, Child-Pugh classification, and proportion of clear cells (Table 2).
Table 1.
Univariate analysis of clinicopathological variables associated with the prognosis of PCCCL
| Parameters | Cases | Median survival time (95% CI) (mo) | χ2-value | P-value |
| AFP range (ng/mL) | ||||
| ≥ 200 | 26 | 31 (27.302-34.698) | 1.413 | 0.285 |
| < 200 | 38 | 49 (42.211-55.789) | ||
| Liver cirrhosis (+) | ||||
| Positive | 56 | 31 (28.013-33.987) | 6.032 | 0.014 |
| Negative | 8 | 43 (38.303-47.697) | ||
| Capsule formation | ||||
| Positive | 46 | 61 (53.106-68.894) | 10.241 | 0.001 |
| Negative | 18 | 19 (16.690-21.310) | ||
| Number of nodules | ||||
| Single | 44 | 61 (53.014-68.986) | 4.028 | 0.045 |
| Multiple | 20 | 11 (9.675-12.325) | ||
| Child-Pugh classification | ||||
| A | 35 | 60 (51.023-68.977) | 11.330 | 0.003 |
| B or C | 21 | 25 (23.302-28.698) | ||
| Vascular invasion | ||||
| Positive | 30 | 27 (23.346-30.654) | 11.755 | 0.001 |
| Negative | 34 | 41 (35.437-46.563) | ||
| lymph node metastases | ||||
| Positive | 12 | 31 (26.012-35.988) | 0.023 | 0.880 |
| Negative | 52 | 40 (37.342-44.658) | ||
| TNM staging | ||||
| I, II | 12 | 43 (39.211-46.789) | 16.192 | 0.001 |
| III, IV | 52 | 27 (25.371-28.629) | ||
| Proportion of clear cells | ||||
| ≥ 70% | 36 | 41 (35.535-46.465) | 7.342 | 0.006 |
| < 70% | 28 | 19 (16.964-21.036) | ||
PCCCL: Primary clear cell carcinoma of the liver.
Table 2.
Multivariate analysis of clinicopathological variables associated with the prognosis of PCCCL
| Parameters | Regression coefficient | Wald value | P value | RR (95% CI) |
| Proportion of clear cells | 1.409 | 6.898 | 0.009 | 4.090 (1.430-11.702) |
| Capsule formation | -1.364 | 5.172 | 0.023 | 0.256 (0.079-0.828) |
| Vascular invasion | 1.686 | 9.923 | 0.002 | 5.395 (1.890-15.398) |
| Child-Pugh classification | 1.917 | 4.119 | 0.042 | 6.798 (3.253-17.334) |
Cumulative recurrence-free survival rates and disease-free prognosis: The life table and Kaplan-Meier method, calculated the cumulative recurrence-free survival rates and the difference between two groups were analyzed by the log-rank test. The 1-, 3- and 5-year disease-free survival rates were 71.82%, 40.63% and 25.00% (mean ± SD, 34.56 ± 3.93 mo; median, 33.0 mo). Kaplan-Meier univariate analysis showed that the disease-free prognosis of the patients with capsule formation, better liver function, negative HCVAb, no vascular invasion, and solitary tumor were better than the patients with no capsule formation, poor liver function, positive HCVAb, vascular invasion and multiple tumors. Patients with no capsule formation, poor liver function, positive HCVAb, vascular invasion and multiple tumors were prone to metastasis and/or recurrence.
