Abstract
Context
It is unclear whether fat mass (FM) and lean mass (LM) differ in the way they influence cortical bone development in boys and girls.
Objective
The aim of the study was to investigate the contributions of total body FM and LM to parameters related to cortical bone mass and geometry.
Design/Setting
We conducted a longitudinal birth cohort study, the Avon Longitudinal Study of Parents and Children.
Participants
A total of 4005 boys and girls (mean age, 15.5 yr) participated in the study.
Outcome Measures
We measured cortical bone mass, cortical bone mineral content (BMCC), cortical bone mineral density, periosteal circumference (PC), and endosteal circumference by tibial peripheral quantitative computed tomography.
Results
LM had a similar positive association with BMCC in boys and girls [regression coefficients with 95% confidence interval (CI); P for gender interactions: boys/girls, 0.952 (0.908, 0.997); P = 0.85]. However, the mechanisms by which LM influenced bone mass differed according to gender because LM was positively associated with PC more strongly in girls [boys, 0.579 (0.522, 0.635); girls, 0.799 (0.722, 0.875); P < 0.0001], but was only associated with cortical bone mineral density in boys [boys, 0.443 (0.382, 0.505); girls, 0.014 (−0.070, 0.097); P < 0.0001]. There was a stronger positive association between FM and BMCC in girls [boys, 0.227 (0.185, 0.269); girls, 0.355 (0.319, 0.392); P < 0.0001]. This reflected both a greater positive association of FM with PC in girls [boys, 0.213 (0.174, 0.253); girls, 0.312 (0.278, 0.347); P = 0.0002], and a stronger negative association with endosteal circumferencePC [boys, −0.059 (−0.096, 0.021); girls, −0.181 (−0.215, −0.146); P < 0.0001].
Conclusions
Whereas LM stimulates the accrual of cortical bone mass to a similar extent in boys and girls, FM is a stronger stimulus for accrual of cortical bone mass in girls, reflecting a greater tendency in females for FM to stimulate periosteal growth and suppress endosteal expansion.
Sexual dimorphism in skeletal development is well recognized and is likely to contribute to gender differences in fracture risk (1), exemplified by the considerably higher hip fracture risk in elderly women (2). At the hip, these differences have been reported to emerge during the adolescent growth spurt and to comprise largely of greater periosteal apposition in boys leading to greater femoral neck width and bending strength (3). More rapid periosteal bone formation in boys, leading to greater cortical bone size, has also been reported at the midfemur (prepubertal children and young adults combined) (4), midtibia (peripubertal children) (5), and distal tibia and femoral neck (18 yr olds) (6). Lean mass (LM) is one of the strongest determinants of bone mass throughout life, largely reflecting adaptation of skeletal modeling to loading. During puberty, rapid changes in LM occur that are thought to play a major role in bone mass acquisition in childhood and may also contribute to gender differences in this process (7, 8).
Whether fat mass (FM) also affects skeletal development and contributes to gender differences thereof is more controversial. Obese children have been reported to have a lower bone mass for a given weight in several previous studies (9–13). In contrast, in a study in which indices of proximal femur geometry were derived from hip dual-energy x-ray absorptiometry (DXA) scans in overweight adolescents, FM was not found to influence any skeletal parameter independently of LM (14). In a study of 18 obese and 30 nonobese children, bone age in the former group was more advanced, but bone mineral density (BMD) was similar (15). On the other hand, in the Avon Longitudinal Study of Parents and Children (ALSPAC), FM was positively related to bone mass as reflected by bone mineral content (BMC) in 3032 children at the age of approximately 10 yr as measured at the total body and upper and lower limbs (16). In contrast, in a study based on 300 subjects aged 13–21 yr, FM was not found to be related to either leg or lumbar spine bone mass or femoral or spinal cross-sectional area as measured by quantitative computed tomography (17). A recent instrumental variable analysis in which we reexamined the relationship between fat and bone mass in ALSPAC using genetic markers of obesity appeared to confirm our initial observation that FM is an important positive determinant of bone mass acquisition in childhood (18).
The association between FM and bone mass in ALSPAC was largely explained by an effect on bone size, which persisted after adjusting for height, suggesting that effects on periosteal growth are primarily responsible (16). Although peripheral quantitative computed tomography (pQCT) provides a more direct approach for evaluating periosteal growth based on measurement of periosteal circumference (PC) of the diaphysis of long bones such as the radius and tibia, conflicting results have been obtained from previous studies. In the GOOD cohort comprising 1086 18-yr-old men, a positive association was observed between FM and PC at the tibia, whereas no association was seen at the radius (19); in 115 18-year-old females, FM was unrelated to PC of either the tibia or radius (20); and in 677 men aged 25–45 yr (comprising 296 sibling pairs), FM was inversely related to PC at both the tibia and radius (21). Cortical bone mass is also affected by cortical thickness reflecting the rate of endosteal expansion relative to that of periosteal growth, and cortical BMD (BMDC) reflecting the rate of cortical remodeling and mineralization. pQCT studies suggest that FM may affect these other determinants of cortical bone mass. For example, in GOOD, FM was positively related to cortical area and endosteal circumference (EC) and inversely related to BMDC (19). In a study of 296 boys and girls ages 5–19 yr, fat area was inversely related to BMDC at the radius in pubertal females only (22).
Taken together, these studies suggest that FM may influence several determinants of cortical bone mass. Precise findings varied between studies, possibly reflecting differences in gender, maturational status, and skeletal site under examination. These inconsistencies may also reflect the limited power of smaller studies to accurately evaluate associations in the presence of multiple factors used for adjustment. In addition, these analyses generally failed to take account of potentially important functional relationships that are likely to exist between PC, cortical thickness, and BMDC, but which are yet to be fully described. In the present study, we aimed to identify independent influences of FM and LM on individual determinants of cortical bone mass and to examine the extent to which these effects differ between boys and girls. We used data from the ALSPAC cohort, which is ideally suited to this purpose because its large size enables statistical models using multiple variables to be built with reasonable accuracy. Moreover, the age of the cohort (mean age, 15.5 yr) ensures that the majority of cohort participants are in the advanced stages of puberty, simplifying model development.
Subjects and Methods
Study population
ALSPAC is a geographically based birth cohort study investigating factors influencing the health, growth, and development of children. All pregnant women resident within a defined part of the former county of Avon in southwest England with an expected date of delivery between April 1991 and December 1992 were eligible for recruitment; approximately 14,000 women from this group were enrolled (23) (http://www.alspac.bristol.ac.uk). Ethical approval was obtained from the ALSPAC Law and Ethics committee and relevant local ethics committees. Data in ALSPAC is collected by self-completion postal questionnaires sent to parents, by linkage to computerized records, by abstraction from medical records, and from examination of the children at research clinics.
Variables
BMCC, BMDC, and cortical bone area (BAC) were measured using the Stratec XCT2000L (Stratec, Pforzheim, Germany) in 4502 participants, of which 88 were excluded due to the presence of major motion artifacts (2127 boys, 45 exclusions) (Table 1). PC, EC, and cortical thickness were derived from circular ring model. A threshold routine was used for defining cortical bone, which specified a voxel with a density greater than 650 mg/cm3 as cortical bone. Total body FM and LM were measured using DXA in 5157 participants, of which 465 were excluded due to major artifacts, the most common of which was a failure to include the whole body within the scan field (2458 boys, 262 exclusions) (GE Lunar Prodigy, Madison, WI). Height was measured using a Harpenden stadiometer (Holtain Ltd., Crymych, Wales, UK). All densitometric and anthropometric measures were taken in the 15.5-yr research clinic. Pubertal status, which was based on an annual Tanner stage questionnaire, was obtained from the mean of breast and pubic hair self-ratings in girls and by pubic hair rating alone in boys. Date of birth and gender information was obtained from birth notification, and date of the scan was recorded automatically, allowing age at scan to be calculated.
TABLE 1.
Descriptive characteristics
| Mean | sd | 25th percentile | Median | 50th percentile | |
|---|---|---|---|---|---|
| Age (yr) | |||||
| M | 15.47 | 0.27 | 15.30 | 15.41 | 15.56 |
| F | 15.49 | 0.30 | 15.31 | 15.43 | 15.58 |
| Height (cm) | |||||
| M | 174.19 | 7.44 | 169.60 | 174.50 | 179.00 |
| F | 164.60 | 6.07 | 160.60 | 164.50 | 168.70 |
| LM (kg) | |||||
| M | 49.61 | 6.56 | 45.58 | 49.77 | 53.82 |
| F | 36.94 | 3.88 | 34.35 | 36.66 | 39.25 |
| FM (kg) | |||||
| M | 10.52 | 7.15 | 5.64 | 8.21 | 12.80 |
| F | 18.39 | 7.58 | 13.10 | 16.99 | 21.98 |
| BMDC (mg/cm3) | |||||
| M | 1074.76 | 34.78 | 1053.14 | 1077.40 | 1100.39 |
| F | 1124.56 | 22.96 | 1111.02 | 1126.67 | 1140.06 |
| BAC (mm2) | |||||
| M | 352.77 | 52.92 | 317.37 | 351.75 | 387.11 |
| F | 308.49 | 40.52 | 280.69 | 306.86 | 334.31 |
| BMCC (mg) | |||||
| M | 327.96 | 46.37 | 296.51 | 327.75 | 357.62 |
| F | 274.37 | 36.13 | 249.42 | 272.73 | 296.85 |
| PC (mm) | |||||
| M | 76.14 | 5.34 | 72.63 | 75.99 | 79.50 |
| F | 69.37 | 4.85 | 66.10 | 68.99 | 72.34 |
| EC (mm) | |||||
| M | 40.87 | 5.83 | 37.05 | 40.34 | 44.10 |
| F | 36.87 | 5.36 | 33.49 | 36.24 | 39.59 |
| CT (mm) | |||||
| M | 5.61 | 0.69 | 5.17 | 5.63 | 6.07 |
| F | 5.17 | 0.58 | 4.79 | 5.18 | 5.55 |
Table shows descriptive characteristics for 1851 boys and 2154 girls undergoing pQCT scans. CT, Cortical thickness.