DISCUSSION
PCCCL is a particular and relatively rare histological type of HCC. Microscopically, it is similar to the clear cell cancers (kidney, ovarian or adrenal), which makes it difficult to differentiate from the metastatic clear cell cancers of the liver. Murakata et al[11] have recommended hepatocyte antibody as a screening immunostain in working up a clear cell tumor in the liver when diagnostic histological criteria of HCC are absent. In this setting, it distinguishes PCCCL from other clear cell malignancies with a sensitivity of 90% and specificity of 100%. Some other studies have indicated in situ hybridization for albumin mRNA as a useful method to distinguish PCCCL from other clear cell tumors metastasizing to the liver[12]. In the present study, we made the diagnosis using features that point toward the diagnosis of HCC. This study integrated the patient’s pathological features, biopsy, and clinical manifestations, imaging studies, endoscope bile stasis and postoperative long-term follow-up to make a clear diagnosis[4,13]. There was no misdiagnosis in our study. Some authors consider < 30% of clear cells within the tumor as sufficient[9], whereas others diagnose PCCCL when the tumor contains > 30% clear cells, however, tumors with clear cells ranging from 90% to 100% are extremely rare[14]. We used the diagnostic criteria generally accepted by pathologists in China to diagnose PCCCL, that is, only when it contained > 50% clear cells[10]. In our further studies, we formed a group according to whether the clear cell count was 70% of all cells. We found that the group with > 70% clear cells had significantly longer survival (χ2 = 7.432, P = 0.006). This shows that the prognosis was related to the proportion of clear cells. The greater the number of clear cells, the better the prognosis.
Surgical resection is an effective way to achieve favorable outcomes and long-term survival of patients with PCCCL. Lao et al[15] have reported 1- and 3-year survival rates of 76.5% (13/18) and 47.1% (8/18), in all 13 surgical resection patients; the longest survival was 97 mo, and surgical resection was an effective treatment to achieve long-term survival. Compared with HCC, PCCCL has a slower development process, good differentiation, lower grade malignancy, and easier capsule formation, therefore, the tumor is more limited and prone to resection. Surgical resection is the most important means of achieving long-term survival. If there is recurrence after resection, tumor re-resection is possible, but if it cannot be removed, development is slower than for HCC. In the present study, there were 24 patients in the surgical resection and chemotherapy group; the median survival period was 38.2 mo, and the median survival of the curative surgical resection group was 39.1 mo. The difference between these two groups was not significant (χ2 = 0.196, Ρ = 0.658), which indicated that postoperative adjuvant chemotherapy with calcium folinate and tegafur was not sensitive to PCCCL and had no obvious effect on the survival time of patients. Other postoperative chemotherapy regimens for PCCCL were not investigated in this study. The prognosis of patients with postoperative chemotherapy requires further study.
Pecorella et al[16] have reported that a 35-year-old patient who was treated with liver transplantation survived for 17 mo, which was lower than the median survival in our study. Emile et al[17] have shown that prognosis was better in a large series of transplanted Caucasian patients with PCCCL than in those with other liver malignancies. In the present study, the prognosis of patients with surgical resection was better than for HCC, which may be related to better tumor differentiation, capsule formation, less vascular invasion and lymph node metastasis, and high resectability rate. The prognosis of patients with PCCCL is still controversial. Many studies have reported PCCCL has better prognosis than other HCCs[8,18]. Lai et al[9] have reported that the outcome for patients with PCCCL is better than those with common-type cancers, and survival improves with an increasing proportion of clear cells. Conversely, other investigators have found that the prognosis of patients with PCCCL is similar to that of their common-type counterparts and perhaps even worse[19,20]. Yang et al[14] have reported that the 3- and 5-year survival rate was 54.5% and 33.3%, respectively, which was slightly lower than the rate for non-PCCCL patients (including HCC). However, all these data failed to disclose any statistical significance, or were not statistically analyzed according to the number of cases. Our study confirmed the former results in a series of postoperative patients, and showed significantly higher 1-, 3- and 5-year survival rates in PCCCL patients. The Kaplan-Meier method showed that capsule formation, preoperative liver function, HCV infection, large vascular invasion and multiple tumor occurrences were related to disease-free survival. The prognosis of patients in the PCCCL group was related to clear cell ratio, preoperative liver function, liver cirrhosis, HCV infection, capsule formation, large vascular invasion and multiple tumor occurrences. In this study, lymph node metastasis did not significantly affect survival, which may have been related to the comparatively small number of cases in this study, therefore, we need to increase the number of sample cases for further study. Cox multivariate analysis showed that clear cell ratio, capsule formation, preoperative liver function and large vascular invasion were independent risk factors for survival. In this study, capsule formation of PCCCL was different from the clinical characteristics of HCC. Capsule formation may limit tumor growth and spread and is conducive to tumor resection and treatment. Lower malignancy and better differentiation of clear cells may have contributed to the improved prognosis. The higher the proportion of clear cells, the better was the prognosis. Preoperative Child-Pugh classification was an independent risk factor for survival. High HCV prevalence led to poor liver function and shorter survival.