Statistical analyses
Descriptive statistics are presented as means and sd, and as medians and lower and upper quartiles points. Sex-specific unadjusted effects of exposures on outcomes are presented as per sd increase in exposure associated with per sd increase in the outcome and as 95% confidence intervals (95% CI). P values are presented with respect to the gender interaction, and when there is no evidence for a gender interaction, the best linear unbiased estimate is presented in the table legend with sex-specific estimates within the tables. Models were adjusted using relevant confounding factors that were identified using directed acyclic graphs. Due to the measurement and estimation procedure of variables from the pQCT, not all analyses are presented with estimates of precision due to their mechanistic determination. All missing data were treated as missing at random, and complete case analysis was performed in analyses. Analyses were conducted using STATA 9.2 (StataCorp LP, College Station, TX).
Results
A total of 4005 subjects (46% boys) were identified with valid pQCT, DXA, and height information that formed the basis for this study; of these, approximately 95% were in advanced puberty as defined by Tanner stages 4 or 5. Subjects were a mean age of 15.5 yr. As expected, boys were taller and had a greater LM, whereas FM was higher in girls (Table 1). BMCC was higher in boys compared with girls, mirroring their greater cortical bone size as reflected by BAC, which in turn reflected their greater PC and cortical thickness. The tendency for greater BAC of boys to increase BMCC was partly offset by their lower BMDC.
To determine whether these gender differences in cortical bone geometry could be explained by those in body composition subsequently, we examined the relationship between LM, FM and BMCC. Height was included in these regression models, because this represents an important determinant of BMCC, particularly in boys (standardized regression coefficients, 0.627 and 0.462 in boys and girls, respectively). In addition, inclusion of height in the model reduced the pathway between FM and LM, helping to determine the independent influence of these two parameters (standardized regression coefficient for the association between FM and LM reduced from 0.119 to 0.05 after height adjustment in boys, and from 0.168 to 0.104 after height adjustment in girls). LM was strongly related to BMCC to a similar extent in boys and girls (coefficient, 0.952; P = 0.854 for gender interaction). A positive relationship was also present between BMCC and FM, but this association was considerably stronger in girls (coefficients, 0.227 and 0.355 in boys and girls, respectively) (Table 2).
TABLE 2.
Determinants of BMCC
| Exposure | Adjusting | Sex | β | 95% CI | P | Sex interaction |
|---|---|---|---|---|---|---|
| Height | M | 0.627 | 0.588, 0.666 | 0.0001 | 0.0001 | |
| F | 0.462 | 0.418, 0.506 | 0.0001 | |||
| LM | M | 0.956 | 0.919, 0.993 | 0.0001 | 0.0390 | |
| F | 1.028 | 0.971, 1.085 | 0.0001 | |||
| Height | M | 0.955 | 0.900, 1.011 | 0.0001 | 0.8584 | |
| F | 0.947 | 0.872, 1.021 | 0.0001 | |||
| FM | M | 0.330 | 0.282, 0.378 | 0.0001 | 0.0077 | |
| F | 0.417 | 0.375, 0.458 | 0.0001 | |||
| Height | M | 0.227 | 0.185, 0.269 | 0.0001 | 0.0001 | |
| F | 0.355 | 0.319, 0.392 | 0.0001 |
Table shows results for regression between LM, FM, and BMCC, with or without adjustment for height, in 1851 boys and 2154 girls. Pooled coefficients: LM adj Height and β = 0.952; 95% CI, 0.908, 0.997; P = 0.0001.