In summary, postoperative chemotherapy with calcium folinate and tegafur had no obvious effect on survival time of patients with PCCCL. Patients with a high clear cell ratio had improved prognosis. Capsule formation, poor preoperative liver function, HCV infection, large vascular invasion, and multiple tumor occurrence were risk factors for metastasis and postoperative recurrence of PCCCL. Patients with capsule formation, no large vascular invasion, high clear cell ratio, and better liver function had improved prognosis.
COMMENTS
Background
Primary clear cell carcinoma of the liver (PCCCL) is a type of primary hepatocellular carcinoma (HCC), which is characterized pathologically by diffuse clear cells of the tumor, and a clear cytoplasm that does not stain with hematoxylin-eosin. At present, treatment and prognosis of PCCCL have not been reported widely in the literature. Its treatment and clinical prognostic features have not been fully clarified.
Research frontiers
PCCCL is a particular histological type of HCC; PCCCL is not frequent and has been reported to account for 7.5%-12.5% of all liver cancer cases. Treatment and clinical prognostic features have not been fully clarified. The research hot topics are how to treat PCCCL, and its independent prognostic risk factors. Surgical resection is an effective way to achieve favorable outcomes and long-term survival of patients with PCCCL.
Innovations and breakthroughs
Previously, there have been more case studies of PCCCL, and large sample studies have been rare. The present study found that postoperative chemotherapy with calcium folinate and tegafur had no obvious effect on patient survival. The study found that the higher the proportion of clear cells, the better the prognosis. The authors also found that the clear cell ratio, capsule formation, preoperative liver function, and vascular invasion were independent prognostic risk factors, which had not been reported previously.
Applications
The study results suggest that postoperative chemotherapy with calcium folinate and tegafur has no obvious effect on patient survival. Surgical resection is an effective way to achieve favorable outcomes and long-term survival of patients with PCCCL.
Terminology
Hepatectomy is a treatment approach that involves the surgical removal of part or all of the liver for therapeutic purposes. Postoperative chemotherapy is the use of certain drugs to further treat cancer after surgery according to certain symptoms and physical signs.
Peer review
This is a good retrospective study which analyzed therapeutic strategies for patients with PCCCL, who were undergoing liver resection. The authors operated on 64 patients with this infrequent type of HCC within the past 6 years. The major oncological and surgical issues are discussed in the introduction and discussion.
Acknowledgments
The authors thank Dr. Young Tang, Department of Pancreatic Oncology, Cancer Hospital of Tianjin Medical University, for help in preparing the manuscript and the statistical analysis.
Footnotes
Peer reviewers: Martin K Schilling, MD, FRCS, Professor of Surgery, Chairman, Department of General, Visceral, Vascular and Pediatric Surgery, University of Saarland, Kirrbergerstrasse, Homburg, D-66424, Germany; Takashi Kobayashi, MD, PhD, Department of Surgery, Showa General Hospital, 2-450 Tenjincho, Kodaira, Tokyo 187-8510, Japan
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
References
- 1.Chin'ombe N, Chavhunduka E, Matarira HT. Seroprevalence of HBV and HCV in primary hepatocellular carcinoma patients in Zimbabwe. Infect Agent Cancer. 2009;4:15. doi: 10.1186/1750-9378-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barazani Y, Hiatt JR, Tong MJ, Busuttil RW. Chronic viral hepatitis and hepatocellular carcinoma. World J Surg. 2007;31:1243–1248. doi: 10.1007/s00268-007-9041-3. [DOI] [PubMed] [Google Scholar]
- 3.But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652–1656. doi: 10.3748/wjg.14.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan A, Yeh SH, Liu CJ, Cheung C, Chen PJ. Viral hepatocarcinogenesis: from infection to cancer. Liver Int. 2008;28:175–188. doi: 10.1111/j.1478-3231.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 5.Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866–3884. doi: 10.1038/sj.onc.1209550. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Qiang L, Huikai L, Butt K, Wang PP, Hao X. Factors associated with disease survival after surgical resection in Chinese patients with hepatocellular carcinoma. World J Surg. 2006;30:439–445. doi: 10.1007/s00268-005-0608-6. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi A, Saito H, Kanno Y, Abe K, Yokokawa J, Irisawa A, Kenjo A, Saito T, Gotoh M, Ohira H. Case of clear-cell hepatocellular carcinoma that developed in the normal liver of a middle-aged woman. World J Gastroenterol. 2008;14:129–131. doi: 10.3748/wjg.14.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai CL, Wu PC, Lam KC, Todd D. Histologic prognostic indicators in hepatocellular carcinoma. Cancer. 1979;44:1677–1683. doi: 10.1002/1097-0142(197911)44:5<1677::aid-cncr2820440522>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Liu JH, Tsai HL, Hsu SM, Hu DH, Chen SS. Clear cell and non-clear cell hepatocellular carcinoma: a case report and literature review. Kaohsiung J Med Sci. 2004;20:78–82. doi: 10.1016/S1607-551X(09)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakata LA, Ishak KG, Nzeako UC. Clear cell carcinoma of the liver: a comparative immunohistochemical study with renal clear cell carcinoma. Mod Pathol. 2000;13:874–881. doi: 10.1038/modpathol.3880156. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira AM, Erickson LA, Burgart LJ, Lloyd RV. Differentiation of primary and metastatic clear cell tumors in the liver by in situ hybridization for albumin messenger RNA. Am J Surg Pathol. 2000;24:177–182. doi: 10.1097/00000478-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kawagishi N, Shirahata Y, Ishida K, Satoh K, Enomoto Y, Akamatsu Y, Sekiguchi S, Fukumori T, Fujimori K, Satoh A, et al. Hepatic resection of giant metastatic tumor from clear cell carcinoma of the ovary. J Hepatobiliary Pancreat Surg. 2005;12:155–158. doi: 10.1007/s00534-004-0957-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int. 1996;46:503–509. doi: 10.1111/j.1440-1827.1996.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 15.Lao XM, Zhang YQ, Jin X, Lin XJ, Guo RP, Li GH, Li JQ. Primary clear cell carcinoma of liver--clinicopathologic features and surgical results of 18 cases. Hepatogastroenterology. 2006;53:128–132. [PubMed] [Google Scholar]
- 16.Pecorella I, Ciardi A, Aiello E, Piras MR, Farci C, Di Tondo U. Clear cell hepatocellular carcinoma treated with liver transplantation. Pathologica. 1994;86:307–310. [PubMed] [Google Scholar]
- 17.Emile JF, Lemoine A, Azoulay D, Debuire B, Bismuth H, Reynès M. Histological, genomic and clinical heterogeneity of clear cell hepatocellular carcinoma. Histopathology. 2001;38:225–231. doi: 10.1046/j.1365-2559.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 18.Salvucci M, Lemoine A, Saffroy R, Azoulay D, Lepère B, Gaillard S, Bismuth H, Reynès M, Debuire B. Microsatellite instability in European hepatocellular carcinoma. Oncogene. 1999;18:181–187. doi: 10.1038/sj.onc.1202279. [DOI] [PubMed] [Google Scholar]
- 19.Shah S, Gupta S, Shet T, Maheshwari A, Wuntkal R, Mohandas KM. Metastatic clear cell variant of hepatocellular carcinoma with an occult hepatic primary. Hepatobiliary Pancreat Dis Int. 2005;4:306–307. [PubMed] [Google Scholar]
- 20.Adamek HE, Spiethoff A, Kaufmann V, Jakobs R, Riemann JF. Primary clear cell carcinoma of noncirrhotic liver: immunohistochemical discrimination of hepatocellular and cholangiocellular origin. Dig Dis Sci. 1998;43:33–38. doi: 10.1023/a:1018859617522. [DOI] [PubMed] [Google Scholar]