To explore these relationships between LM, FM, and BMCC, we then examined associations between indices of body composition and different components of BMCC. The latter is influenced by three potentially independent biological processes, namely periosteal expansion (reflected by PC), endosteal relative to periosteal expansion (reflected by ECPC, which is closely related to cortical thickness), and intracortical remodeling/mineralization (reflected by BMDC). As predicted, the contribution of these parameters to the overall variation in BMCC increased as additional variables were added (r2 = 0.62, 0.95, and 0.99, respectively, for BMCC = PC, BMCC = PC + ECPC, and BMCC = PC + ECPC + BMDC).
Regression models for PC were adjusted for height, which was strongly related (coefficient, 0.509 in boys and girls) (Table 3). LM was positively related to PC, with a stronger association evident in girls (coefficients, 0.579 and 0.799 in boys and girls, respectively). FM was also positively related to PC to a greater extent in girls (coefficients, 0.213 and 0.312 in boys and girls, respectively). Because EC was related to both height and PC, ECPC was also adjusted for height (Table 3). LM was inversely related to ECPC to a similar extent in boys and girls (coefficient, −0.597 for boys and girls combined). FM was also inversely associated with ECPC, but in this instance the association was considerably stronger in girls (coefficients, −0.059 and −0.181 in boys and girls, respectively).
TABLE 3.
Determinants of cortical bone size
| Exposure | Adjusting | Sex | β | 95% CI | P | Sex interaction |
|---|---|---|---|---|---|---|
| PC | ||||||
| Height | M | 0.510 | 0.474, 0.546 | 0.0001 | 0.9307 | |
| F | 0.508 | 0.466, 0.549 | 0.0001 | |||
| LM | M | 0.690 | 0.652, 0.728 | 0.0001 | 0.0001 | |
| F | 0.984 | 0.925, 1.044 | 0.0001 | |||
| Height | M | 0.579 | 0.522, 0.635 | 0.0001 | 0.0001 | |
| F | 0.799 | 0.722, 0.875 | 0.0001 | |||
| FM | M | 0.297 | 0.252, 0.341 | 0.0001 | 0.0041 | |
| F | 0.383 | 0.344, 0.422 | 0.0001 | |||
| Height | M | 0.213 | 0.174, 0.253 | 0.0001 | 0.0002 | |
| F | 0.312 | 0.278, 0.347 | 0.0001 | |||
| EC | ||||||
| Height | M | 0.244 | 0.197, 0.290 | 0.0001 | 0.0018 | |
| F | 0.355 | 0.302, 0.407 | 0.0001 | |||
| PC | M | 0.799 | 0.765, 0.833 | 0.0001 | 0.0049 | |
| F | 0.869 | 0.834, 0.903 | 0.0001 | |||
| Height, fat, lean | M | 1.124 | 1.086, 1.162 | 0.0001 | 0.5769 | |
| F | 1.111 | 1.074, 1.147 | 0.0001 | |||
| LM | M | 0.218 | 0.165, 0.272 | 0.0001 | 0.0001 | |
| F | 0.510 | 0.427, 0.593 | 0.0001 | |||
| Height, PC | M | −0.605 | −0.655, −0.555 | 0.0001 | 0.5328 | |
| F | −0.580 | −0.650, −0.509 | 0.0001 | |||
| FM | M | 0.180 | 0.127, 0.234 | 0.0001 | 0.9201 | |
| F | 0.184 | 0.137, 0.231 | 0.0001 | |||
| Height, PC | M | −0.059 | −0.096, −0.021 | 0.0022 | 0.0001 | |
| F | −0.181 | −0.215, −0.146 | 0.0001 |
Table shows result for regression between height, LM, FM, and PC, with or without adjustment for height, in 1851 boys and 2154 girls. Pooled coefficients PC, height β = 0.509; 95% CI, 0.482, 0.536; P = 0.0001. Pooled coefficients EC, PC adj height/FM/LM β = 1.117; 95% CI, 1.088, 1.146; P = 0.0001; LM adj height/PC β = −0.597, 95% CI, −0.642, −0.553; P = 0.0001; FM β = 0.182, 95% CI, 0.147, 0.218; P = 0.0001.
In analyzing relationships between body composition and BMDC, regression models were adjusted for height (which was positively related to BMDC in boys but not girls), PC (which was inversely related to BMDC particularly in girls), and EC (which was inversely related to BMDC to a similar extent in both sexes) (Table 4). In this adjusted model, LM was positively related to BMDC in boys, whereas there was no association in girls (coefficients, 0.443 and 0.014 in boys and girls, respectively). Very weak associations were observed between FM and BMDC (coefficient, 0.046 for boys and girls combined).
TABLE 4.
Determinants of BMDC
| Exposure | Adjusting | Sex | β | 95% CI | P | Sex interaction |
|---|---|---|---|---|---|---|
| Height | M | 0.208 | 0.170, 0.245 | 0.0001 | 0.0001 | |
| F | −0.035 | −0.078, 0.007 | 0.1001 | |||
| PC | M | −0.038 | −0.077, 0.000 | 0.0526 | 0.0001 | |
| F | −0.221 | −0.260, −0.181 | 0.0001 | |||
| Height, EC, fat, lean | M | −0.182 | −0.255, −0.109 | 0.0001 | 0.0102 | |
| F | −0.057 | −0.131, 0.017 | 0.1306 | |||
| EC | M | −0.237 | −0.270, −0.204 | 0.0001 | 0.4192 | |
| F | −0.256 | −0.289, −0.223 | 0.0001 | |||
| Height, PC, fat, lean | M | −0.207 | −0.257, −0.157 | 0.0001 | 0.2859 | |
| F | −0.240 | −0.292, −0.188 | 0.0001 | |||
| LM | M | 0.344 | 0.302, 0.386 | 0.0001 | 0.0001 | |
| F | −0.097 | −0.162, −0.031 | 0.0037 | |||
| PC, height, EC | M | 0.443 | 0.382, 0.505 | 0.0001 | 0.0001 | |
| F | 0.014 | −0.070, 0.097 | 0.7466 | |||
| FM | M | 0.059 | 0.016, 0.102 | 0.0068 | 0.0084 | |
| F | −0.017 | −0.055, 0.020 | 0.3682 | |||
| PC, height, EC | M | 0.046 | 0.018, 0.074 | 0.0014 | 0.6862 | |
| F | 0.041 | 0.002, 0.080 | 0.0409 |
Table shows association between height, PC, EC, LM, FM, and BMDC, with or without adjustment for other relevant confounding factors, in 1851 boys and 2154 girls. Pooled coefficients: EC β = −0.246; 95% CI, −0.270, −0.223; P = 0.0001; EC adj height/PC/FM/LM, β = −0.219; 95% CI, −0.255, −0.183; P = 0.0001; FM adj PC/height/EC β = 0.046; 95% CI, 0.018, 0.074; P = 0.0014.
Next, we examined the relative contribution of effects of LM and FM on BMCC acting via PC, ECPC, and BMDC by deriving coefficients for these three distinct pathways. Pathway coefficients were calculated based on regressions from two sets of relationships, namely FM/LM vs. PC/ECPC/BMDC (Tables 3 and 4) and PC/ECPC/BMDC vs. BMCC (see Table 5). Where there was no evidence of a sex interaction, the pooled estimate (shown in the table legend) was used in the calculation of each path. In boys, the ECPC pathway makes the strongest contribution to the influence of LM on BMCC as judged by magnitude of the pathway coefficient, with an additional important contribution from the PC pathway and a smaller contribution from the BMDC pathway (Fig. 1A). In girls, coefficients representing the contributions of the ECPC and PC pathways to the effect of LM on BMCC were broadly equivalent to that of the ECPC pathway in boys, whereas there was no evidence of a contribution via BMDC.
TABLE 5.
Relative contributions of PC, EC, and BMDC to BMCC
| Exposure | Adjusting | Sex | β |
|---|---|---|---|
| PC | EC, BMDC | M | 1.525 |
| F | 1.456 | ||
| EC | PC, BMDC | M | −0.832 |
| F | −0.789 | ||
| BMDC | EC, PC | M | 0.228 |
| F | 0.191 |
Table shows the independent associations between PC, EC, BMDC, and BMCC in 1851 boys and 2154 girls.
FIG. 1.
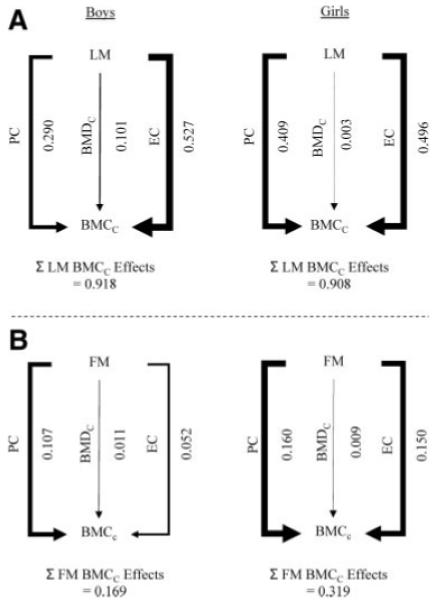
Pathway coefficients for the effects of LM (A) and FM (B) on BMCC via PC, EC adjusted for PC, and BMDC in boys and girls. The sum of these pathways, representing the overall influence of LM or FM on BMCC is shown at the bottom. Line thickness depicts overall effect size.
In terms of effects of FM on BMCC, in boys, this primarily involved the PC pathway, with a smaller contribution via ECPC and a negligible contribution from BMDC (Fig. 1B). In girls, the coefficient for the PC pathway between FM and BMCC was approximately 50% stronger than in boys; moreover, that for the ECPC pathway was 190% higher in girls compared with boys and almost equivalent to that of the PC pathway.
In sensitivity analyses, models investigating the effects of FM on bone parameters were refitted adjusting for LM, height, and interactions with sex. Similar associations were observed with primary analyses. The FM effect on bone parameters was stronger in girls compared with boys: FM and BMCC, coefficients, 0.222 (95% CI, 0.187, 0.257) and 0.275 (95% CI, 0.244, 0.307) for boys and girls, respectively (P = 0.027 for gender interaction); FM and PC, coefficients, 0.210 (95% CI, 0174, 0.247) and 0.245 (95% CI, 0.211, 0.278) for boys and girls, respectively (P = 0.2 for gender interaction); FM and ECPC, coefficients, −0.097 (95% CI, −0.132, −0.062) and −0.160 (95% CI, −0.192, −0.128) (P = 0.009 for gender interaction).
Discussion
In this analysis of the relationship between body composition and pQCT parameters at the midtibia in a large cohort of adolescent boys and girls, a strong positive association was observed between LM and BMCC, consistent with previous studies suggesting that LM represents an important determinant of cortical bone mass accrual in childhood (7, 22, 24). Considering the gender differences in LM which we found (approximately 2.5 sd higher in boys compared with girls) and the strength of the relationship between LM and cortical bone mass (approximately 1 sd increase in cortical bone mass per sd increase in LM), if anything, the gender difference in cortical bone mass evident at this age (approximately 1–1.5 sd higher in boys compared with girls) was smaller than predicted from these relationships. In terms of possible factors acting to increase bone mass in girls relative to boys, FM, which is considerably higher in girls, was also found to be positively related to cortical bone mass, particularly in girls. Taken together, these observations suggest that whereas the greater LM in boys contributes to their greater cortical bone mass, this effect is partly counteracted by the greater FM in girls.
In terms of the mechanisms by which body composition influences cortical bone mass, previous studies have largely focused on influences on periosteal expansion. For example, several lines of evidence suggest that LM exerts a positive influence on periosteal expansion, leading to an increase in overall bone size (7, 19, 21, 22). However, in the present analyses, LM appeared to increase cortical bone mass predominantly through reduced ECPC, at least in boys. Taken together, our results suggest that any tendency for LM to increase cortical bone mass by increasing PC is partly offset by associated increases in EC, and that substantial gains in cortical bone mass only occur when there is coexistent suppression of endosteal expansion. That LM is inversely related to ECPC is consistent with a previous report in the GOOD study that LM is positively related to cortical thickness (which is inversely related to ECPC) in 18-yr-old men (19). Interestingly, increases in BMDC also contributed to the positive effect of LM on cortical bone mass, at least in boys. Although the mechanism involved in this action remains unclear, any tendency for LM to suppress resorption, as presumably underlies reduced endosteal expansion, might also be expected to increase BMDC as a consequence of reduced intracortical remodeling, if such an activity extends to the cortex itself.
Contrary to the case for LM, which showed similar associations with BMCC in both genders, the association between FM and BMCC was somewhat stronger in girls compared with boys. The relationship between FM and BMCC was partly accounted for by an effect on periosteal expansion as reflected by PC, which also showed a stronger relationship with FM in girls compared with boys. Moreover, suppression of endosteal expansion, as reflected by ECPC, also contributed to the positive relationship between FM and BMCC, particularly in girls. On the other hand, FM had little effect on cortical bone mineralization, as reflected by BMDC, in either gender.
The present findings are consistent with our previous report in the same cohort of a positive association between FM and bone mass as measured by DXA, which we interpreted as a positive effect of FM on periosteal expansion based on equivalent association of FM with height-adjusted bone area (16). These results are also in keeping with those from the GOOD cohort that FM is positively associated with PC of the tibia in young adult men (19). However, in contrast to the GOOD cohort, the present study included both genders, enabling us to evaluate sexual dimorphism in terms of how body composition affects cortical bone development. Moreover, we are not aware of any previous reports that FM acts to inhibit endosteal expansion independently of any effect on periosteal apposition. On the other hand, our findings are seemingly at odds with the recent report by Taes et al. (21) that FM is inversely associated with both radial and tibial PC. Other than differences in maturational status, one potential explanation for these apparently conflicting findings is that in their analyses of associations between pQCT parameters and FM, Taes et al. (21) adjusted for both height and body weight, which might lead to spurious results as a result of overadjustment for confounding factors. However, in sensitivity analyses intended to explore this question in our dataset, we found that when associations between BMCC and FM were adjusted for LM as well as height, broadly similar results were obtained to our primary analysis.
Because the present study represents a cross-sectional analysis, it is difficult to infer causality. Nevertheless, the positive associations between FM and pQCT parameters that we observed may well represent a causal pathway between fat and bone, based on results of our recent instrumental variable analysis of associations between FM, bone mass as measured by DXA, and genetic markers of obesity (18). One potential pathway by which FM influences cortical bone mass accrual is as a consequence of weight-bearing activity. In support of this possibility, in the GOOD study, relationships between FM and PC were considerably weaker for radial as opposed to tibial measures (19). On the other hand, in our previous DXA analysis, positive relationships were seen between FM and upper as well as lower limb bone mass (16).
To the extent that FM represents a direct stimulus for accrual of cortical bone, the precise pathway is currently unclear. One possible route is factors produced by adipocytes that subsequently act on the skeleton. Whereas leptin has, if anything, been found to be inversely related to cortical bone parameters, because these analyses were adjusted for both FM and LM, the issue of overadjustment alluded to above may also apply here (19). Alternatively, adiponectin could play a role because this is apparently inversely related both to FM and bone mass (25, 26). Other pathways related to insulin resistance that are up-regulated in obesity could also be involved, such as IGF-I, which is recognized as a potent stimulator of periosteal bone growth in animal models (27). However, whereas insulin resistance is a feature of central obesity (28), in further analyses, we observed equivalent associations between BMCC and peripheral and truncal FM (results not shown). Our finding that FM is related to cortical bone mass most strongly in girls also raises the possibility of an interaction with sex hormones such as estrogen. This may apply particularly to inhibitory effects of FM on endosteal expansion, for which we observed that differential gender effects were most pronounced, and which action estrogen has previously been reported to share (29). Adipocytes are known to express aromatase enzymes that are responsible for maintaining estrogen levels in postmenopausal women. Any tendency for increased FM to enhance estrogenic activity in adolescent girls represents a possible mechanism for the inverse association between FM and endosteal expansion that we observed, particularly in females.
In summary, we examined relationships between FM, LM, and pQCT parameters as measured at the midtibia in 4005 boys and girls (mean age, 15.5 yr). Although LM was the major determinant of BMCC, FM also exerted an important positive influence, particularly in girls, in which the effect was approximately 70% greater than in boys. Subsequent pathway analysis revealed that FM has a stronger tendency both to stimulate periosteal expansion and to reduce endosteal expansion in girls compared with boys. Further studies are justified to examine the biological basis for these apparent effects of FM at the endosteal and periosteal envelopes and to explore possible clinical implications, such as whether development of the female skeleton is preferentially affected by conditions such as anorexia nervosa associated with reduced FM.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
The UK Medical Research Council, the Wellcome Trust, and the University of Bristol provide core support for ALSPAC. Salary support for A.S. is provided by Wellcome Trust Grant ref 079960, which also funded the pQCT scans. This publication is the work of the authors who serve as guarantors for the contents of this paper.
Abbreviations
- BAC
Cortical bone area
- BMC
Bone mineral content
- BMCC
cortical BMC
- BMD
bone mineral density
- BMDC
cortical BMD
- CI
confidence interval
- DXA
dual-energy x-ray absorptiometry
- EC
endosteal circumference
- FM
fat mass
- LM
lean mass
- PC
periosteal circumference
- pQCT
peripheral quantitative computed tomography
Footnotes
Disclosure Summary: A.S. and J.H.T. have nothing to declare.
References
- 1.Seeman E. Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med. 2003;349:320–323. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C. Osteoporosis—an epidemiological perspective: a review. J R Soc Med. 1989;82:753–757. doi: 10.1177/014107688908201217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forwood MR, Bailey DA, Beck TJ, Mirwald RL, Baxter-Jones AD, Uusi-Rasi K. Sexual dimorphism of the femoral neck during the adolescent growth spurt: a structural analysis. Bone. 2004;35:973–981. doi: 10.1016/j.bone.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Högler W, Blimkie CJ, Cowell CT, Kemp AF, Briody J, Wiebe P, Farpour-Lambert N, Duncan CS, Woodhead HJ. A comparison of bone geometry and cortical density at the mid-femur between prepuberty and young adulthood using magnetic resonance imaging. Bone. 2003;33:771–778. doi: 10.1016/s8756-3282(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 5.Kontulainen SA, Macdonald HM, Khan KM, McKay HA. Examining bone surfaces across puberty: a 20-month pQCT trial. J Bone Miner Res. 2005;20:1202–1207. doi: 10.1359/JBMR.050214. [DOI] [PubMed] [Google Scholar]
- 6.Nieves JW, Formica C, Ruffing J, Zion M, Garrett P, Lindsay R, Cosman F. Males have larger skeletal size and bone mass than females, despite comparable body size. J Bone Miner Res. 2005;20:529–535. doi: 10.1359/JBMR.041005. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald H, Kontulainen S, Petit M, Janssen P, McKay H. Bone strength and its determinants in pre- and early pubertal boys and girls. Bone. 2006;39:598–608. doi: 10.1016/j.bone.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 8.Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord. 2000;24:627–632. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 10.Lazcano-Ponce E, Tamayo J, Cruz-Valdez A, Díaz R, Hernández B, Del Cueto R, Hernández-Avila M. Peak bone mineral area density and determinants among females aged 9 to 24 years in Mexico. Osteoporos Int. 2003;14:539–547. doi: 10.1007/s00198-002-1363-2. [DOI] [PubMed] [Google Scholar]
- 11.Mobley SL, Ha E, Landoll JD, Badenhop-Stevens NE, Clairmont A, HGoel P, Matkovic V. Children with bone fragility fractures have reduced bone mineral areal density at the forearm and hip and higher percent body fat. J Bone Miner Res. 2005;20(Suppl 1):S34. [Google Scholar]
- 12.Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J Bone Miner Res. 2001;16:1337–1342. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- 13.Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–207. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 14.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–576. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998;83:3469–3475. doi: 10.1210/jcem.83.10.5204. [DOI] [PubMed] [Google Scholar]
- 16.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janicka A, Wren TA, Sanchez MM, Dorey F, Kim PS, Mittelman SD, Gilsanz V. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–147. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 18.Timpson NJ, Sayers A, Davey-Smith G, Tobias JH. How does body fat influence bone mass in childhood? A Mendelian randomisation approach. J Bone Miner Res. 2009;24:522–533. doi: 10.1359/jbmr.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorentzon M, Landin K, Mellström D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006;21:1871–1878. doi: 10.1359/jbmr.060814. [DOI] [PubMed] [Google Scholar]
- 20.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 21.Taes YE, Lapauw B, Vanbillemont G, Bogaert V, De Bacquer D, Zmierczak H, Goemaere S, Kaufman JM. Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab. 2009;94:2325–2331. doi: 10.1210/jc.2008-2501. [DOI] [PubMed] [Google Scholar]
- 22.Fricke O, Sumnik Z, Tutlewski B, Stabrey A, Remer T, Schoenau E. Local body composition is associated with gender differences of bone development at the forearm in puberty. Horm Res. 2008;70:105–111. doi: 10.1159/000139153. [DOI] [PubMed] [Google Scholar]
- 23.Golding J, Pembrey M, Jones R. ALSPAC-the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15:74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 24.Foley S, Quinn S, Jones G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone. 2009;44:752–757. doi: 10.1016/j.bone.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Jeffery AN, Murphy MJ, Metcalf BS, Hosking J, Voss LD, English P, Sattar N, Wilkin TJ. Adiponectin in childhood. Int J Pediatr Obes. 2008;3:130–140. doi: 10.1080/17477160801954538. [DOI] [PubMed] [Google Scholar]
- 26.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 27.Tobias JH, Chow JW, Chambers TJ. Opposite effects of insulin-like growth factor-I on the formation of trabecular and cortical bone in adult female rats. Endocrinology. 1992;131:2387–2392. doi: 10.1210/endo.131.5.1425437. [DOI] [PubMed] [Google Scholar]
- 28.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2:231–237. doi: 10.1242/dmm.001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Alén M, Nicholson PH, Halleen JM, Alatalo SL, Ohlsson C, Suominen H, Cheng S. Differential effects of sex hormones on peri- and endocortical bone surfaces in pubertal girls. J Clin Endocrinol Metab. 2006;91:277–282. doi: 10.1210/jc.2005-1608. [DOI] [PubMed] [Google Scholar]


